Prohibitin Protein Expression During Spermatogenesis in the Large Yellow Croaker,Larimichthys crocea
2022-10-24LINChenwenGAOXinmingNIJieZHANGShengshuoLIUChengLUOShengyuDUChenZHENGXuebinHOUCongcongTANGDaojunZHANGChundanandZHUJunquan
LIN Chenwen, GAO Xinming, NI Jie, ZHANG Shengshuo, LIU Cheng, LUO Shengyu, DU Chen, ZHENG Xuebin, HOU Congcong, TANG Daojun, ZHANG Chundan, and ZHU Junquan
Prohibitin Protein Expression During Spermatogenesis in the Large Yellow Croaker,
LIN Chenwen#, GAO Xinming#, NI Jie, ZHANG Shengshuo, LIU Cheng, LUO Shengyu, DU Chen, ZHENG Xuebin, HOU Congcong, TANG Daojun, ZHANG Chundan, and ZHU Junquan*
Key Laboratory of Applied Marine Biotechnology by the Ministry of Education, School of Marine Sciences, Ningbo University, Ningbo 315211, China
Mitochondria are important for animals’ fertility, and their morphologies and functions during spermatogenesis are un- der investigation. However, the molecular mechanism that regulates the mitochondrial dynamic during spermatogenesis is still un- known. In this study, the cytological features of spermatogenesis were investigated in. In addition, the structure and function of prohibitin (PHB), which is associated with mitochondrial structure and dynamic, was also investigated. The full- length cDNA and protein (PHB) from thegene () contained 1625 base pairs and 271 amino acids, respec- tively.PHB had a conserved primary structure that resulted in a transmembrane, SPFH (the analogous region of proteins stomat- ins, prohibitins, flotillins and HflK/C), and coiled-coil domains. It was detected at high levels in the muscle, liver, and heart, and at intermediate levels in the testis, gill, and brain.mRNA expression wasdetected in spermatogenic cells by fluorescencehybridization. An immunofluorescenceassay revealed that PHB protein was localized in the mitochondria during spermatogenesis. Specifically, PHB expression was detected in the perinuclear cytoplasm of spermatogonia, spermatocytes, and spermatids in the early developmental stage, and mainly localized on one side of the nuclei in the cytoplasm of spermatids in a middle developmental stage, and finally on the sperm midpiece. Western blotting showed that PHB was located in the extracted mitochondria protein fraction but not in the cytoplasm protein fraction of testes. Conclusively, these results indicated that PHB was expressed in the mitochondria dur- ing spermatogenesis.In addition, the study explained the mitochondrial dynamic during fish spermatogenesis and proposed a possi- ble relationship among PHB, spermatogenesis, and male fertility.
spermatogenesis; ultrastructure; prohibitin; expression pattern; subcellular localization
1 Introduction
Reproduction is necessary for the perpetuation of living beings. As infertility happens occasionally in some animal individuals, the process and mechanism of gametogenesis of animals are investigated. In aquaculture industry, fish reproduction is involved in fry breeding that directly re- lated to fish gametogenesis. Therefore, the process and me- chanism of fish gametogenesis is an important field of re- search.
Mitochondria are required for male gametogenesis, na-mely spermatogenesis, the process of sperm productionfrom spermatogonia. Mitochondrial defects caused by agene mutation, hypoxia, or an environmental pollutant can result in abnormal spermatogenesis and even male infer- tility (Hales and Fuller, 1997; Huang., 2015; Quan., 2016). In addition, spermatogenesis is regulated by the mitochondria shape, which is dynamic and can adapt to the different metabolic levels in spermatogenic cells. For instance, in adult rat testes (), orthodox mitochondria were observed in spermatogonia and lepto- tene spermatocytes, while condensed mitochondria were detected in spermatocytes at later stages and spermatids, possibly because it was more efficient in producing ATP (Ramalho-Santos., 2009; Rato., 2012). In, mitochondria shape changes from round- ed to an onion-like shape by elongation, fusion, and swell-ing during spermiogenesis (Haseeb., 2018). To under- stand the mitochondria dynamic during spermatogenesis, the functions of some mitochondrial proteins in sperma- togenesis including GASZ, mitofusin 1 (MFN1), mitofusin 2 (MFN2), and dynamin-related protein 1 were investigatedat a molecular level (Honda and Hirose, 2003; Zhang., 2016; Varuzhanyan., 2019). For instance, conditional deletion of the mousegene in the testis that plays a role during mitochondrial fusion caused male in- fertility (Zhang., 2016; Varuzhanyan., 2019), indicating the importance of mitochondrial fusion in sper- matogenesis. However, the molecular mechanism that re- gulates the mitochondrial dynamic and physiology during spermatogenesis is still not completely understood and needs further studied.
Prohibitin (PHB) is an important protein for the mito-chondria morphology and physiology. PHB in the inner mi-tochondrial membrane (IMM) usually forms a complex with PHB2. The complex regulates the membrane protein de- gradation by the association with the m-AAA protease(Steglich., 1999); serves as a chaperone for newly syn- thesized proteins (Nijtmans., 2000, 2002); stabilizes the mtDNA by interacting with the TFAM protein, a com- ponent of the mitochondrial nucleoids (Wang and Bogen- hagen, 2006; Bogenhagen., 2008); maintains the tu- bular cristae, and promotes mitochondrial fusion (Merk- wirth., 2008; Merkwirth and Langer, 2009). These PHB functions are important for mitochondrial biogenesis, metabolic regulation, and cell migration, survival, and pro-liferation (Artal-San and Tavernarakis, 2009; Merkwirth and Langer, 2009). In addition, PHB was also detected in the plasma membrane, inhibiting inflammatory responses and mediating Ras-Raf signaling (Sharma and Qadri, 2004; Rajalingam and Rudel, 2005), and in cell nucleus, regulat- ing the gene transcription (Mishra., 2005, 2006; Thu- aud., 2013). Therefore, the PHB function depends on its location. In reproductive biology, PHB was involved inspermatogenesis and related to sperm quality. For instance,PHB deficiency resulted in abnormal spermatogenesis in(Sanz., 2003)Low levels of PHB inwere associated with low-mo- tility spermatozoa (Xiong., 2018). Meanwhile, in hu- mans, they were related to asthenospermia and oligoas- thenospermia (Wang., 2012). Furthermore, Chai(2017) noted the low level of PHB increased the concen- tration of mitochondrial reactive oxygen species and lipid peroxidation in sperm, lowering the sperm quality. Thus,these results promoted the study of the PHB function in spermatogenesis.
The aquaculture production of,an economically important species of marine fish of the or- der Perciformes and family Sciaenidae, has been ranked top one in the maricultured fishes in 2017 in China with a production of 176600 tons. For this reason,is an important model fish in marine research and its reproduc- tive biology has been widely researched, including the his- tological characteristics of gonadal development and itsbreeding cycle and artificial seedling production (Su., 1997; Fang., 2003; Liu, 2004; You., 2012), whe- reas the molecular mechanisms related to spermatogene- sis are still unknown.
In the present study, we observed the ultrastructure cha- racteristic ofspermatogenesis and analyzed the characteristic, expression, subcellular localization, and pos- sible functions of PHB during spermatogenesisThis studyserves as a basis for future research of the mechanisms of PHB inspermatogenesis
2 Materials and Methods
2.1 Animals and Tissues
All animalexperiments in this paper were performed in accordance with the guidelines and procedures approved by the Animal Care and Use Committee of the Ningbo Uni-versity and the Zhejiang Laboratory Animal Research Cen- ter.
Malefish were sampled from the Xiangshan Fishery (Ningbo City, Zhejiang Province, China). Nine tis- sues (muscles, kidneys, testes, hearts, intestines, spleens, gills, livers, and brains) were harvested, immediately fro- zen in liquid nitrogen, and stored at −80℃ for RNA and protein extraction. Testis sections were fixed in 2.5% glu- taraldehyde in 0.1molL−1phosphate buffered saline (PBS; pH 7.4) for transmission electron microscopy (TEM) vi- sualization, and in 4% paraformaldehyde in 0.1molL−1PBS for fluorescenthybridization (FISH) and im- munofluorescence (IF) analyses.
2.2 Electron Microscopic Analysis of Spermato- genesis in L. crocea
TheTEM experiments characterized the ultrastructural features of spermatogenesis inThe fixed testes in 2.5% glutaraldehyde 0.1molL−1PBS at 4℃ for 24h were rinsed in 0.1molL−1PBS three times for 45min, then fixed in 1% osmium tetroxide in the dark for 1h, and finally washed in 0.1molL−1PBS. The samples subsequen- tly were dehydrated by immersion into increasing concen- trations of ethanol and acetone, treated with an acetone- araldite mixture, and embedded in araldite. Ultrathin sec- tions were obtained with the LKB-α ultramicrotome and stained with uranyl acetate and lead citrate. The ultrathin sections were examined and photographed with an H-7650 transmission electron microscope (Hitachi).
2.3 Full-Length cDNA Cloning of the phb Gene
Total RNA was extracted from Stage IV testis by using the TRIzol Reagent (Invitrogen, USA), according to the manufacturer instructions, subjected to3’ and 5’ RACE re- verse transcription by using the 3’ full RACE Amplifica- tion (Takara, Japan) and Smart RACE cDNA AmplificationKits (Clontech, USA), respectively. The reverse transcribed products (cDNA) were stored at −25℃ for subsequent PCR.
The intermediate segment sequence of(Gen- Bank accession number: XM_010740095.3) was confirm- ed by PCR and gene sequencing. Then, we designed the specific primers by using Primer Premier 5.0 software for RACE (Table 1). The touch-down PCR used for 3’/5’ RACEwas conducted as follows: 94℃ for 5min; 8 cycles of 94℃ for 30s, 62/69℃ for 30s (decreased by 0.5℃/cycle) and 72℃ for 1min; 27 cycles of 94℃ for 30s, 58/65℃ for 30s and 72℃ for 1min; and then a final extension at 72℃ for 10min. These PCR products were separated, iden- tified, and sequenced, based on the method described by Zhang(2017). We assembled the intermediate seg- ment and the clonal 3’ and 5’ cDNA segment sequences and obtained the full-length cDNA of.

Table 1 Primer and probe sequence used in this study for phb cDNA full-length cloning and fluorescentin situ hybridization
2.4 Structure and Property Predictions, Sequence Alignment, and Phylogenetic Analysis
The predictions ofPHB protein structure and proper-ty were conducted with online tools: the Sequence Manipu-lation Suite (http://www.bio-soft.net/sms/) was used for pre- dicting the primary structure; the ProtParam tool (https://web. expasy.org/protparam/) was used for calculating the molecu- lar weight and the isoelectric point; the online tool (https://embnet.vital-it.ch/software/TMPRED_form.html) was usedfor predictingtransmembrane segments; the online tool (https://embnet.vital-it.ch/software/COILS_form.html) was used for detecting coiled-coil domains; and the I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) was usedfor predicting the tertiary structure. Sequence alignment and phylogenetic analyses were performed with the software Vector NTI and Mega 5.1, respectively. The amino acid (aa) sequences of PHB proteins were downloaded from Na- tional Center of Biotechnology Information (https://www. ncbi.nlm.nih.gov/), and their GenBank accession number were listed in Table 2.
Table 2 PHB proteins information and corresponding GenBank accession numbers used in this study
2.5 Quantitative Analysis of Lc-phb mRNA Expression
Real-time quantitative PCR (qPCR) was used for ana- lyzing themRNA expression. The total RNA, ex- tracted from muscles, kidneys, testes, hearts, intestines, spleens, gills, livers, and brains (collected from three male fish), was reversed transcribed by using the Prime-Script®RT reagent Kit (Takara, Japan). The reverse transcription products (cDNA) were stored in −25℃ until performing the qPCR experiment. The primers used for qPCR are listed in Table 1, and thegene was employed as house- keeping gene, based on Luo(2019). The qPCR as- say was conducted in the Rotor-Gene 6000 System. The reaction system contained 5μL 1:25 diluted cDNA, 1μL each primer (10μmolL−1), 3μL PCR-grade water, and 10μL Master Mix (Genestar, China). The qPCR was conducted at 95℃ for 4min, followed by 40 amplification cycles (de- naturation at 95℃ for 10s, annealing at 60℃ for 15s, and extension at 72℃ for 15s). All samples were run in trip- licates on the same plate. The comparative delta-delta Ct method was applied for analyzing the gene expression ofin different tissues. The liver was selected as thecomparator samples for determining the relative mRNAexpression levels ofin different tissues. All data are expressed as the mean±SD (standard deviation;=3). The one-way analysis of variance (ANOVA) with Tukey- Kramer Honest Significant Difference test was used to de- termine significant differences.<0.05 was considered sta- tistically significant differences.
2.6 Fluorescence in situ Hybridization (FISH) Analysis
The frozen testis sections were fixed in 4% paraformal- dehyde in PBS at 4℃ for 20h, then washed in 0.1molL−1PBS, incubated with 0.5molL−1sucrose-PBS (pH 7.4) over-night at 4℃, finally embedded in O.C.T. compound, and frozen at −80℃. The embedding blocks were sliced into 5-μm-thick frozen sections using a sliding microtome and placed on the RNase-free poly-lysine-coated slides, which were stored at −80℃ until further use. The probe (Table 1), conjugated to fluorescein isothiocyanate, was synthesized by Generay Biotech, Co., Ltd. (Shanghai, China). Thehy- bridization experiments were conducted based on Xurecommendations (2018). The negative control groups were incubated with a sense probe. The sections were observed and photographed with the Zeiss laser scanning confocal microscope (LSM880; Carl Zeiss, Germany).
2.7 Immunofluorescence (IF) Analysis
The mouse anti-human PHB antibody (Abzoom, Ame- rica) was applied in this experiment. Its specificity was determined by western blotting (WB) experiment accord- ing to Zhang. (2017). One single protein band (29 kDa) was detected in advance to certify the IF experiment can be conducted using this antibody. The experiments were conducted according to Zhang. (2017). The primary antibody (1:100 dilution) used in this study was mouseanti-human PHB antibody, and the secondary antibody (1:500 dilution) was Alexa Fluor 555-labelled donkey anti- mouse IgG (H+L; Beyotime, China). Meanwhile the mito- chondrial dye (MitoTracker Deep Green FM; 1:2000 dilu- tion) was added in the reaction solution. The negative con- trol samples were incubated without the addition of pri-mary antibodies. The fluorescence signal was then observed and photographed with the Zeiss laser scanning confocal microscope (LSM880; Carl Zeiss, Germany).
2.8 Extraction of Mitochondria and Detection of PHB Protein
Mitochondria and cytoplasm were extracted from Stage IV testes with the tissue mitochondria isolation Kit (Be- yotime, China) following the manufacturers’ instructions. The WB experiments were cnducted according to Zhang(2017) to detect PHB levels (with mouse anti-hu- man PHB antibody) in the mitochondrial and cytoplasmic protein fractions. The cytochromeoxidase subunit I (COXI) and β-tubulin proteins were used to confirm the efficient separation of mitochondria and cytoplasm, respectively. Rabbit anti-COX I and rabbit anti-β-tubulin antibodies were purchased from Abzoom (America) and Beyotime (China), respectively.
3 Results
3.1 Cytologic Characterization of Spermatogenesis
spermatogenesis included the proliferation and differentiation of spermatogonia, two cycles of meiosis, and spermiogenesis, during which spermatids develop intosperm. Cytological features of spermatogenic cells are de- scribed below.
3.1.1 Spermatogonia
Spermatogonia, usually are near the basement membrane and adhere to Sertoli cells, are the largest male germ cells with oval nuclei and with one or more nucleoli (Fig.1). Abundant mitochondria concentration was found in the cy- toplasm of spermatogonia. The nuage, a unique germ-line organelle with high electron density in the cytoplasm, was observed near the nuclear membranes or randomly in cy- toplasm (Fig.1). In addition, several mitochondria associ- ated with a nuage, described as an intramitochondrial ce- ment, formed a mitochondrial cluster. Mitochondria shapeswere mainly circular or oval and infrequently clubbed with the tubular cristae (Fig.1).
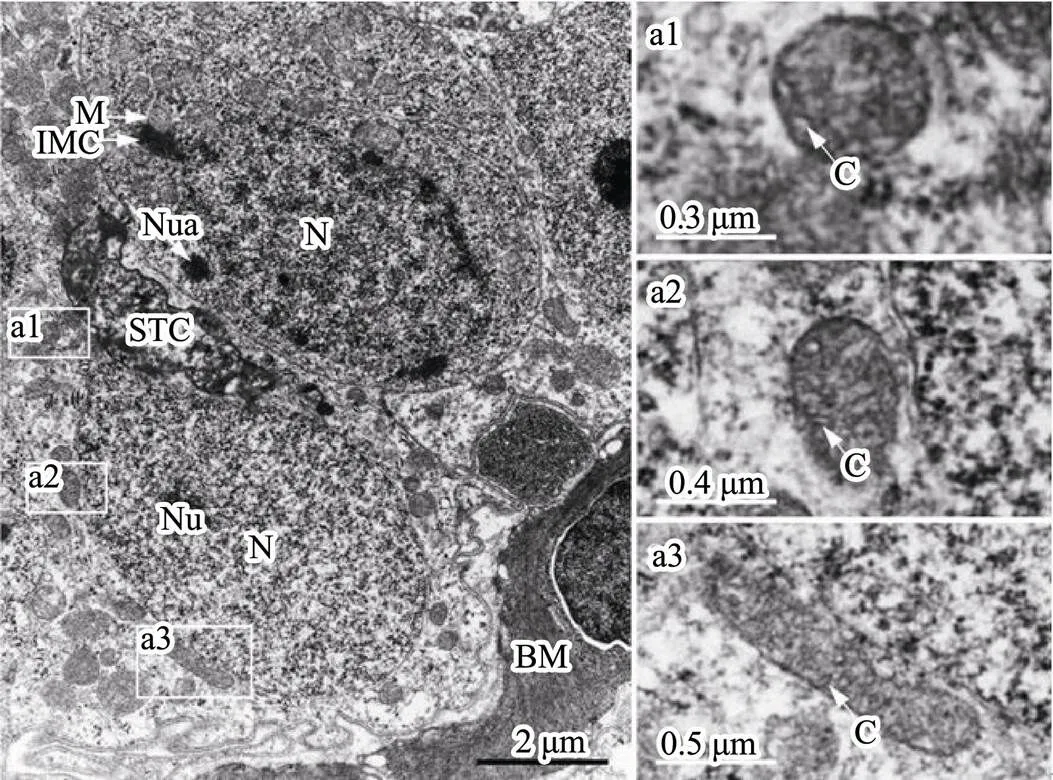
Fig.1 Ultrastructure of spermatogonia. (a1–a3) Representa- tive mitochondria in spermatogonia. BM, basement mem- brane; C, mitochondrial cristae; IMC, intramitochondrial cement; M, mitochondria; N, nucleus; Nu, nucleolus; Nua, nuage; STC, Sertoli’s cells.
3.1.2Spermatocyte
Primary spermatocytes, arising from the differential sper- matogonia, had a little volume than spermatogonia. The chromosome presented different features owing to the dif- ferent developmental stages of the primary spermatocytes during meiosis (Figs.2A–E). During meiosis prophase, theleptotene, zygotene, pachytene, and diplotene cells were observed. Mitochondria were randomly distributed in the cytoplasm. In some cells, several mitochondria associated with intramitochondrial cement were observed, similarly to the findings obtained with spermatogonia (Figs.2B). Theshapes of mitochondria were circular, elliptic, clubbed, and occasionally irregular with tubular cristae (Figs.2J–M).
Secondary spermatocytes were smaller with a shorter life, and underwent meiotic division faster than the primary ones (Figs.2G–I).
3.1.3 Spermiogenesis and sperm
Spermiogenesis, the last stage of spermatogenesis, is a process by which spermatids mature into spermatozoids. Based on the cell size, nucleus shape, chromatin conden- sation degree, and mitochondrial distribution characteris- tics, we divided the spermatids into three developmental stages during spermiogenesis, including early spermatid, middle spermatid, and late spermatid. The early spermatid was smaller than the second spermatocyte after meiosis (Fig.3A1). The initial spermatid formed from the second meiosis had a high electron density in the nuclei (Fig.3A2). With development, the chromatin was well-distributed in the nuclei (Fig.3A3). Mitochondria, mainly oval or ellip- tic with the tubular cristae, were randomly distributed in the perinuclear cytoplasm (Figs.3A3–A4). Flagellum was also observed at this stage (Fig.3A2). The middle sper- matid nucleus had high chromatin concentration, were uni- formly distributed, and the posterior nuclear fossa was al- ready formed (Fig.3B1). Cell polarity was detected. One side of the posterior nuclear fossa was abundant in cyto- plasm, and contained merged mitochondria and vesicles (Figs.3B2–B3). Mitochondria were mainly oval (Figs.3 B3–B4). In addition, a cytoplasmic bridge among sper- matids was detected (Fig.3B1). The late spermatid was sig- nificantly smaller than the middle spermatid, and its chro- matin was highly concentrated with a graininess texture (Figs.3C1–C3). Mitochondria gathered in the future sperm midpiece in a rounded shape (Figs.3C3–C4). In fact, the morphology and volume of the late spermatids were all si- milar to those of the mature spermatozoa, except for hav- ing more residual cytoplasm. After reducing the cytoplasm,mature sperm were then generated. With the rupture of sper-matocysts, mature sperm, composed of head and tail, were released into the lobular cavity; meanwhile, the sub-round- ed mitochondria with thin tubular cristae gathered in the midpiece (Figs.3D1–D4).
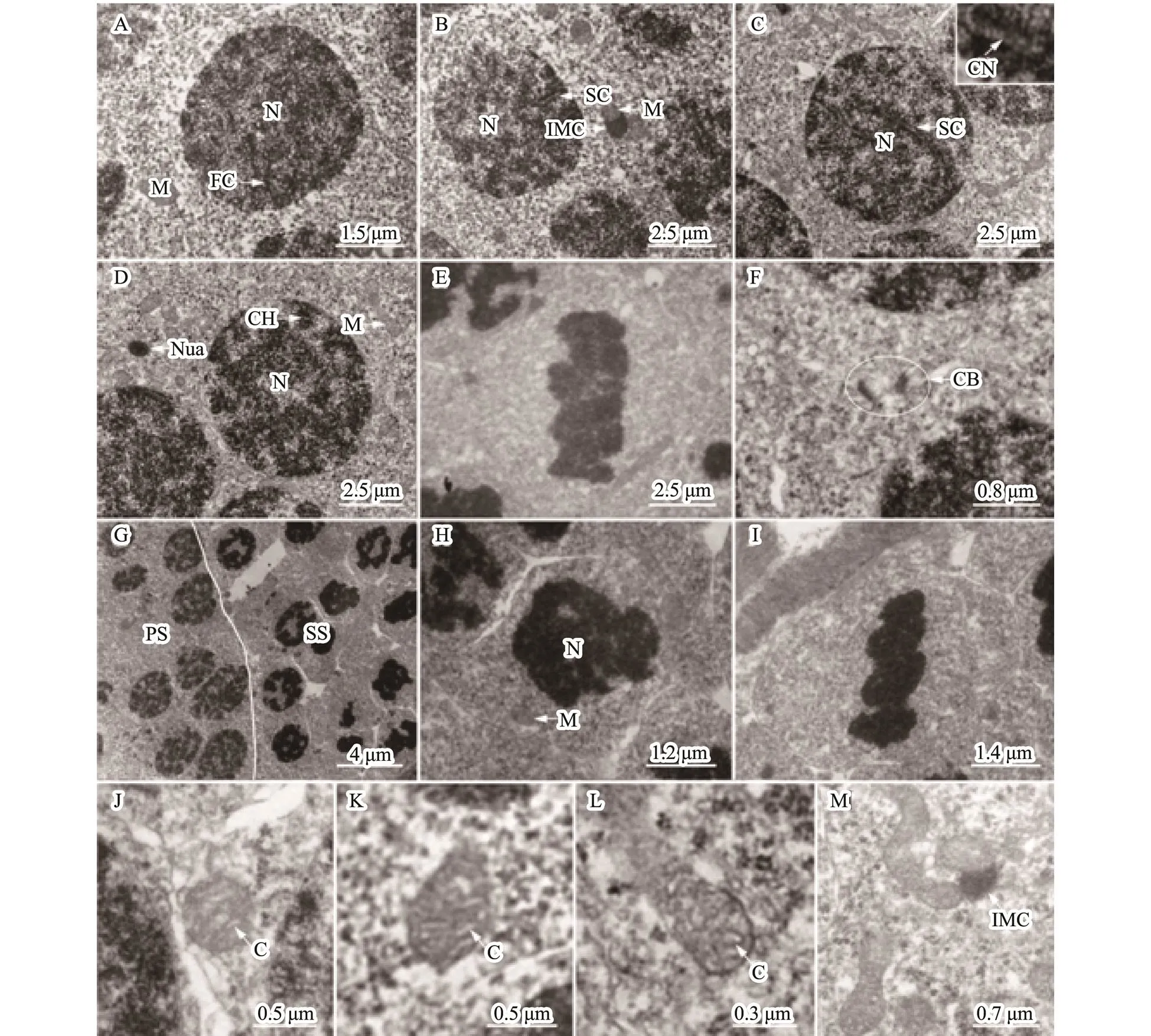
Fig.2 Ultrastructure of spermatocyte. (A–F) Primary spermatocyte. The volume of primary spermatocytes smaller than spermatogonia. The chromosome has adopted different forms due to the different stages during meiosis. (A) Leptotene cell. The chromosome as a fibriform with one end linking the nuclear membranes. (B) Zygotene cell. The synaptonemal complex was formed by the pair of homologous chromosome and emerged near the nuclear membranes. (C) Pachytene cell. The synapsis was completed. A combination nodule was observed in the middle site of the synaptonemal complex. (D) Diplotene cell. The homologous chromosomes were separated and only associated by some chiasmas. (E) Metaphase I cell. The chromosome gathered in the equatorial plane. (F) Cytoplasmic bridge in primary spermatocytes. (G) Primary spermatocytes (PS) and secondary spermatocytes (SS). (H) Secondary spermatocytes. (I) Metaphase II cell. The volume, especially that of secondary spermatocytes was reduced, compared to that of primary spermatocytes (H). Secondary spermatocytes quickly performed the second meiosis. Therefore, the secondary spermatocytes were usually in meta- phase II (J). Mitochondria were randomly distributed in the cytoplasm of spermatocytes and also associated with IMC in the cytoplasm. (J–L) Representative mitochondrial morphology in spermatocytes. (M) Occasional mitochondrial mor- phology observed inspermatocyte. Mitochondria consisted of circular, elliptic, clubbed, and occasionally irregularly shaped with the tubular cristae in spermatocyte. C, mitochondrial cristae; CB, cytoplasmic bridge; CH, chiasma; FC, fi- briform chromosome; IMC, intermitochondrial cement; M, mitochondria; N, nucleus; Nua, nuage; Nu, nucleolus; SC, synaptonemal complex; CN, combination nodule.
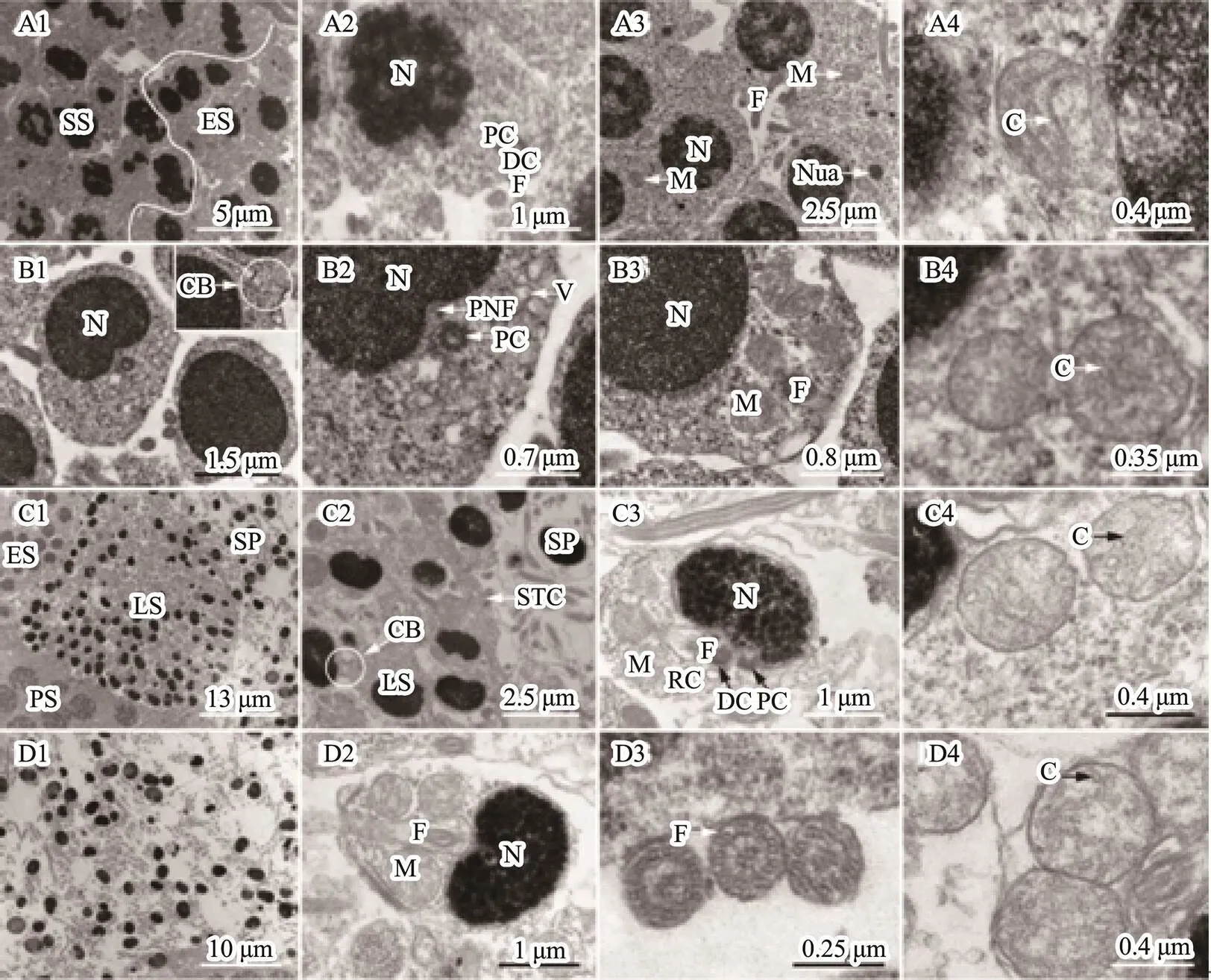
Fig.3 Ultrastructure of spermatids and spermatozoon. (A1–A4) Early spermatid. The initial spermatid formed from the second meiosis had a high electron density in the nuclei (A1, A2). With development, the chromatin was well-distributed in the nuclei (A3). Mitochondria were randomly distributed in the cytoplasm mainly with circular shape and tubular cris- tae (A3, A4). The flagellum started to form (A2). (B1–B4) Middle spermatid. The chromatin was higher condensed than that in the early spermatids, and well-distributed in the nuclei. The posterior nuclear fossa was formed. The cytoplasm was mainly distributed on one side of the cell, where mitochondria and vesicles gathered. (C1–C4) Late spermatid. The chromatin was more condensed than observed in the middle spermatid with small particles. The volume of the nucleus was significantly small. The cytoplasm was not completely eliminated, and the spermatid package in the cysts by Sertoli’s cells. (D1–D4) Sperm. Mature sperm was released in the lobular cavity (D1), consisting of head and tail (D2). The axial filament in the flagellum had a ‘9+2’ structure (D3). The mitochondria gathered in the midpiece of the sperm tail with a circular shape and several tubular cristae (D4). C, mitochondrial cristae; CB, cytoplasmic bridge; DC, distal centriole; ES, Early spermatid; F, flagellum; LS, late spermatid; M, mitochondrion; N, nucleus; Nua, nuage; PC, proximal centriole; PNF, posterior nuclear fossa; RC, residual cytoplasm; SP, sperm; SS, secondary spermatocytes; STC, Sertoli’s cells.
3.2 Characteristic of Lc-phb Full-Length cDNA and Predicted Lc-PHB Protein
Full-length cDNA ofgene is 1625bp (GenBank accession number: MG712297), which consists of a 77bp 5’ untranslated region (UTR), a 735bp 3’ UTR, and an 813 bp open reading frame sequence, encoding 271aa (Fig.4A).
PHB protein has a computed molecular weight of 29.8kDa and theoretical isoelectric point of 5.39. Ala, Arg, Glu, Gly, Leu, Val, Ile, and Ser were dominant amino acidswith percentages of 9.2%, 7.0%, 8.1%, 7.0%, 11.1%, 6.6%, and 6.6%, respectively. ThePHB aa sequence analysis predicted three function domains: N-terminal transmem- brane domain (7–25aa), SPFH domain (26–220aa), and coiled-coil domain (185–205aa; Fig.4 B), and thetertiary structure is shown in Fig.4C.
In addition, the aa sequence alignment was conducted to analyze the conservation of thePHB primary structure. As shown in Fig.4D, the primary structure of PHB protein was highly conserved among vertebrates and invertebrates. The similarities ofPHB with its homologues in,,,,,,,,, andare 94.9%, 94.9%, 95.6%, 94.5%, 98.9%, 98.2%, 85.8%, 86.4%, 84.6%, and 78.9%, respectively. The result indicated that the PHB protein was conserved among animals.
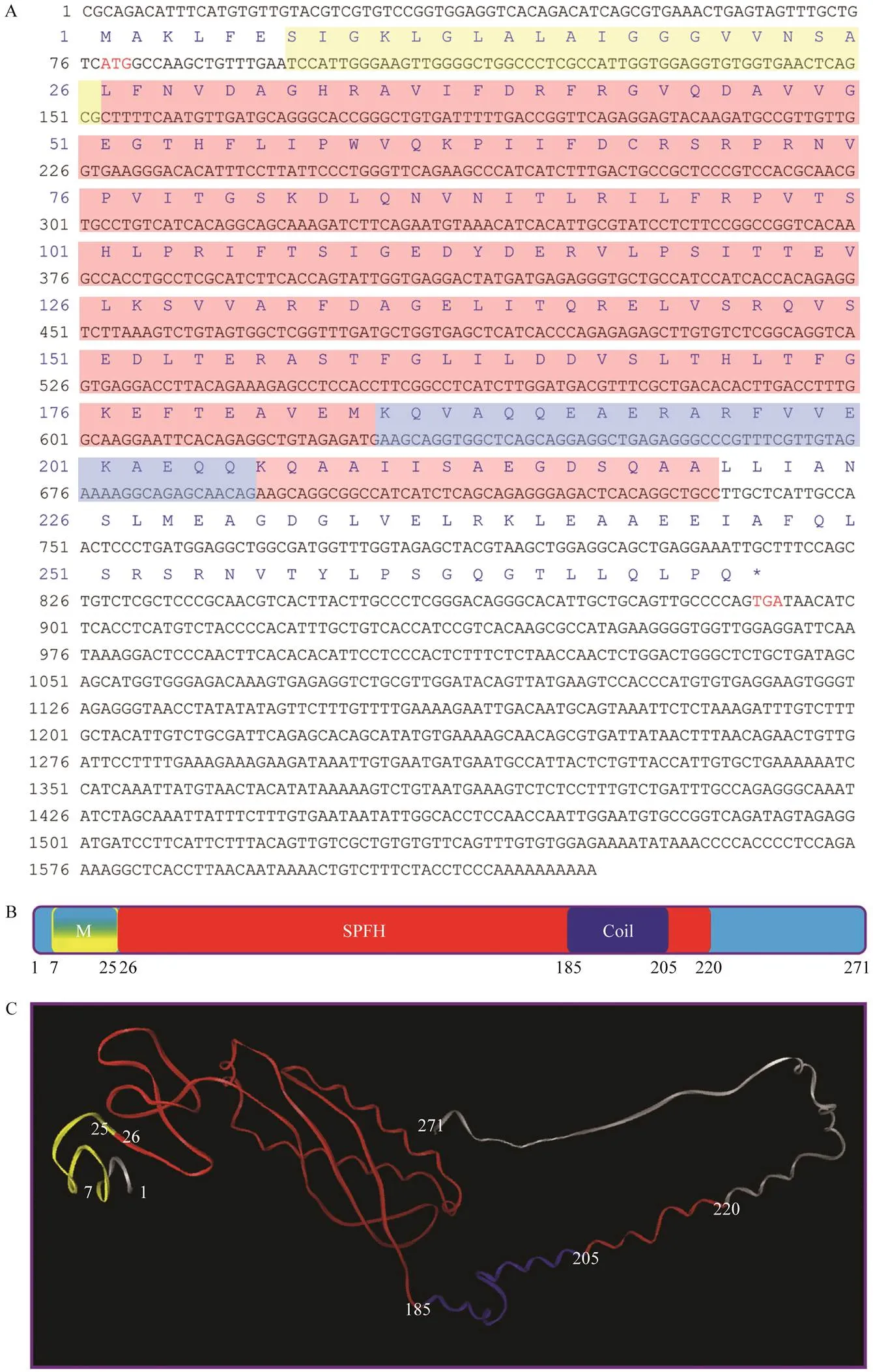
Fig.4 Full-length cDNA and conservative structure of PHB. (A) Full-length cDNA of Lc-phb geneand primary structure of Lc-PHB protein. The initiation and termination codons were ATG and TGA (marked red word), respectively. The sequences labeled by the yellow, red, and light blue shades encoded the transmembrane/hydrophobic, SPFH, and coiled-coil domains, respectively. (B) Predicted function domains of Lc-PHB. The N-terminal transmembrane domain labeled in yellow corresponded to amino acids 7–25. The SPFH domain labeled in red corresponded to amino acids 26–220. The coiled-coil labeled in blue corresponded to amino acids 185–205. (C) Predicted tertiary structure of Lc-PHB. M, transmembrane domain; SPFH, SPFH domain; Coil, coiled-coil domain.
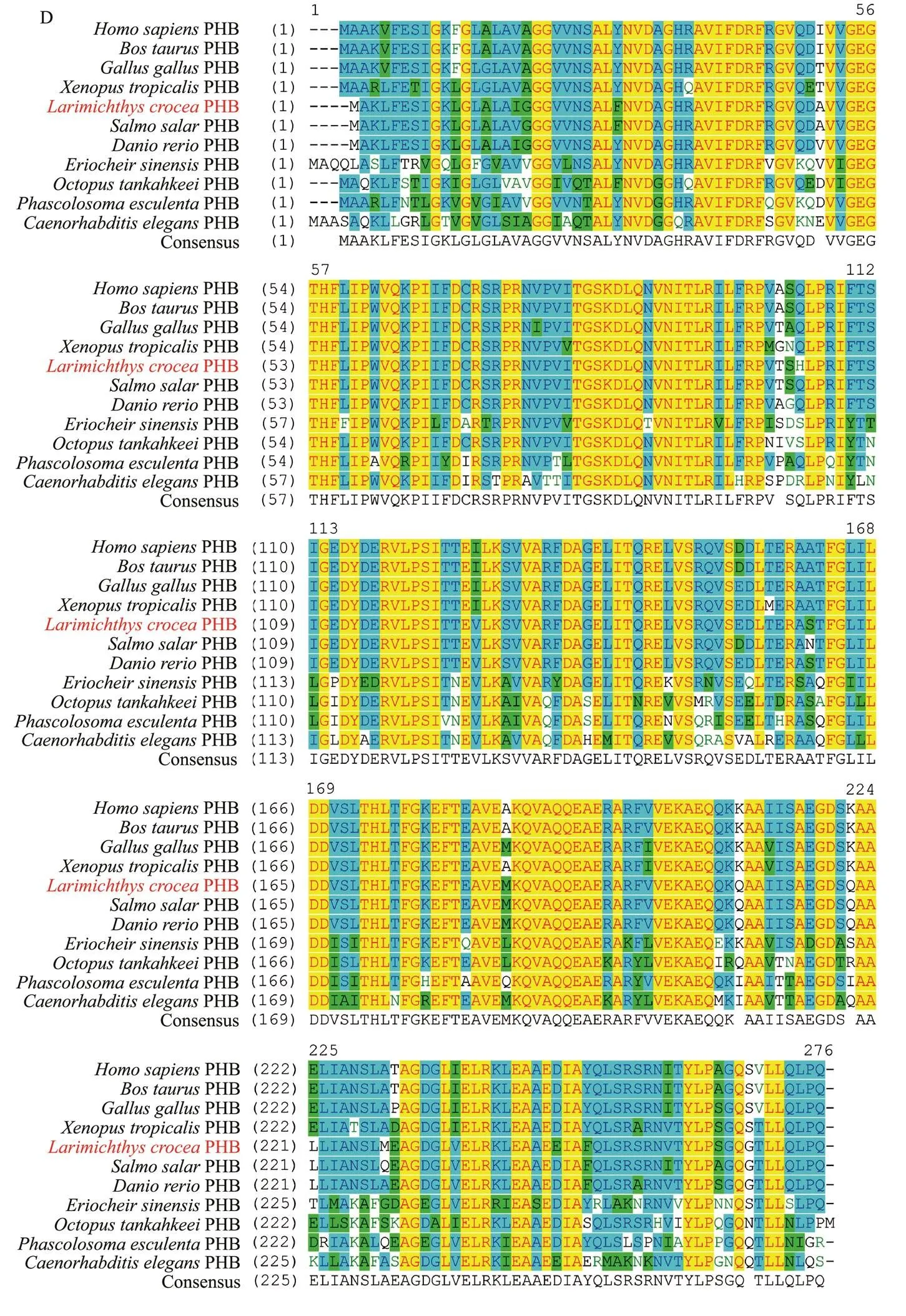
Fig.4 (D) Sequence alignment of PHB amino acids among L. crocea and other species. The regions in yellow showed the same amino acids sequence; in light blue represented more than 50% similarity; in green represented a lower similarity.
3.3 Phylogenetic Analysis of PHB
The phylogenetic analysis revealed an evolutionary re- lationship between PHB proteins (Fig.5).PHBtoge- ther with its homologues in vertebrates formed a branch that was the closest to the fish PHB among the examined species. The evolutionary relationship of thePHB pro- tein agreed with that of.
3.4 Expression Pattern of Lc-phbin Different Tissues
As seen in Fig.6A,mRNA was expressed in liver, testis, kidney, spleen, brain, gill, intestine, muscle, and heart. However, according to qPCR, the muscle, liver, and heart contained the highermRNA expression; while, testis, gill, and brain expressed intermediate levels, and the intestine, kidney, and spleen were correlated with the lower expression.
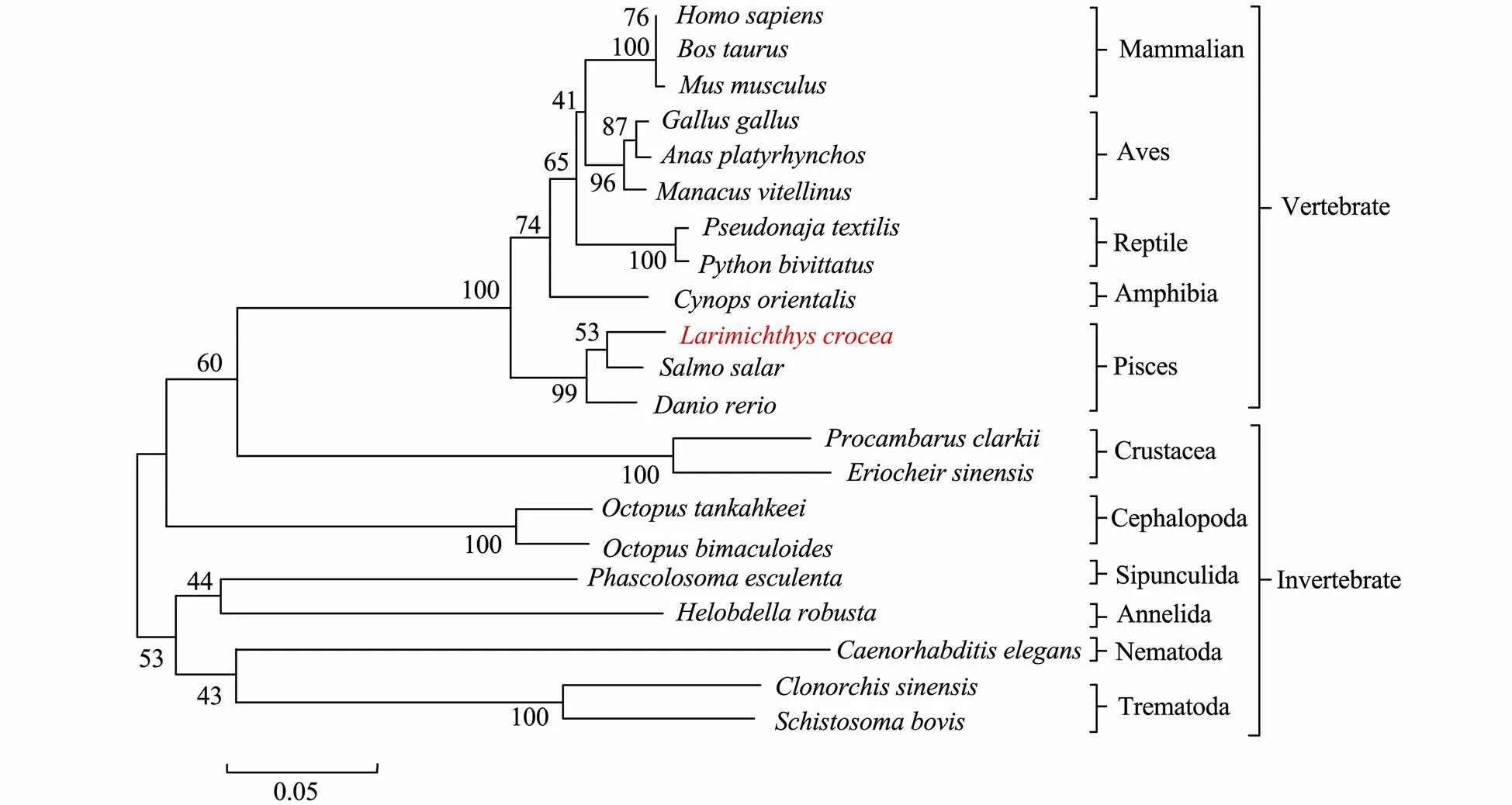
Fig.5 Phylogenetic tree based on the amino acid sequences of PHB proteins. The Mega 5.1 software was used for constructing this NJ phylogenetic tree. Lc-PHB formed a sub-branch with fish PHB.
3.5 Expression of Lc-phb mRNA During Spermatogenesis
As observed in Fig.6B, FISH detected the expression of-mRNA in spermatogenic cells.mRNA was detected in the perinuclear cytoplasm of spermatogonia, spermatocytes, and early spermatids, and in the sperm mid- piece (Fig.6B).
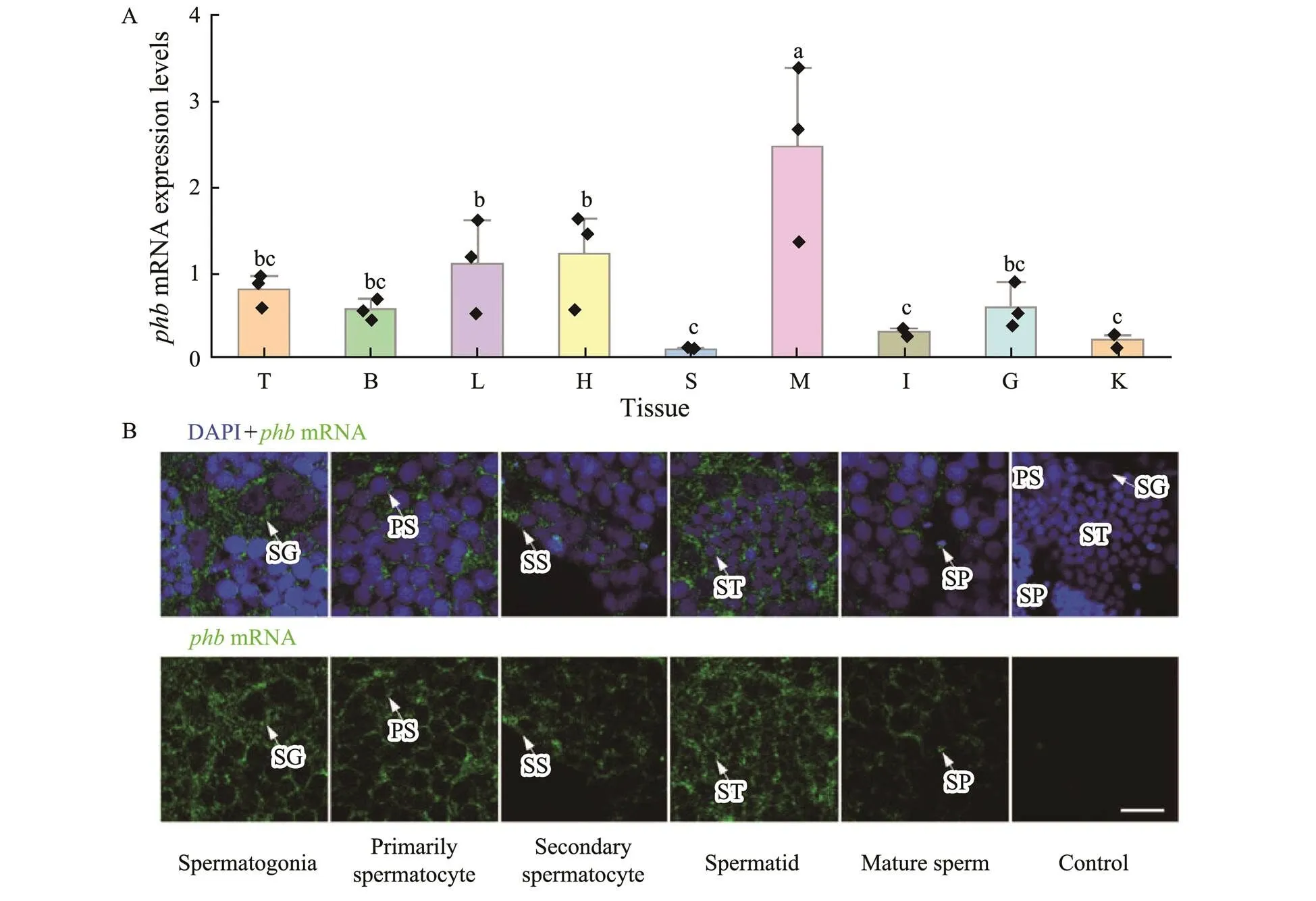
Fig.6 Expression pattern of Lc-phb mRNA. (A) Expression of Lc-phb mRNA in different tissues (n=3). Different letters above columns indicate significant differences (P<0.05). T, testis; B, brain; L, liver; H, heart; S, spleen; M, muscle; I, in- testine; G, gill; K, kidney. (B) FISH assay showing the expression of Lc-phb mRNA in spermatogenic cells. Blue indicates the nuclei strained by DAPI and green indicates the Lc-phb mRNA signals. Lc-phb mRNA was detected in spermatogonia, primarily spermatocyte, secondary spermatocyte, spermatid and sperm. No positive signals were detected in the control group. SG, spermatogonia; PS, primary spermatocyte; SS, secondary spermatocyte; ST, spermatid; SP, sperm. The scale bar is 10µm.
3.6 Temporal and Spatial Distribution of Lc-PHB and Mitochondria During Spermatogenesis
As observed in Fig.7, IF assay showed thatPHB pro- tein was localized in the mitochondria present in theper- inuclear cytoplasm of the spermatogonia (Figs.7A1–A4), primarily spermatocytes (Figs.7B1–B4), second spermato- cytes (Figs.7C1–C4), and early spermatids (Figs.7D1–D4). With the development of spermatids, the-PHB protein was mainly localized in the mitochondria present in one side of the middle spermatid (Figs.7E1–E4). In the sperm, the-PHB and mitochondria were present in the mid- piece (Figs.7F1–F5).
To further confirm the-PHB protein location in the mitochondria of spermatogenic cells, WB experiments were performed. As shown in Fig.8, COX I expression was de- tected only in the mitochondrial protein fraction. Contra- rily, we detected tubulin protein in the cytoplasm but not in the mitochondrial fraction. This result indicated that the separation of mitochondria and cytoplasm protein fractions was successful. Subsequently, we only detected PHB pro- tein in the mitochondrial protein fraction. This result and those from the IF experiment revealed the expression and mitochondria location ofPHB during spermatogenesis.
4 Discussion
4.1 Fish Spermatogenesis
Fishspermatogenesis included cystic and semi-cystic types (Mattei., 1993). In the cystic type, spermato- genic cellsdevelop into sperm in cysts(spermatocysts). Meanwhile, in the semi-cystic type, cystsopen before the sperm matures and therefore partial spermatogenic cells de- velop into sperm in the tubule lumen (Mattei., 1993). The cystic type spermatogenesis occurs in most teleosts, such as in the model organism(Rupik., 2011)The semi-cystic type is rare in fish but was observedin(Characidae; Andrade., 2001) andsp. (Ophididae; Mattei., 1993).In, the spermatogenic cells develop into cysts until they pro- duce sperm. Spermatogenesis, therefore, is cystic type. In cystic type, the cysts are formed from Sertoli cells surround-ing individual spermatogonia near the basement membrane. With the proliferation and differentiation of spermatogo- nia, the Sertoli cells enclose within a cyst (Uribe., 2014).In the presence of the same spermatocysts, the cytoplasmic bridge, a structure connecting adjacent cells, is possibly in- volved in the synchronism of spermatogenic cells by shar- ing RNA, protein, and other components (Ventelä.,2003). In our study, we observed the typical cytoplasmic bridge in spermatocytes and spermatids (Figs.2F, 3B1), in- dicating that this cytoplasmic bridge plays an important role in exchanging material and guaranteeing the synchronous development of spermatogenic cells in. In addi- tion, we proposed that the cytoplasmic bridge may be also necessary for the survival of spermatogenic cells in the tes- tis. Spermatogenic cells mainly rely on Sertoli’s cells for nutrition. For spermatogenic cells in the cysts that do notinteract directly with Sertoli’s cells, the cytoplasmic bridge may be an important way for feeding the spermatogenic cells.
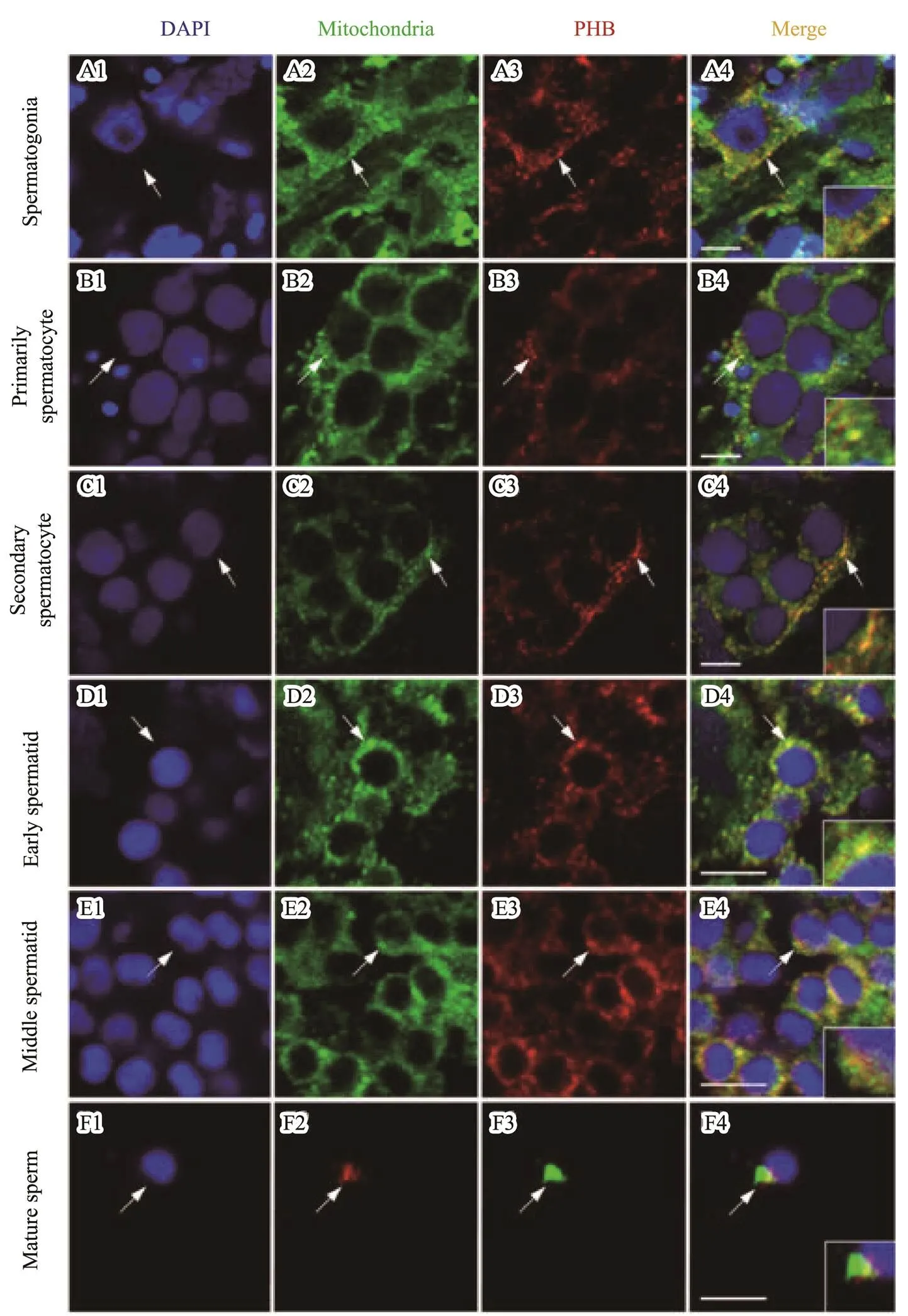
Fig.7 IF assay showed the expression pattern of Lc-PHB during spermatogenesis. Lc-PHB protein was present in the mi- tochondria located in the cytoplasm around the nuclei in spermatogonia (A1–A4), primarily spermatocyte (B1–B4), secondary spermatocyte (C1–C4), and early spermatid (D1–D4), mainly on one side of nuclei in the cytoplasm of mid- dle spermatid (E1–E4), and in the midpiece of mature sperm (F1–F4). No positive signals were detected in the control group (date not shown). The scale bars are 5µm.
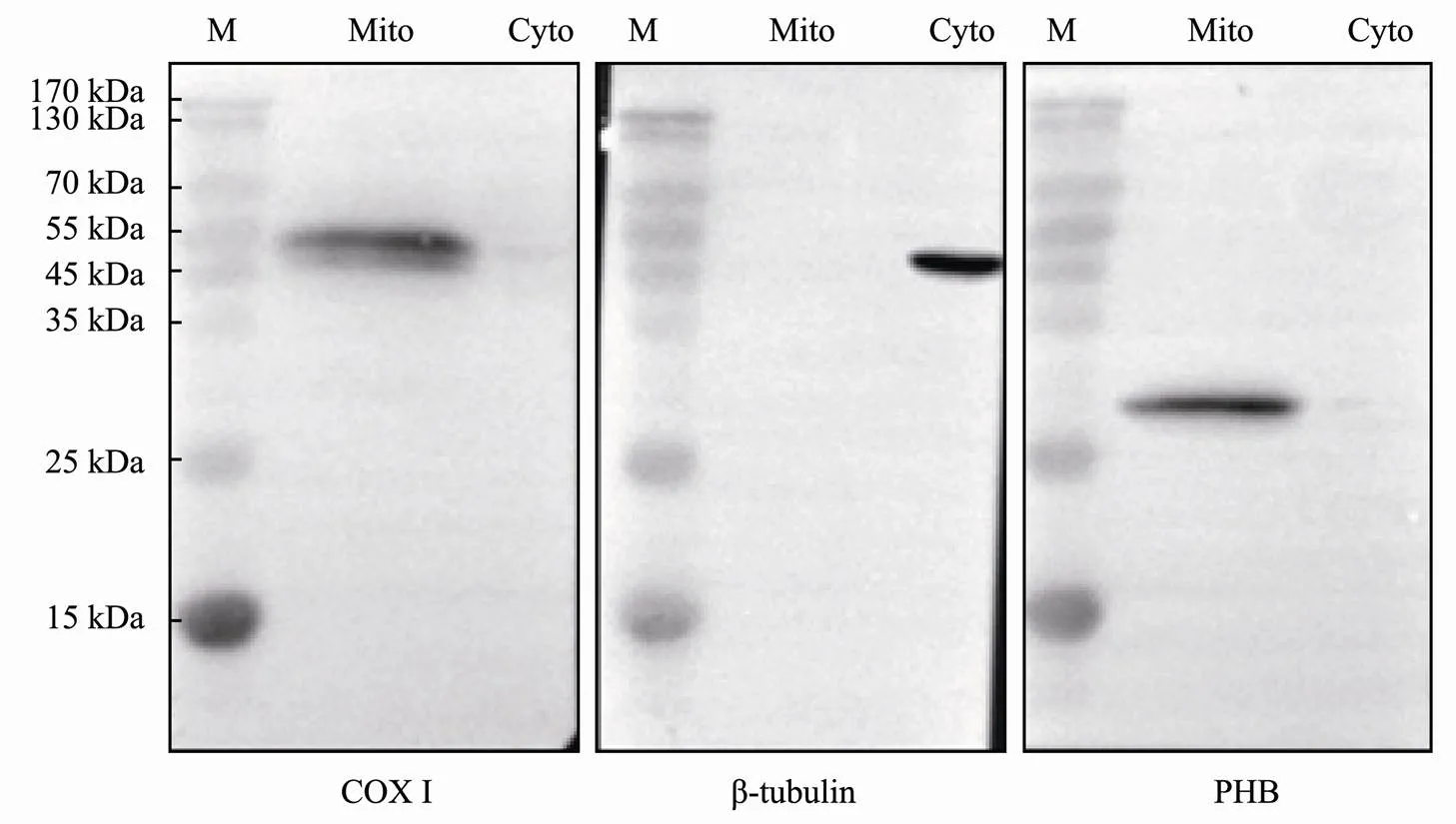
Fig.8 WB assay showed the intracellular location of Lc-PHB in Stage IV testis.COX I protein was detected in Mito, and β-tubulin protein was detected in Cyto, characterizing both protein fractions and purities. PHB was detected in Mito, con- firming that it was located in the mitochondria. M, protein marker; Mito, mitochondria protein fraction purified from Stage IV testis; Cyto, total protein extracted from cytoplasmic protein fraction (16µglane−1).
Mitochondria play important roles in spermatogenesis by regulating the physiological status and providing en- ergy for spermatogenic cells (Vertika., 2020). Mito- chondria maintain their characteristic structures and regu- late them during spermatogenesis. They regulate the sper- matogenic cells’ metabolism levels by promoting cell de- velopment and differentiation (Ramalho-Santos., 2009; Rato., 2012; Haseeb., 2018), a requirement for animal spermatogenesis and male fertility (Varuzhanyan., 2019). For instance, Varuzhanyan(2019) found that depletion of MFN1 and MFN2 proteins inhibited a me-tabolic shift during meiosis and therefore arrested the sper- matogenesis, highlighting the importance of the normal and dynamic mitochondrial morphology during spermatogene-sis. In fish, although the fish spermatogenesis has been ex- tensively reported (Stanley, 1969; Quagio-Grassiotto and Oliveira, 2008; Magalhaes., 2011; Rupik., 2011; Ribes., 2015), the detailed description of the dyna- mic mitochondria and the regulation of this mechanism were not reported. In this study, we observed the mitochon- drial morphological changes during spermatogenesis in. The mitochondria always maintained the tubular cristae; however, from spermatogonia to spermatocyte, themitochondrial morphology became diverse in shape, and mainly was round in spermatid and sperm. A further under- standing of the relationship between mitochondrial dyna- mic and spermatogenic cells’ metabolism levels inis required.
4.2 PHB Protein Structure and mRNA Expression Characterization
PHB is an important protein that regulates the mitochon- drial dynamic and was involved in animal spermatogene- sis. In this study, we cloned the full-length cDNA ofgene and analyzed its expression after investigating the cy-tological features of spermatogenesis inThrough sequence alignment, we determined that the primary struc- ture of PHB was conservedamong different animals (Fig.4D). This result suggested that PHB may have conserved func- tions and play essential functions in animals; the mutation of thegene usually affects the survival of organisms. The importance of PHB was confirmed by previous research,while the knocking down of PHB expression can cause em- bryo death (Artal-Sanz and Tavernarakis, 2009b). We fur- ther predicted the function domain of PHB and detected the potential transmembrane, coiled-coil, and SPFH do- mains (Fig.4). These domains were also predicted in yeast PHB (Back., 2010),-PHB (Hou., 2018),- PHB (Mao., 2012), and Human PHB (Winter., 2007). In yeast, PHB forms PHB complex with PHB2. The transmembrane domain anchors this complex to the IMM (Tatsuta., 2005; Winter., 2007; Back., 2010),and the coiled-coil domain allows the PHB1 and PHB2 in-teraction (Tatsuta., 2005; Winter., 2007; Back., 2010). Based on the protein structure analysis, we hypothesized a conservedPHB function and location in the mitochondria.
According to qPCR experiment, high expression ofwas detected in multiple tissues, including muscle, liver,and heart. Similarly, thegene was ubiquitously express- ed in multiple tissues of(Mao., 2012),(Jin., 2016),and(Hou., 2018). Artal-Sanz and Tavernarakis (2009a) summarized the PHB expression features and suggested its higher levels in tissues that heavily rely on mitochon- drial function. Thus, the higher expression ofgene in muscle, liver, and heart may indicate that these tissues relyheavily on mitochondrial function in. In addition, in the present study, the detection ofgene expression in spermatogenic cells at different stages by FISH indicatedthat PHB may play a role in the development of spermato- genic cells.
4.3 Intracellular Location and Putative Functions of PHB During Spermatogenesis
A relationship between PHB and animals’ fertility was reported. PHB deletion caused abnormal spermatogenesis inand down-regulation of PHB expression may result in sperm mitochondrial damage and low sperm qua-lity (Sanz., 2003; Wang., 2012; Chai., 2017). However, the molecular mechanism of PHB on spermato- genesis is inadequate understanding. To study the impor- tance of the mitochondria during spermatogenesis and the multiple physiological functions of PHB in mitochondria, we investigated the spermatogenesis in.PHBwas detected in the cytoplasm of spermatogenic cells at different stages and localized within the mitochondria (Fig.7).The PHB and mitochondria were finally located in thesperm midpiece (Figs.7F1–F4). WB assay verified thatPHB was located in the mitochondria extracted from Stage IV testis (Fig.8). These results confirmed the mito- chondrial location of PHB and provided insights about thecorrelation of PHB absence with abnormal spermatogene- sis, low sperm quality, and semen deficiency. 1) Are the tubular mitochondrial cristae and the long clubbed mito- chondrial morphology related to the mitochondrial location of PHB in spermatogenic cells? In mouse embryonic fi- broblasts (MEFs), Merkwirth(2008) found the loss of PHB resulted in the swelling and even the disappearance of mitochondria cristae. Meanwhile, the lack of PHB causedmitochondrial fragment inmuscle cell (Sanz., 2003), MEFs(Merkwirth., 2008), HeLa cells (Kasashima., 2006), and PC12 neural cells (Ander- son., 2018), indicating the importance of PHB in mi- tochondrial fusion. Therefore, our results about the expres-sion and mitochondrial location of PHB provided evidence that PHB may associate with observed tubular mitochon- drial cristae and long clubbed mitochondria in spermato- genic cells as observed in.Given the necessity of mitochondrial fusion for meiosis of spermatocytes (Va- ruzhanyan., 2019), a deduction is that PHB may also be necessary for meiosis in spermatocytes by promoting mitochondrial fusion. 2) Is the PHB expression important for the proliferation and survival of spermatogenic cell? In MEFs and mouse endothelial cells, the loss of PHB im- paired cell proliferation (Merkwirth., 2008; Schlei- cher., 2008). Similar conclusions were also reported in cancer cells (Sánchez-Quiles., 2010; Jiang., 2015). In addition, PHB plays roles in cell survival and in- hibiting apoptosis (Peng., 2015). Spermatogonia are the only cells that can increase the sperm count by proliferation and differentiation during spermatogenesis. Thus, spermatogonia proliferation is a prerequisite to generate enough spermatozoa in animals (Uribe., 2014). Our re- sults about the expression of PHB during spermatogenesis indicated a possible function of PHB in spermatogonia pro- liferation and spermatogenic cells survival. In humans, Wang(2012) reported lower PHB expression in oligoasthe- nospermia. The lower expression of PHB could affect the proliferation of spermatogonia, maiosis of spermatocyte and increased the apoptosis of spermatogenic cells, and there- fore resulted in a reduced sperm number and finally caused oligospermatism. The confirmation of these events is impor-tant for understanding the relationship among PHB, mito- chondria, and male fertility.
5 Conclusions
We performed an ultrastructural characterization of germ cells inIn addition, we analyzed the expression pattern and possible functions of mitochondrial functional regulatory protein PHB during spermatogenesis. This ge- ne was expressed in a testis. FISH revealed its expression in spermatogenic cells. Moreover, we detected PHB expres-sion in mitochondria of spermatogenic cells, including sper-matogonia, spermatocyte, spermatid, and sperm. These re-sults indicated that 1) PHB may be a potential protein to regulate the mitochondrial morphology during spermato- genesis; 2) PHB may be involved in the proliferation and survival of spermatogenic cells. This conjecture can better explain the relationship between PHB absence with oligo- spermatism and abnormal spermatogenesis in animals. Fur- ther evidence about the conserved mitochondrial function of PHB in spermatogenic cells ofis currently un- der investigation.
Acknowledgements
This research was funded by the Natural Science Foun- dation of Zhejiang Province (No. LY18C190007), the Na- tural Science Foundation of China–Zhejiang Joint Fund for the Integration of Industrialization and Informatization (No.U1809212), the Natural Science Foundation of Ningbo City (No. 2018A610228), the Scientific and Technical Project of Zhejiang Province (No. 2021C02069-1), the Scientific and Technical Project of Ningbo City (No. 2021Z002), the Scientific Research Foundation of Ningbo University (No. XYL19023), and the Collaborative Innovation Center for Zhejiang Marine High-efficiency and Healthy Aquaculture, the K. C. Wong Magna Fund in Ningbo University.
Anderson, C. J., Kahl, A., Qian, L., Stepanova, A., Starkov, A., Manfredi, G.,., 2018. Prohibitin is a positive modulator of mitochondrial function in PC12 cells under oxidative stress., 146: 235-250.
Andrade, R.F., Bazzoli, N., Rizzo, E., and Sato, Y., 2001. Con- tinuous gametogenesis in the neotropical freshwater teleost,(Pisces: Characidae).,33 (5): 524-532.
Artal-Sanz, M., and Tavernarakis, N., 2009a. Prohibitin and mi- tochondrial biology., 20: 394-401.
Artal-Sanz, M., and Tavernarakis, N., 2009b. Prohibitin couples diapause signalling to mitochondrial metabolism during age- ing in., 461 (7265): 793-797.
Back, J. W., Sanz, M. A., de Jong, L., de Koning, L. J., Nijtmans, L. G., de Koster, C. G.,., 2010. A structure for the yeast prohibitin complex: Structure prediction and evidence from chemical crosslinking and mass spectrometry., 11: 2471-2478.
Bogenhagen, D. F., Rousseau, D., and Burke, S., 2008. The layer-ed structure of human mitochondrial DNA nucleoids., 283: 3665-3675.
Chai, R. R., Chen, G. W., Shi, H. J., Martin-DeLeon, P. A., and Chen, H., 2017. Prohibitin involvement in the generation of mitochondrial superoxide at complex I in human sperm., 21: 121-129.
Fang, J. Z., Chu, M. B., Xiao, Q., Chen, X. Z., and Yu, H., 2003. Morphological studies on the early development of large yel- low croaker,(Richardson)., 27 (6): 1-6 (in Chinese with English abstract).
Hales, K. G., and Fuller, M. T., 1997. Developmentally regulatedmitochondrial fusion mediated by a conserved, novel, predicted GTPase., 90 (1): 121-129.
Haseeb, A., Chen, H., Huang, Y. F., Yang, P., Sun, X. J., Iqbal, A.,., 2018. Remodelling of mitochondria during spermioge- nesis of Chinese soft-shelled turtle ()., 30 (11): 1514-1521.
Honda, S., and Hirose, S., 2003. Stage-specific enhanced expres- sion of mitochondrial fusion and fission factors during sper- matogenesis in rat testis., 311 (2): 424-432.
Hou, C. C., Gao, X. M., Ni, J., Mu, D. L., Yang, H. Y., Liu, C.,., 2018. The expression pattern and potential functions of PHB in the spermiogenesis of.652: 25-38.
Huang, S. P., Cui, Y. Q., Guo, X. J., Wang, L., Li, S. Y., Lu, Y.,.,2015. 2,2’,4,4’-Tetrabromodiphenyl ether disrupts spermatoge-nesis, impairs mitochondrial function and induces apoptosis ofearly leptotene spermatocytes in rats., 51: 114-124.
Jiang, L., Dong, P., Zhang, Z., Li, C., Li, Y., Liao, Y.,., 2015. Akt phosphorylates prohibitin 1 to mediate its mitochon- drial localization and promote proliferation of bladder cancer cells., 6 (2): e1660.
Jin, J. M., Hou, C. C., Tan, F. Q., and Yang, W. X., 2016. The po- tential function of prohibitin during spermatogenesis in Chi- nese fire-bellied newt., 363: 805-822.
Kasashima, K., Ohta, E., Kagawa, Y., and Endo, H., 2006. Mito- chondrial functions and estrogen receptor-dependent nuclear translocation of pleiotropic human prohibitin 2., 281: 36401-36410.
Liu, J. F., 2004. A study on twice maturity characteristic of cul- tured large yellow croaker in one year., 3: 200-204 (in Chinese with Eng- lish abstract).
Luo, S., Gao, X., Ding, J., Liu, C., Du, C., Hou, C.,., 2019. Transcriptome sequencing reveals the traits of spermatogene- sis and testicular development in large yellow croaker ()., 10 (12): 958.
Magalhaes, A. L. B., Andrade, R. F., Gomes, B. V. C., Perini, V. R., Rizzo, E., and Bazzoli, N., 2011. Ultrastructure of the se- micystic spermatogenesis in the South American freshwater characid(Teleostei, Characiformes)., 27 (4): 1041-1046.
Mao, H. T., Wang, D. H., Lan, Z., Zhou, H., and Yang, W. X., 2012. Gene expression profiles ofin testes of(-) revealing its possible role during spermiogenesis., 39: 5519-5528.
Mattei, X., Siau, Y., Thiaw, O. T., and Thiam, D., 1993. Peculiari- ties in the organization of testis ofsp. (Pisces Te- leostei). Evidence for two types of spermatogenesis in teleost fish., 43 (6): 931-937.
Merkwirth, C., and Langer, T., 2009. Prohibitin function within mitochondria: Essential roles for cell proliferation and cristae morphogenesis., 1793: 27-32.
Merkwirth, C., Dargazanli, S., Tatsuta, T., Geimer, S., Löwer, B., Wunderlich, F. T.,., 2008. Prohibitins control cell prolix- feration and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria., 22: 476-488.
Mishra, S., Murphy, L. C., and Murphy, L. J., 2006. The prohi- bitins: Emerging roles in diverse functions., 10: 353-363.
Mishra, S., Murphy, L. C., Nyomba, B. L., and Murphy, L. J., 2005. Prohibitin: A potential target for new therapeutics., 11: 192-197.
Nijtmans, L. G. J., de Jong, L., Sanz, M. A., Coates, P. J., Berden, J. A., Back, W. J.,., 2000. Prohibitins act as a membrane- bound chaperone for the stabilization of mitochondrial proteins., 19 (11): 2444-2451.
Nijtmans, L. G. J., Sanz, M. A., Grivell, L. A., and Coates, P. J., 2002. The mitochondrial PHB complex: Roles in mitochondrial respiratory complex assembly, ageing and degenerative disease., 59 (1): 143-155.
Peng, Y. T., Chen, P., Ouyang, R. Y., and Song, L., 2015. Multi- faceted role of prohibitin in cell survival and apoptosis., 20 (9): 1135-1149.
Quagio-Grassiotto, I., and Oliveira, C., 2008. Sperm ultrastruc- ture and a new type of spermiogenesis in two species of Pi- melodidae, with a comparative review of sperm ultrastructure in Siluriformes (Teleostei: Ostariophysi)., 247 (1): 55-66.
Quan, C., Shi, Y. Q., Wang, C., Wang, C. M., and Yang, K. D., 2016. p,p’-DDE damages spermatogenesisphospholipid hy- droperoxide glutathione peroxidase depletion and mitochondria apoptosis pathway., 31 (5): 593-600.
Rajalingam, K., and Rudel, T., 2005. Ras-Raf signaling needs pro- hibitin., 4: 1503-1505.
Ramalho-Santos, J., Varum, S., Amaral, S., Mota, P. C., Sousa, A. P., and Amaral, A., 2009. Mitochondrial functionality in repro- duction: From gonads and gametes to embryos and embryonic stem cells., 15 (5): 553-572.
Rato, L., Alves, M. G., Socorro, S., and Duarte, A. I., 2012. Meta- bolic regulation is important for spermatogenesis., 9 (6): 330-338.
Ribes, E., Cheema, M., González-Romero, R., Lloris, D., Ausió, J., and Saperas, N., 2015. Spermiogenesis and biflagellate sper- matozoon of the teleost fish(Mycto- phiformes, Myctophidae): Ultrastructure and characterisation of its sperm basic nuclear proteins., 361 (2): 619-632.
Rupik, W., Huszno, J., and Klag, J., 2011. Cellular organisation of the mature testes and stages of spermiogenesis in(Cyprinidae; Teleostei)–Structural and ultrastructural stu- dies.,42 (8): 833-839.
Sánchez-Quiles, V., Santamaría, E., Segura, V., Sesma, L., Prieto, J., and Corrales, F. J., 2010. Prohibitin deficiency blocks pro- liferation and induces apoptosis in human hepatoma cells: Mole- cular mechanisms and functional implications., 10: 1609-1620.
Sanz, M. A., Tsang, W. Y., Willems, E. M., Grivell, L. A., Lemire, B. D., van der Spek, H.,., 2003. The mitochondrial pro- hibitin complex is essential for embryonic viability and germ- line function in., 278 (34): 32091-32099.
Schleicher, M., Shepherd, B. R., Suarez, Y., Fernandez-Hernan- do, C., Yu, J., Pan, Y.,., 2008. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mito- chondrial function and senescence., 180: 101-112.
Sharma, A., and Qadri, A., 2004. Vi polysaccharide oftargets the prohibitin family of molecules in intes- tinal epithelial cells and suppresses early inflammatory responses., 101: 17492-17497.
Stanley, H. P., 1969. An electron microscope study of spermio- genesis in the teleost fish oligocottus maculosus., 27 (3): 230-243.
Steglich, G., Neupert, W., and Langer, T., 1999. Prohibitins regu- late membrane protein degradation by the m-AAA protease in mitochondria., 19: 3435-3442.
Su, Y. Z., Zheng, Z. Y., You, L., Shi, X. W., Liu, J. F., Wong, Z. C.,., 1997. A study on techniques of artificial propagation and breeding of large yellow croaker,(Richardson)., 5: 21-27 (in Chi- nese with English abstract).
Tatsuta, T., Model, K., and Langer, T., 2005. Formation of mem- brane-bound ring complexes by prohibitins in mitochondria.
, 16: 248-259.
Thuaud, F., Ribeiro, N., Nebigil, C. G., and Désaubry, L., 2013. Prohibitin ligands in cell death and survival: Mode of action and therapeutic potential., 20 (3): 316- 331.
Uribe, M. C., Grier, H. J., and Mejía-Roa, V., 2014. Comparative testicular structure and spermatogenesis in bony fishes., 4 (3): e983400.
Van der Weyden, L., Arends, M. J., Chausiaux, O. E., Ellis, P. J., Lange, U. C., Surani, M. A.,., 2006. Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of sper- matogenesis., 26 (9): 3595- 3609.
Varuzhanyan, G., Rojansky, R., Sweredoski, M. J., Graham, R. L. J., Hess, S., Ladinsky, M. S.,., 2019. Mitochondrial fusion is required for spermatogonial differentiation and meio- sis., 8: e51601.
Ventelä, S., Toppari, J., and Parvinen, M., 2003. Intercellular organelle traffic through cytoplasmic bridges in early sperma- tids of the rat: Mechanisms of haploid gene product sharing., 14: 2768-2780.
Vertika, S., Singh, K. K., and Rajender, S., 2020. Mitochondria, spermatogenesis, and male infertility–An update.,54: 26-40.
Wang, M. J., Ou, J. X., Chen, G. W., Wu, J. P., Shi, H. J., Chen, H.,.,2012. Does prohibitin expression regulate sperm mito- chondrial membrane potential, sperm motility, and male fer- tility?, 7 (3): 513-519.
Wang, Y., and Bogenhagen, D. F., 2006. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane., 281: 25791-25802.
Winter, A., Kämäräinen, O., and Hofmann, A., 2007. Molecular modeling of prohibitin domains., 68: 353-362.
Xiong, Z., Zhang, H., Huang, B., Liu, Q., Wang, Y., Shi, D.,., 2018. Expression pattern of prohibitin, capping actin protein of muscle Z-line beta subunit and tektin-2 gene in Murrah buf-falo sperm and its relationship with sperm motility., 31 (11): 1729-1737.
Xu, Y. R., Fan, Y. S., and Yang, W. X., 2017. Mitochondrial pro- hibitin and its ubiquitination during spermatogenesis of the swimming crab., 627: 137-148.
You, X. R., Cai, M. Y., Jiang, Y. H., and Wang, Z. Y., 2012. His- tological observation on gonadal sex differentiation in large yellow croaker ()., 36 (7): 1057-1064.
Zhang, D. D., Gao, X. M., Zhao, Y. Q., Hou, C. C., and Zhu, J. Q., 2017. The C-terminal kinesin motor KIFC1 may participate in nuclear reshaping and flagellum formation during spermioge- nesis of., 43: 1351-1371.
Zhang, J. J., Wang, Q., Wang, M. S., Jiang, M. X., Wang, Y. S., Sun, Y.,., 2016. GASZ and mitofusin-mediated mitochon-drial functions are crucial for spermatogenesis.,17 (2): 220-234.
January 6, 2021;
April 27, 2021;
May 1, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
#The two authors contributed equally to this work.
. E-mail:zhujunquan@nbu.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Variations in Dissolved Oxygen Induced by a Tropical Storm Within an Anticyclone in the Northern South China Sea
- Intensity of Level Ice Simulated with the CICE Model for Oil-Gas Exploitation in the Southern Kara Sea, Arctic
- Learning the Spatiotemporal Evolution Law of Wave Field Based on Convolutional Neural Network
- Development and Control Strategy of Subsea All-Electric Actuators
- Acoustic Prediction and Risk Evaluation of Shallow Gas in Deep-Water Areas
- In Situ Observation of Silt Seabed Pore Pressure Response to Waves in the Subaqueous Yellow River Delta
