Acute asthma exacerbation after SARS-CoV-2 vaccine(Sinovac®):a case report
2022-10-30FatihUzerAykutCilli
Fatih Uzer,Aykut Cilli
Department of Pulmonology,Akdeniz University School of Medicine,Antalya,Turkey
Abstract A 76-year-old female received a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (CoronaVac,Sinovac®,Beijing,China) and subsequently experienced chest discomfort.A computed tomography performed 1 day after vaccination showed multiple inf iltrations in both lungs and ground-glass shadows in both lung f ields.Her f ingertip oxygen saturation was 81% and there was widespread wheezing on physical examination.Based on these f indings,the patient was hospitalized with a preliminary diagnosis of drug-induced pneumonitis and acute asthma exacerbation due to a SARS-CoV-2 vaccine.During her hospitalization,40 mg/d systemic steroid,4 times a day salbutamol nebulized,2 L/min inhaled oxygen therapy and 400 mg/d moxif loxacin intravenous were administered for 5 days.One month later,the thorax computed tomography scan revealed that the previous f indings were almost completely regressed.
Key words: adverse event;allergy;computed tomography;inf iltrations;SARS-CoV-2;side eff ect;systemic inf lammation;vaccine
INTRODUCTION
Coronavirus disease 2019 (COVID-19) f irst appeared in China in December 2019 and was soon declared as a pandemic.The disease has negative eff ects on economics,health systems and also society,around the world.Shortly after the disease emerged,relevant studies began all over the world,to f ind a medication to treat the disease and to develop a vaccine to prevent it.Germany,UK,USA,Russia and China developed the f irst vaccines,and these vaccines have been used in many diff erent parts of the world.Turkey,along with Brazil,is one of the f irst countries to import and use the vaccine Sinovac®from China.It is reported that the vaccine’s protection rate is 50-83.5%.1-4Until now,vaccine-related side eff ects such as pain at the vaccination site,rash,systemic allergic reaction,cough and fever have been reported.5-8We present a patient who presented to our outpatient clinic 1 day after the vaccination with the complaint of severe shortness of breath.
CASE REPORT
A 76-year-old female patient,who did not have a history of close contact with a person with COVID-19,admitted to the outpatient clinic with the complaint of shortness of breath one day after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (CoronaVac,Sinovac®,Beijing,China).On admission,the patient’s temperature was 36.5°C,respiratory rate was 25 breaths per minute,heart rate was 116 beats per minute,f ingertip oxygen saturation (Phillips,Beijing,China) was 81% and there was widespread wheezing in both lung f ields.Other systemic examinations were normal.The patient’s previous medical history included bronchial asthma,diabetes mellitus,and hypertension,and her family history was unremarkable.Her body mass index was 32.2 kg/m2.She did not smoke and had not previously suff ered an allergic reaction to any medicine or vaccination.In laboratory tests,hemoglobin was measured as 12.2 mg/dL,white blood cell count was 12,600/mm3,eosinophil was 9%,sedimentation was 40 mm/h,C-reactive protein was 25 mg/L and brain natriuretic peptide was 181 ng/L.In the arterial blood gas sample taken in room air,pH was measured as 7.45,partial pressure of oxygen 43 mmHg,partial pressure of carbon dioxide 35 mmHg.
Inf iltrations were seen in chest radiography at bilateral lower zones,predominantly peripheral (Figure 1).In the computed tomography (CT);peribronchial thickening in peribronchial subpleural distances,ground-glass shadows,and areas of increased ventilation at the lobular level were observed in all zones and in both lungs (Figure 2).Ejection fraction was 65%in echocardiography,and no pathological change was detected.The SARS-CoV-2 reverse transcriptase ribonucleic acid test for COVID-19 was detected as negative.
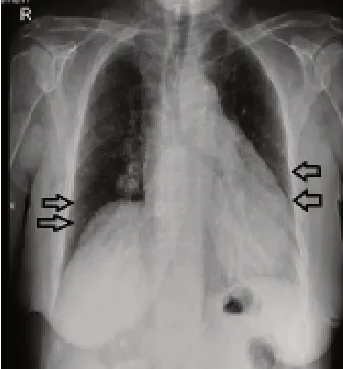
Figure 1:Chest X-ray of the patient:inf iltration at bilateral low zones.
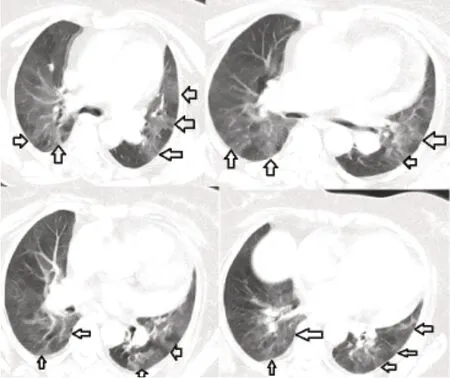
Figure 2:Thorax CT of the patient immediately after the vaccination.
The patient was hospitalized with a preliminary diagnosis of drug-induced pneumonitis and acute asthma exacerbation.During her hospitalization,40 mg/d methylprednisolone (Prednol®,Istanbul,Turkey),4 times a day salbutamol (Ronkotol®,Ankara,Turkey) nebulized and 2 L/min inhaled oxygen therapy and 400 mg/d moxif loxacin (Avelox®,Kırklareli,Turkey) intravenous were administered for 5 days.On the 5thday,the patient’s complaints regressed signif icantly,and she was discharged with a follow-up appointment after 1 month.One month later,the thorax CT scan revealed that the previous f indings were almost completely regressed and there were signs of small airway diseases (Figure 3).The patient,who had no additional complaints,was advised to come to her routine check-ups for asthma.
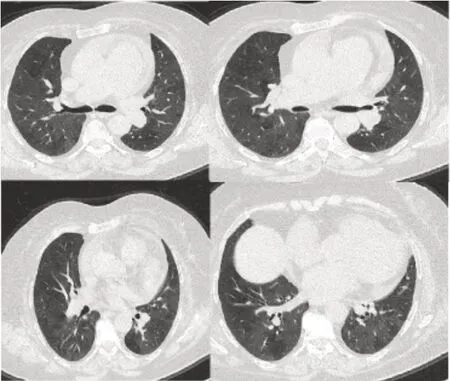
Figure 3:Thorax CT of the patient after 1 month.
DISCUSSION
As COVID-19 vaccines were started to be administered widespread,a wider spectrum of side eff ects started to be seen.Local side eff ects such as increased temperature,redness and pain at the vaccination site are quite common,while systemic side eff ects are less commonly seen.5-8We think that the patient,who developed shortness of breath one day after the vaccination and had inf iltrations in the CT of the thorax,had these symptoms and f indings due to the vaccine.Although there is no such side eff ect def ined in the literature due to CoronaVac(Sinovac®,Beijing,China) vaccine,there is a case of systemic inf lammatory response syndrome reported by Steinberg et al.9due to mRNA-1273 SARS-CoV-2 vaccine.Our patient’s clinic did not fulf ill the systemic inf lammatory response syndrome criteria.However,as the patient’s complaints started right after vaccination and there were inf iltrative images present on chest radiography,these may support that the patient had a systemic inf lammatory response.
Numata et al.10published a case report in 2018,in which,the patient developed pulmonary inf iltrates after receiving seasonal f lu vaccine.They diagnosed the patient with druginduced pneumonitis with the trans-bronchial lung biopsy performed,and followed up without treatment.They reported that the patient’s f indings resolved completely after 6 months.Although pathological examination was not performed in our case,we thought that the suddenly developed inf iltrations might be a reaction to the vaccine.Computed tomography of the patient,whose complaints disappeared after a short-term steroid treatment,inf iltration was almost completely regressed in the CT performed 1 month later.
To conclude,COVID-19 vaccines are fairly new vaccines.It is apparent that as the number of vaccinations increases,side eff ects related to the vaccine will emerge in more detail.We think that in patients developing sudden shortness of breath after vaccination,this entity should be taken into consideration.
Author contributions
FU wrote the initial draft,and completed the f inal draft;FU and AC participated in the literature search and proofread the initial draft.Both authors read and approved the f inal manuscript for publication.
Conflicts of interest
No.
Financial support
None.
Institutional review board statement
In Turkey,case reports do not require ethical approval.
Declaration of participant consent
Written informed consent was obtained from the patient.
Reporting statement
This study follows the CAse REport (CARE) statement.
Copyright license agreement
The Copyright License Agreement has been signed by both authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Medical Gas Research的其它文章
- Potential therapeutic effect of oxygen-ozone in controlling of COVID-19 disease
- Perioperative melatonin in COVID-19 patients:benefits beyond sedation and analgesia
- Effects of Iranian Polyherbal Syrup (Zufa syrup)on oxygen saturation and clinical symptoms in suspected patients with COVID-19:a triple-blinded,randomized,placebo-controlled trial
- Ozone gas applied through nebulizatıon as adjuvant treatment for lung respiratory dıseases due to COVID-19 infectıons:a prospective randomized trial
- Prediction of diagnosis and prognosis of COVID-19 disease by blood gas parameters using decision trees machine learning model:a retrospective observational study
- COVID-19 incidence and local ozone level:is there any association?
