Synthesis and properties of La1-xSrxNiO3 and La1-xSrxNiO2
2022-10-26MengwuHuo霍梦五ZengjiaLiu刘增家HualeiSun孙华蕾LisiLi李历斯HuiLui刘晖ChaoxinHuang黄潮欣FeixiangLiang梁飞翔BingShen沈冰andMengWang王猛
Mengwu Huo(霍梦五), Zengjia Liu(刘增家), Hualei Sun(孙华蕾), Lisi Li(李历斯), Hui Lui(刘晖),Chaoxin Huang(黄潮欣), Feixiang Liang(梁飞翔), Bing Shen(沈冰), and Meng Wang(王猛)
Center for Neutron Science and Technology,Guangdong Provincial Key Laboratory of Magnetoelectric Physics and Devices,School of Physics,Sun Yat-Sen University,Guangzhou 510275,China
Keywords: nickel oxide,superconductivity
The discovery of superconductivity in film samples of Nd0.8Sr0.2NiO2has drawn much attention on nickel oxide materials.[1]In fact, LaNiO3was studied in 1983 before the discovery of superconductivity in copper oxide materials.La2-xSrxNiO4were investigated because of the structural similarity to the high temperature superconducting (SC) cuprate perovskites La2-xSrxCuO4. Superconductivity has also been predicted in nickelate heterostructures like LaNiO3/LaMO3(Mis a trivalent cation,like Al or Ga)superlattices,[2,3]while unconventional superconductivity has not been realized in nickel oxide materials with the oxidation states of Ni3+and Ni2+.[4–6]It was suggested that superconductivity may exist in low spin oxidation state of Ni1+with a square coordination with O2-ions.[7–9]However, the low valent state of nickel is difficult to stabilize through conventional high-temperaturesolid state reaction synthesis method.[10,11]
Tuning the valent states of Ni ions in nickelate thin film heterostructures by combining CaH2reductant and carrier doping greatly stimulated scientists to explore superconductivity and other novel physical properties in nickelbased oxides.[1,12–21]Infinite layerRNiO2(R=Nd, La, and Pr) is isostructural to CaCuO2with two-dimensional NiO2planes stacking as NiO2–R–NiO2–Rlayers as shown in Fig.1.CaCuO2is one of the parent compounds of high-temperature superconductors. Through hole-doping the antiferromagnetic(AF)order can be suppressed and superconductivity emerges in (Ca1-xSrx)1-yCuO2-δwith the maximum SC transition temperature ofTc=110 K.[22,23]Furthermore, the spin configuration of Ni1+3d9is similar to that of Cu2+in cuprates.SC domes were observed in films ofR1-xSrxNiO2(R=La,Nd, and Pr),[13–15,24]La1-xCaxNiO2[25]and Nd6Ni5O12.[17]AF spin fluctuations were revealed and unconventional mechanism of superconductivity was proposed.[16,26–28]While the superconductivity observed in nickel-based oxides is all in the form of thin film samples,[1,13–15,17,24,29–32]it is worthwhile to explore superconductivity in bulk samples. Recently bulk samplesR1-xSrxNiO2(R= Sm, Nd)[33–35]and La1-xCaxNiO2[36]were synthesized whereas superconductivity was not detected. So far, electrical and magnetic properties of Sr doped bulk samples of La1-xSrxNiO2have not been reported, where superconductivity has been observed in film samples.
In this paper, we report synthesis and systematic studies of the bulk samples of La1-xSrxNiO3and La1-xSrxNiO2with normal compositions of 0≤x ≤0.4. The actual doping level is around 0≤x ≤0.2 determined from inductively coupled plasma measurements.La1-xSrxNiO3are synthesized using molten KOH as flux. La1-xSrxNiO2are obtained via a topochemical reduction process from La1-xSrxNiO3using CaH2as a reducing agent. Measurements of resistivity, magnetization, and heat capacity are carried out to explore the magnetic and electrical properties. Results of electronic transport show that La1-xSrxNiO3(0≤x ≤0.2) are good metals,while La1-xSrxNiO2exhibit insulating behavior and no superconductivity is observed.
La1-xSrxNiO3with nominal 0≤x ≤0.4 were synthesized using KOH as the flux.[37]La2O3was preheated at 1100°C for one day to remove water then weighed in a glove box filled with argon. Stoichiometric ratios of powders of NiO(99.99%), La2O3(99.9%), and SrCO3(99.99%) were mixed and heated at 1200°C for one day. The flux of KOH was heated at 450°C for 12 h to form O-2via the reaction of 3O2+4OH-⇐⇒4O-2+2H2O,[38]followed by the mixture of the nickelate oxide powders and dwelled at 450°C for 10 h.KOH was removed from powders of La1-xSrxNiO3by deionizing water. Thereafter the powders were dried at 120°C.Finally, dark powders of La1-xSrxNiO3were obtained. The topotactic reduction process was demonstrated in Fig.1. The process was as follows: La1-xSrxNiO3were placed in a crucible and CaH2was wrapped with an aluminum foil. They were sealed in an evacuated silica tube at a molar ratio of 1:4. La1-xSrxNiO2were obtained after heating the mixture at 350°C for 12 h.Fig. 1. Structures of La1-xSrxNiO3(left) and La1-xSrxNiO2(right).Upon low temperature reduction with CaH2as a reducing agent, the powder samples undergo a topotactic transition from the perovskite phase to the infinite-layer phase.
Crystal structures of the samples were investigated by xray diffraction (XRD, Empyrean) at 300 K. The diffraction data were refined by the Rietveld method.[39]The cation contents in powder samples of La1-xSrxNiO3and La1-xSrxNiO2were measured by the inductively coupled plasma (ICP,Atomic Emission Spectrometry, Optima8300). An iodometric titration method was performed to evaluate the content of oxygen in La1-xSrxNiO3.[40]The powder samples were dissolved in HCl with KI (0.1 mol/L) solution. The Ni3+ions will be reduced to Ni2+and iodine is generated during the process. The content of iodine could be determined by Na2S2O3(0.01 mol/L) using starch solution as an indicator. Magnetization and resistivity in the temperature range of 3–300 K and heat capacity measurements in the range of 3–200 K were conducted on a physical property measurement system (PPMS,Quantum Design). The electrical resistivity was measured on a pressed bar sample using a standard 4-prob technique.
The crystal structures of La1-xSrxNiO3and La1-xSrxNiO2are shown in Fig. 1. XRD patterns of the two compounds are depicted in Figs. 2(a) and 2(b), respectively. The XRD patterns of LaNiO3can be indexed as a rhombohedral unit cell and LaNiO2as a tetragonal unit cell as reported previously.[41–44]The XRD patterns for Sr doped compounds indicate that the powder samples are a single phase for La1-xSrxNiO3with 0≤x ≤0.15 and La1-xSrxNiO2with 0.05≤x ≤0.4. To simplify the description, the nominalxis used to present samples with distinct compositions in the article.NiO is identified in La1-xSrxNiO3forx=0.2,0.3,and 0.4. Reflection peaks associated with La2O3can be observed in the XRD pattern of LaNiO2,as shown in Fig.2.Ni impurity can be observed in La1-xSrxNiO2withx=0.3. Variation of the lattice parameters as a function of the Sr contents is not obvious because the sizes of La3+and Sr2+are similar.[35,45]The refined structural parameters are shown in Table 1.
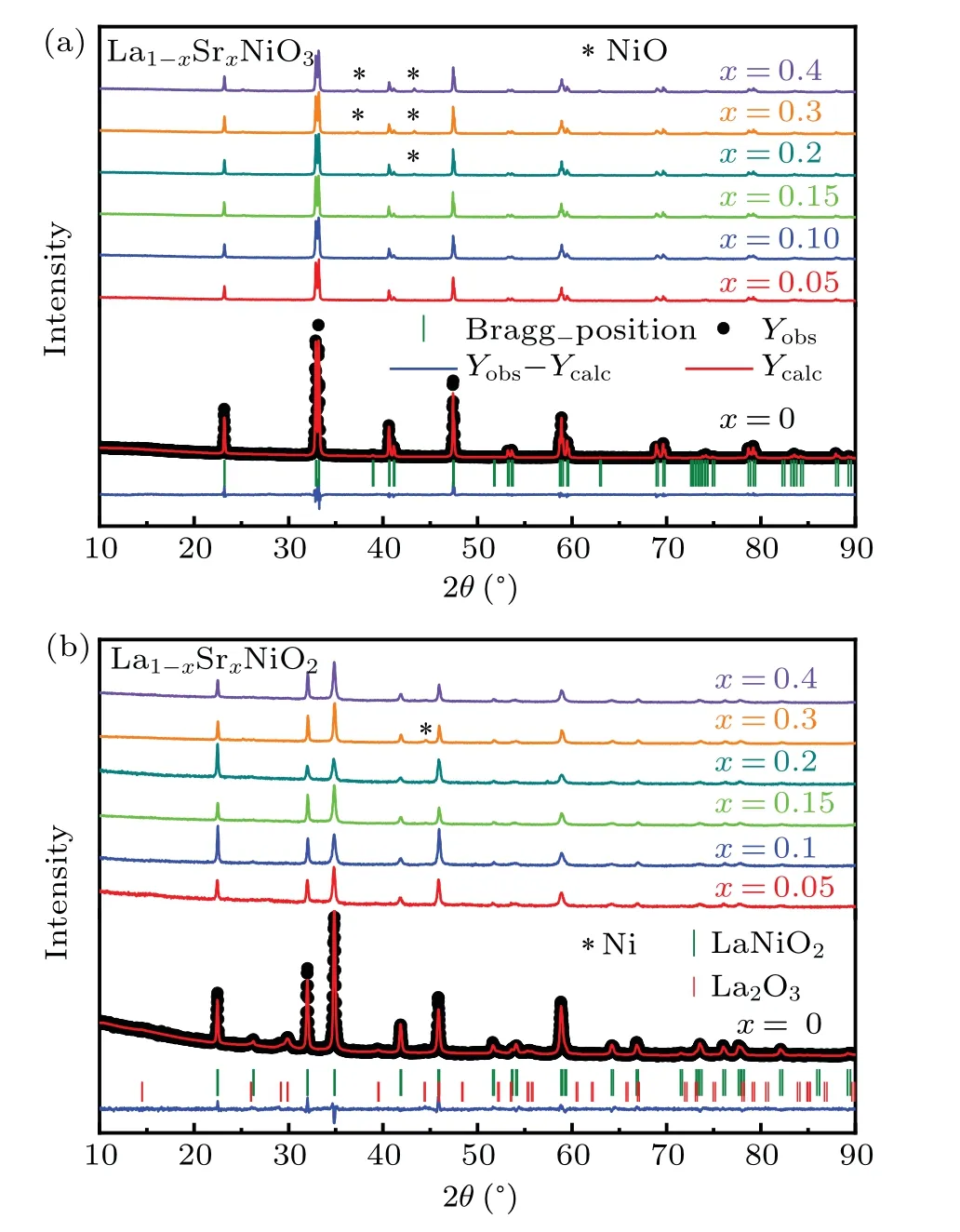
Fig. 2. Ambient powder XRD patterns of (a) La1-xSrxNiO3 and (b)La1-xSrxNiO2 with nominal compositions of 0 ≤x ≤0.4 with corresponding Rietveld fitting results by using the R-3c space group and P4/mmm,respectively. The black dots represent the observed data,the red lines represent the fitting curves,and the blue lines illustrate the differences between the data and fitting.The olive short vertical lines mark the Bragg positions. For comparison the data are shifted vertically. The positions of reflections related to NiO,La2O3,and Ni are indicated.
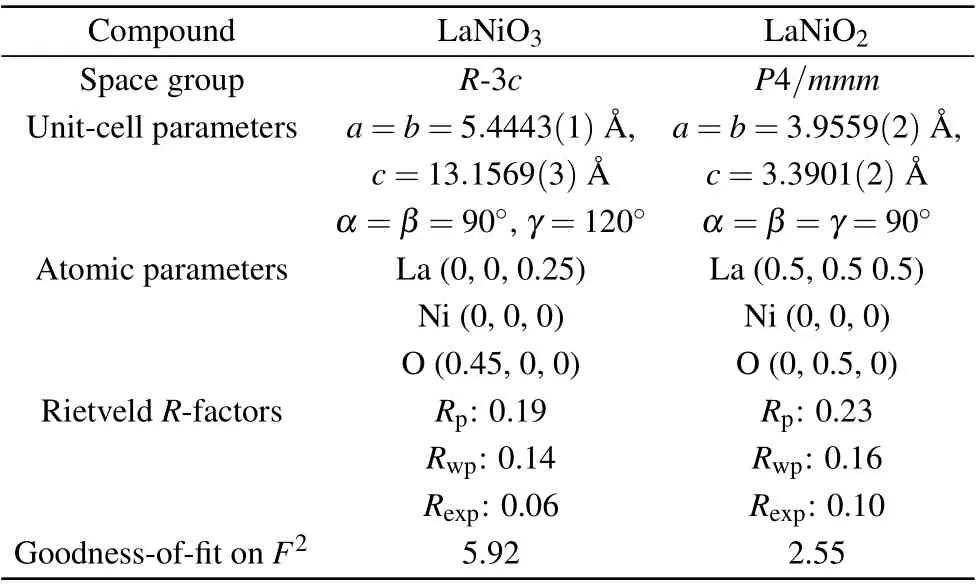
Table 1. Refined structural parameters for LaNiO3 and LaNiO2 at 300 K.
To check the real compositions of the as grown and oxygen removed compounds,we show the actualxverse the nominalxin Fig. 3(a). The relations of the actual and nominal contents of Sr follow the same trend, revealing half of the Sr is successfully doped to the samples. For the nominalx=0.4 compounds, the actual values arex=0.21 and 0.19 for La1-xSrxNiO3and La1-xSrxNiO2, respectively. The big error of the actualxin La0.85Sr0.15NiO3may be due to inhomogeneity of the two samples we measured. The contents of oxygen are further investigated through the iodometric titration method. In this way, the oxygen content is determined from the ratio of Ni3+and Ni2+. The results as shown in Fig.3(b)suggest the existence of 12%oxygen vacancies.
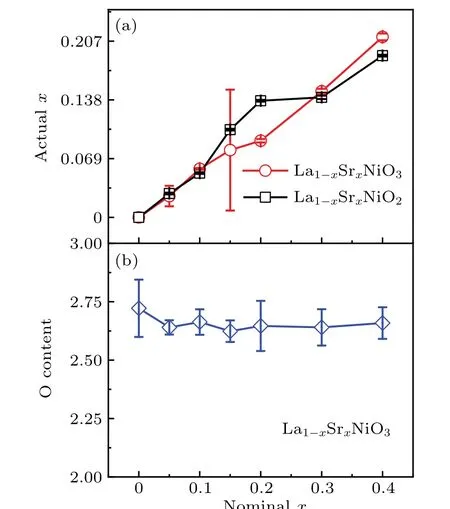
Fig. 3. (a) ICP determined actual x in La1-xSrxNiO3 and La1-xSrxNiO2. (b) Actual oxygen content of La1-xSrxNiO3 measured via the iodometric titration method. The error bars are the standard deviation of the measured results.
Figure 4 displays the temperature dependence of the physical properties of La1-xSrxNiO3.The resistivity and magnetization of LaNiO3are consistent with previous reports that reveal LaNiO3exhibiting a metallic paramagnetic state with large electronic correlations,[46,47]as shown in Figs.4(a)and 4(b). The upturn in resistivity below 50 K has been observed in bulk LaNiO3-δand suggested to result from defection of oxygen during the synthesis process.[48,49]Upon Sr doping,the metallic behavior of La1-xSrxNiO3is remained,while the magnitude of resistivity changes.
Weak kinks can be identified on magnetization at~22 K in doped compounds. The kink is obvious for La0.8Sr0.2NiO3measured withμ0H=0.1 T and zero-field cooled(ZFC)mode as shown in Fig. 4(b). The temperatures of the kinks for different compounds locate at the same temperature. To ascertain the origin of the kinks, we carried out measurements of magnetization and specific heat as a function of temperature at various magnetic fields for La0.8Sr0.2NiO3.The magnetization is gradually suppressed as the magnetic field increases,while the temperature of the anomaly is barely changed[Fig.4(c)].We repeated the process of synthesis and measurements. The kinks in magnetization for the doped samples are repeatable. However, an anomaly around 22 K is not observed on specific heat at 0 T and 5 T magnetic fields as shown in Fig. 4(d). The kinks are absent in Nd1-xSrxNiO3[50]and Sm1-xSrxNiO3.[33]It is possible that the abnormal magnetization in La1-xSrxNiO3at 22 K origins from impurities during the synthesis process.
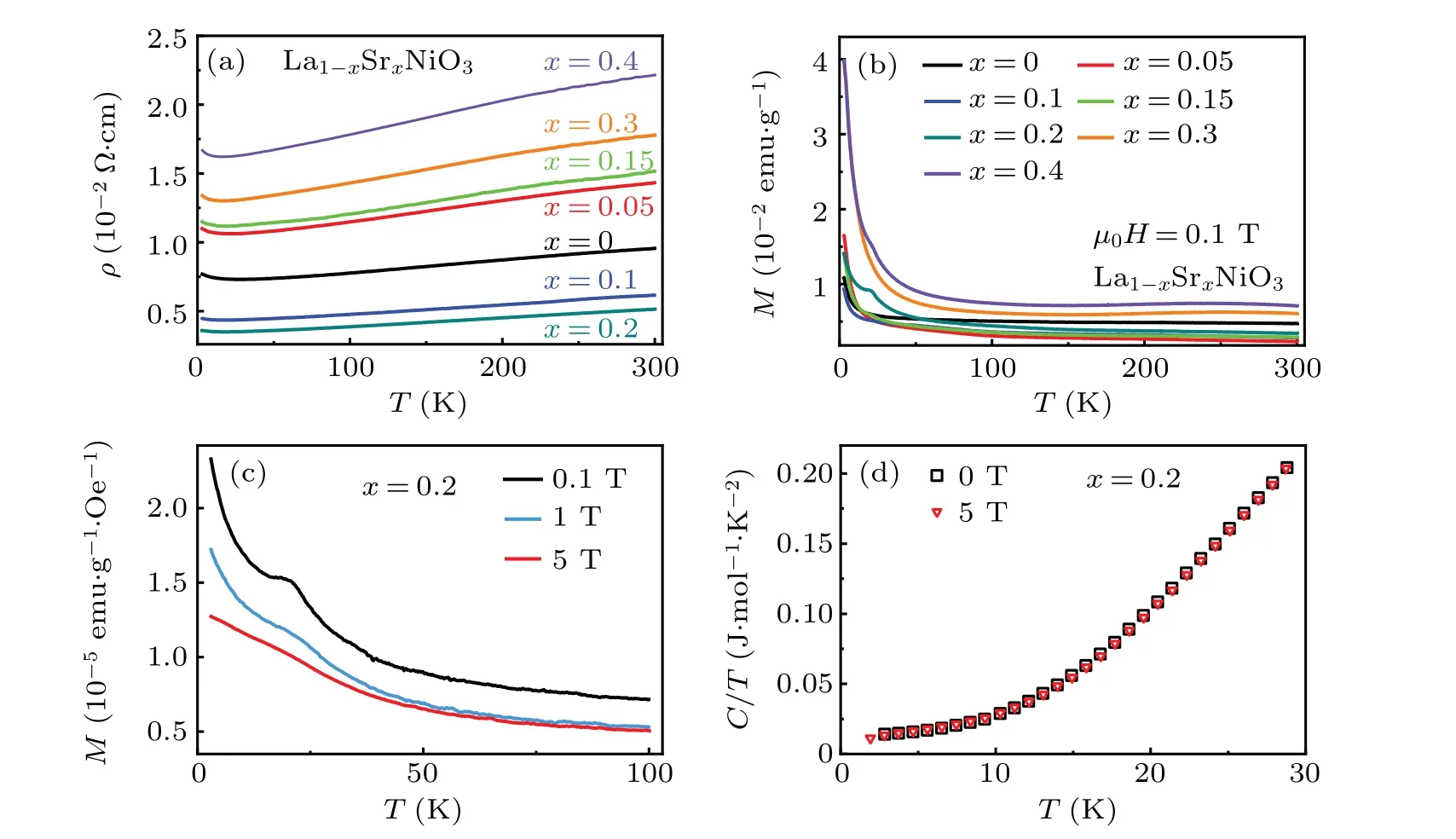
Fig.4. (a)Resistivity versus temperature ρ(T)plots of La1-xSrxNiO3 with nominal compositions of 0 ≤x ≤0.4. (b)Magnetic susceptibility of La1-xSrxNiO3 with a field of μ0H=0.1 T.(c)Magnetization for La0.8Sr0.2NiO3 in ZFC process for magnetic fields of μ0H=0 T,1 T,and 5 T.(d)Specific heat of La0.8Sr0.2NiO3 for 3 K ≤T ≤30 K,and μ0H=0 T and 5 T.
To explore the possible superconductivity in the bulk samples of La1-xSrxNiO2, we performed resistivity and magnetization measurements of La1-xSrxNiO2as shown in Fig. 5. The resistivity versus temperatureρ(T) of La1-xSrxNiO2in zero applied magnetic field in Fig. 4(a) reveals semiconducting-like behavior. The doped Sr does not affect the electronic properties severally. No superconductivity is observed in the bulk samples. In Fig. 5(b), we show the fittings by using the activation-energy modelρ(T)=ρ0exp(E/kBT) through fitting the high temperature resistivity data from 200 K to 300 K, whereρ0is a prefactor andkBis the Boltzmann constant.[34,51–53]The resultant thermal activation energies are between 35–55 meV, as shown in the inset of Fig. 5(b). To explore the magnetic properties of La1-xSrxNiO2,we show magnetization and magnetoresistance as functions of magnetic field and temperature in Figs. 5(c)and 5(d). The magnetization measured in field cooling (FC)deviates from that of ZFC in a wide temperature range, indicating a spin glass state. Figure 5(d)displays temperature dependence of the magnetoresistance(MR)of LaNiO2. The MR effect changes sign from negative at 3 K, 10 K, and 20 K to positive at 30 K and 50 K,similar to the observation in films of Nd0.8Sr0.2NiO2.[34]The sign reversal of the MR upon changing temperature suggests the existence of magnetic order or magnetic correlations. Figure 5(e) shows a typical hysteresis of ferromagnetism(FM)vs.magnetic field at various temperatures measured atT=3 K,50 K,300 K,and 650 K.The FM is reminiscent of the resident nickel in Sr dopedRNiO2.[33,34,54]Therefore,magnetic susceptibility is measured from 300 K to 1000 K to verify the existence of Ni. As shown in Fig. 5(f),the ferromagnetic transition of Ni atTc=627 K is identified in bulk LaNiO2. However, XRD measurements on powder samples of LaNiO2did not reveal the existence of Ni, indicating a low content of Ni impurity in the samples. Thus,the spin-glass like behavior may be due to the Ni impurity. Small amount of Ni in the sample would not change the electronic transport properties. The MR effect should be the intrinsic properties of LaNiO2and induced by magnetic correlations that have been proposed by Raman scattering measurements on NdNiO2[55]and x-ray magnetic linear dichroism measurements on Nd0.8Sr0.8NiO2films.[27]

Fig. 5. (a) Resistivity of La1-xSrxNiO2 as a function of temperature from 3 K to 300 K and (b) fits using the activation-energy model ρ(T)=ρ0exp(E/kBT)from 200 K to 300 K.The inset shows the thermal activation energies against the Sr content. (c)Magnetic susceptibility measured under ZFC and FC conditions for 3 K ≤T ≤400 K and μ0H=0.1 T. (d)Magnetoresistance for LaNiO2 at T =3 K,10 K,20 K,30 K,and 50 K.(e)Magnetization of LaNiO2 at T =3 K,50 K,300 K,and 650 K.(f)Magnetic susceptibility of LaNiO2 from 300 K to 1000 K with μ0H=0.1 T.
As theoretical calculations,RNiO2are metal.[12,56–58]However, resistivity measurements reveal that bulk samples ofRNiO2are semiconductors. It has been suggested that the difference between theoretical predictions and experimental observations could be ascribed to hydrogens that take place the apical oxygen sites to reduce the thermodynamical instability.[59]Small size of hydrogen can insert into the samples and form oxide hydride La1-xSrxNiOyHzwhich exhibit insulating behavior. This has been observed in NdNiOxHy(x~2.3,y~0.7),[60]BaTiO3-xHx,[61]Sr3Co2O4.33H0.84,[62]and LaSrCoO3H0.7.[63]In addition, impurity and incomplete reduction of the apical oxygens inRNiO2could also result in large resistivity.[43,64]It is known that the purity of films is essential for realizing superconductivity in Nd1-xSrxNiO2films.[29,30]The absence of superconductivity in bulk samples may be due to the deviation of O2-ions amount and the existence of Ni impurity. The interfaces between the substrate of SrTiO3and La1-xSrxNiO2films may play a role in the magnetic correlations and emergence of superconductivity.[42,65–67]Further studies on high quality single crystals of La1-xSrxNiO2are required.
In conclusion, we synthesized polycrystalline samples La1-xSrxNiO3and La1-xSrxNiO2with an actual doping level of 0≤x ≤0.2 and characterized their properties. The samples of La1-xSrxNiO3are prepared using KOH as the flux. La1-xSrxNiO2are synthesized by removing the apical oxygen from La1-xSrxNiO3using CaH2as a reducing agent. La1-xSrxNiO3are good metals as expectation. While La1-xSrxNiO2exhibit insulating behavior,differing from theoretical calculations. No evidence of superconductivity is observed. The Ni impurity, resident apical oxygens, inserted hydrogens in bulk La1-xSrxNiO2may mask the fundamental magnetic properties and inhibit the achievement of superconductivity.
Acknowledgements
Work at Sun Yat-Sen University was supported by the National Natural Science Foundation of China (Grant Nos. 12174454, 11904414, 11904416, and U2130101), the Guangdong Basic and Applied Basic Research Foundation(Grant No.2021B1515120015),the Guangzhou Basic and Applied Basic Research Foundation(Grant No.202201011123),and the National Key Research and Development Program of China(Grant No.2019YFA0705702).
杂志排行
Chinese Physics B的其它文章
- Design of vertical diamond Schottky barrier diode with junction terminal extension structure by using the n-Ga2O3/p-diamond heterojunction
- Multiple modes of perpendicular magnetization switching scheme in single spin–orbit torque device
- Evolution of the high-field-side radiation belts during the neon seeding plasma discharge in EAST tokamak
- Phase-matched second-harmonic generation in hybrid polymer-LN waveguides
- Circular dichroism spectra of α-lactose molecular measured by terahertz time-domain spectroscopy
- Recombination-induced voltage-dependent photocurrent collection loss in CdTe thin film solar cell
