A short P-wave duration is associated with incident heart failure in the elderly: a 15 years follow-up cohort study
2022-10-17BozenaOstrowskaLarsLindElenaSciaraffiaCarinaBlomstrLundqvist2
Bozena Ostrowska ✉,Lars Lind ,Elena Sciaraffia ,Carina Blomström-Lundqvist2
1.Department of Medical Sciences,Uppsala University,Uppsala,Sweden;2.School of Medical Science,Faculty of Medicine and Health,Örebro University,Örebro,Sweden
ABSTRACT BACKGROUND Early identification of patients at risk of congestive heart failure (HF) may alter their poor prognosis.The aim was therefore to test whether simple electrocardiographic variables,the P-wave and PR-interval,could predict incident HF.METHODS The PIVUS (Prospective Investigation of the Vasculature in Uppsala Seniors) study (1016 individuals all aged 70 years,50% women) was used to identify predictors of HF.Subjects with prevalent HF,QRS duration ≥ 130 ms,atrial tachyarrhythmias,implanted pacemaker/defibrillator,second-and third-degree atrioventricular block or delta waves at baseline were excluded.Cox proportional hazard analysis was used to relate the PR interval,P-wave duration (Pdur) and amplitude (Pamp),measured in lead V1,to incident HF.Adjustment was performed for gender,RR-interval,beta-blocking agents,systolic blood pressure,body mass index and smoking.RESULTS Out of 836 subjects at risk,107 subjects were diagnosed with HF during a follow-up of 15 years.In the multivariate analysis,there was a strong U-shaped correlation between Pdur in lead V1 and incident HF (P=0.0001) which was significant for a Pdur <60 ms [HR=2.75;95% CI: 1.87-4.06,at Pdur 40 ms] but not for prolonged Pdur.There was no significant relationship between incident HF and the PR-interval or the Pamp.A Pdur <60 ms improved discrimination by 3.7% when added to the traditional risk factors including sex,RR-interval,beta-blocking agents,systolic blood pressure,BMI and smoking (P=0.048).CONCLUSIONS A short Pdur,an easily measured parameter on the ECG,may potentially be a useful marker of future HF,enabling its early detection and prevention,thus improving outcomes.
The prevalence of heart failure (HF) is approximately 1%-2% in the adult population in developed countries.[1]Despite new therapeutic advances,the prognosis remains poor with 64%-66% mortality rate within 5 years.[2]
The P-wave on the surface ECG reflects the electrical atrial depolarization.While normal values of the P-wave duration (Pdur) have not been standardized,cut-offs of 110 or 120 ms have been proposed.[2,3]Prolonged Pdur is caused by a prolonged atrial conduction due to atrial enlargement and/or fibrosis,and is independently associated with atrial fibrillation (AF),ischemic stroke and increased mortality.[4]Less is known about its association with HF.Left atrial (LA) enlargement,a marker of atrial remodeling,has been associated with HF (regardless of the left ventricular ejection fraction) as well as increased mortality in HF patients.[5-8]
A prolonged PR-interval >200 ms is uncommon(0-5.2%) in the healthy population,but prevalent(18%-52%) in patients with HF.[9-10]A prolonged PR interval shortens the left ventricular (LV) diastolic filling time,resulting in a reduced preload and an impaired cardiac output.[11]In a healthy population,a PR prolongation has not been associated with increased cardiovascular (CV) mortality,however,an increased risk for HF and AF has been reported in individuals with CV disease and in elderly.[11,12]
The P-wave amplitude (Pamp) reflects atrial myocardial mass.A high Pamp in leads II and V1 has been associated with incident AF and with right atrial enlargement in pulmonary hypertension,but its association with HF is unclear.[13,14]An increased negative amplitude of the second component of the P-wave in V1 has been correlated with left atrial hypertrophy and both cardiovascular and all-cause mortality.[15-17]
Given this background,we aimed to investigate if simple ECG variables,Pdur,Pamp and PR-interval,are associated with future HF.
METHODS
Study Population and Design
The PIVUS (Prospective Investigation of the Vasculature in Uppsala Seniors) study invited in 2001 all individuals aged 70 years from Uppsala,Sweden to participate.[18]Out of 2025 invited subjects,1016 agreed to participate.The medical history and medications were recorded and a cardiovascular examination including blood pressure,ECG,echocardiographic examination and blood sampling for biomarkers was performed at baseline.The ECG leads(V1 through V6) were recorded digitally during 5 min in the supine position and were then analyzed by a semiautomatic EClysis (AstraZeneca R&D,Molndal,Sweden) software designed to calculate mean amplitudes and intervals with a high accuracy and reliability.[19,20]The EClysis software has been described previously[21].The two-dimensional and Doppler echocardiography was performed with an Acuson XP124 ultrasonic unit (Acuson,California,USA) using a 2.5 MHz transducer.Measurements of LA diameter,interventricular septal (IVS) thickness,posterior wall (PW) thickness,left ventricular (LV)end-diastolic and end-systolic diameters (LVEDD,LVESD) were made with M-mode from the parasternal views,using a leading-edge to leading-edge convention.Left ventricular mass (LVM) was determined by the Penn conversion and further adjusted for height to obtain left ventricular mass index(LVMI).LV volumes were calculated by the Teichholz formula,from which stroke volume (SV) and ejection fraction (LVEF) were calculated.The LV diastolic filling pattern of the mitral inflow during diastole was obtained from the apical projections with the pulsed Doppler.The peak velocity of the early LV filling (E wave) and the peak velocity of atrial filling wave (A wave) were recorded and the E/A-ratio was calculated.Left ventricular isovolumic relaxation time (IVRT) was measured as the time between aortic valve closure and the start of mitral flow using the Doppler signal from the area between the left ventricular outflow tract and mitral flow.A low E/A defined as ≤ 0.8 and a high E/A defined as ≥ 2 were used instead of continuous measures due to the issue of pseudo-normalization.
A re-examination was performed after 5 and 10 years at the age of 75 and 80 years,and included a standard ECG for the detection of atrial fibrillation only.The PIVUS study was approved by the Ethics Committee of the University of Uppsala and all participants gave written informed consent.[22]
The present study population was included from the PIVUS registry after excluding those,who at baseline presented with a diagnosis of HF with reduced or preserved LVEF,implanted pacemaker/defibrillator,atrial tachyarrhythmias,second-and third-degree atrioventricular block,delta waves and QRS complex duration ≥ 130 msecs (Figure 1),leaving 836 individuals for evaluation.
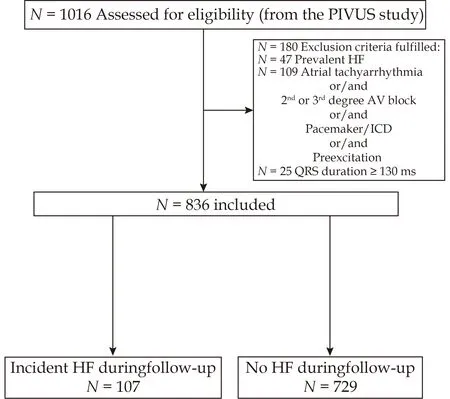
Figure 1 Flowchart for the study design.AF: atrial fibrillation;AV: atrioventricular;HF: heart failure;ICD: implantable defibrillator.
Data on HF (ICD-8 codes 427.00,427.10,428.99,ICD-9,428 and ICD-10 code I50 and I11.0),AF (ICDcodes 427.X,I48.X) and myocardial infarction (MI)(ICD-codes 4109,4110,410.X,I21.X) diagnoses were retrieved from the Swedish Cause of Death Register and the Swedish Hospital Discharge Register,both with a high quality and accuracy.[23]In addition,the diagnoses of AF,HF and MI were validated by an experienced clinician (LL) using the hospital records.Both in-hospital care and death from HF were included in the diagnosis of HF.
Statistical Methods
Cox proportional hazard analysis was used to relate the P-wave indices and PR-intervals to incident HF.Due to the theoretical assumption of non-linear relationships,the ECG variables were modeled as restricted cubic spline function with three knots (10,50 and 90thpercentile).
In the first set of models,adjustment was performed for sex and RR-interval (age same in all subjects).In a second set of models,adjustment was also performed for beta-blocking agents,systolic blood pressure,body mass index (BMI) and smoking.Lipids and diabetes were not related to incident HF in initial models regarding confounders,and were therefore not included as confounders.
C-statistics based on logistic regression was used to evaluate any improvement in discrimination of HF of adding P-wave indices to the above-mentioned risk factors for HF.
After excluding major non-linear relationships between ECG variables and echocardiographic variables,linear regression models were performed between P-wave indices and echocardiographic variables.In the first set of models,adjustment was performed for gender and RR-interval (age same in all subjects).In a second set of models,adjustment was also performed for beta-blocking agents,systolic blood pressure,BMI and smoking.Before this analysis,all investigated echocardiographic variables were inverse rank normalized to avoid skewed distributions.
APvalue <0.05 was regarded as significant.STATA16(Stata inc,College Station,TX) was used for the calculations.
RESULTS
A total of 836 of 1016 subjects were included in the analysis.Characteristics of the study population and distribution of Pdur and PR-intervals at baseline are shown in Table 1 and Figure 2.During the 15.0 years follow-up (10,935 patient-years at risk),107 subjects experienced a HF diagnosis and 222 individuals died (18 deaths due to HF).Levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) at baseline were significantly higher in individuals who later developed HF compared to the rest of the PIVUS population [252.96 ±491.00vs.134.23 ± 137.00 pg/mL,P<0.0001].
The Pdur in V1 was significantly associated with incident HF following adjustment for gender and RR-interval,beta-blocking agents,systolic blood pressure,BMI and smoking (P<0.0001).The relationship was U-shaped (P=0.0006 for non-linearity) with the lowest risk seen at a Pdur of 80 ms,but which turned into a significantly increased risk at Pdur <60 ms (Figure 3).A Pdur of 40 ms had a hazard ratio (HR) of 2.75 (95% CI: 1.87-4.06).Longer Pdur intervals seemed also associated with increased risk for incident HF,but did not reach statistical significance (HR=1.35,95% CI: 0.92-1.99 for Pdur at 100 ms),(Figure 3).A similar U-shaped association was also seen between Pdur in V3 and incident HF (Figure 4),although the association was weaker as compared to Pdur in V1 (P=0.001).
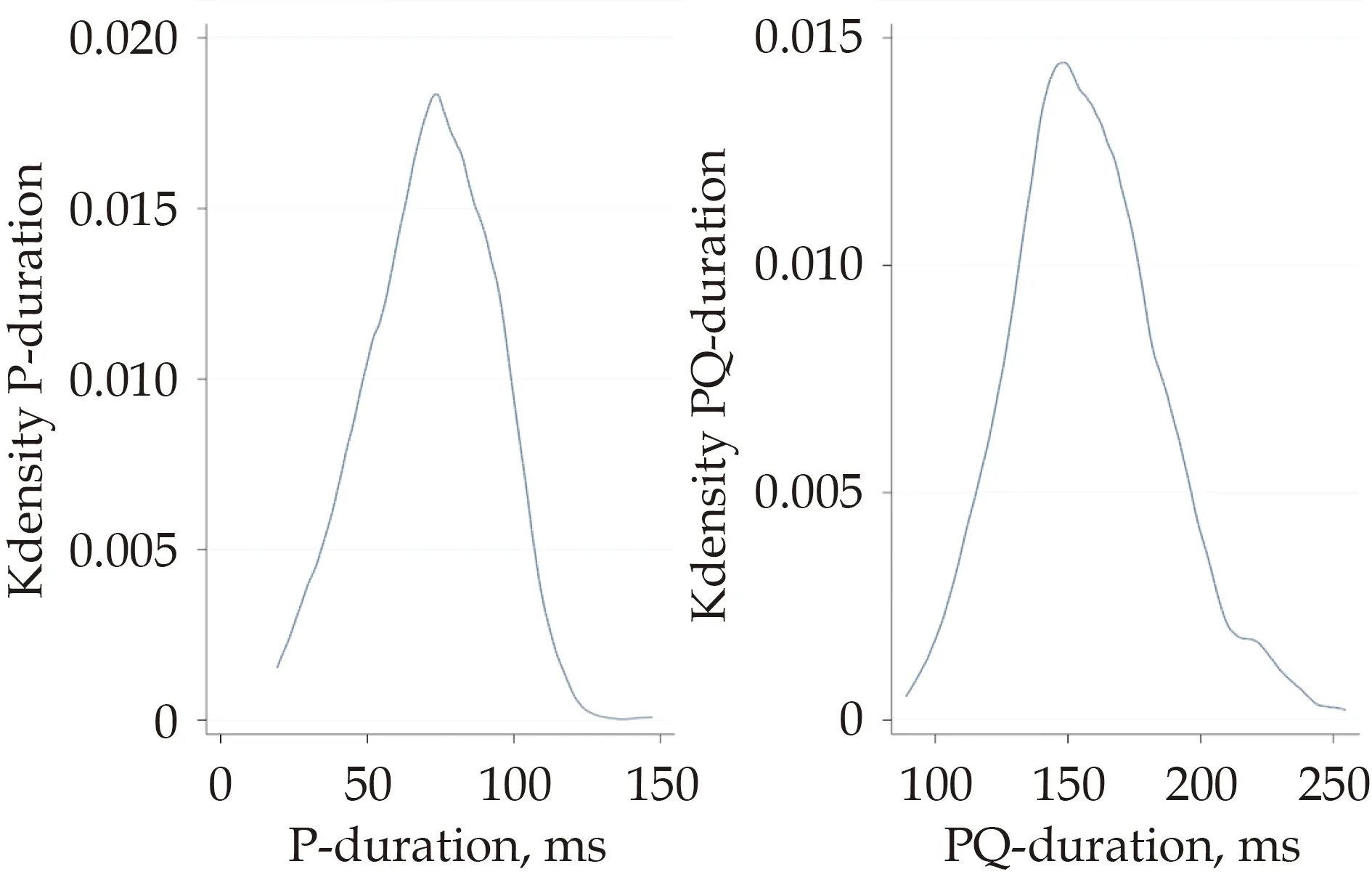
Figure 2 Distribution of P-wave duration and PR-interval duration in the study population at baseline.K density: kernel density estimation;PQ-duration: PQ-interval duration.

Figure 3 Relationship between P-wave duration in lead V1 and incident heart failure.The solid line represents the hazard ratio for incident heart failure.The dashed line represents the 95% confidence interval.
New-onset AF was diagnosed in 148 (17,7 %) subjects during follow-up,36 in whom occurring prior to the HF diagnosis.The mean Pdur at baseline in these subjects was 66 ± 22 ms.Atrial fibrillation was related to incident HF (P<0.0001),but had only a marginal impact on the association between Pdur and incident HF when introduced in the model as a confounder (stillP<0.0001 for Pdur).
Myocardial infarction occurred in 92 (11%) subjects during the follow-up period,23 in whom ocurring prior to the HF.The mean Pdur at baseline in these subjects was 70 ± 24 ms and although it was associated with subsequent HF (P=0.0001) it had only a marginal impact on the relationship between Pdur and incident HF when introduced in the model as a confounder (stillP<0.0001 for Pdur).
A short Pdur (<60 ms) improved the discrimination of future HF by 3.6% when added to a model with traditional risk factors for HF including sex,RRinterval,beta-blocking agents,systolic blood pressure,BMI and smoking [area under curve (AUC)=0.690;95%CI: 0.635-0.744) versus AUC 0.727 (95%CI 0.677-0.776) when including Pdur,P=0.048].
The Pdur in V1 was significantly related to LA diameter,LVEDD and LV mass,but not to LVEF,IVRTor the E/A-ratio (Table 2).When the LA diameter entered the Cox model evaluating the relationship between Pdur and incident HF as a co-variate,no major attenuation in the relationship for Pdur was observed (from Chi2 26.6 to 24.0,stillP<0.0001 for Pdur).Similar finding was seen when LV mass entered the Cox model (from Chi2 26.6 to 21.3,stillP<0.0001 for Pdur).
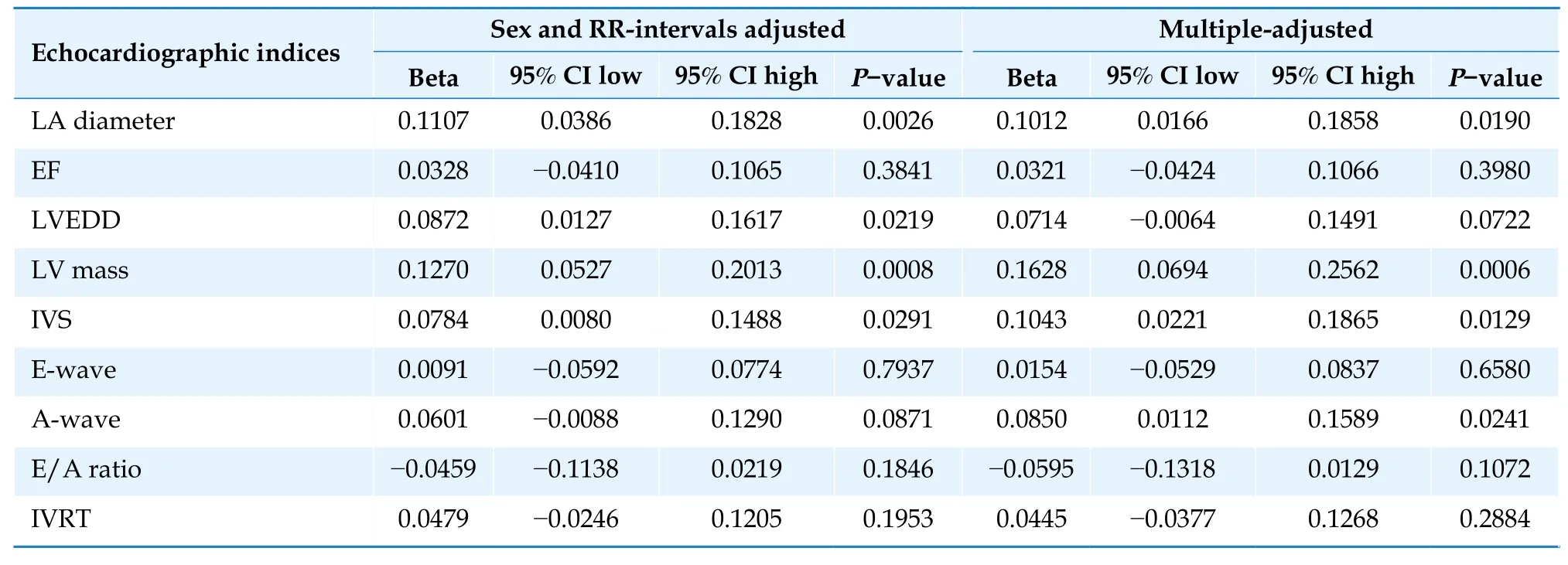
Table 2 Relationship between P-wave duration and echocardiographic indices at baseline.
The PR-interval in V1 was not significantly related to incident HF (P=0.15,Figure 5).Nor were the isolated “PR minus Pdur” interval or the Pamp in V1 or V3,related to incident HF (P=0.80,P=0.83 andP=0.09,respectively).
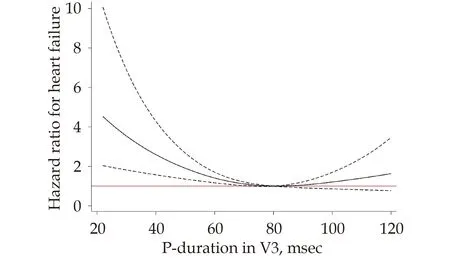
Figure 4 Relationship between P-wave duration in lead V3 and incident heart failure.The solid line represents the hazard ratio for incident heart failure.The dashed line represents the 95% confidence interval.
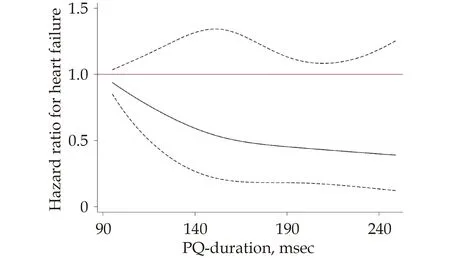
Figure 5 Relationship between PR-interval duration in lead V1 and incident heart failure.The solid line represents the hazard ratio for incident heart failure.The dashed line represents the 95% confidence interval.PQ-duration=PR-interval duration.
DISCUSSION
In the present study of elderly subjects,a short Pdur was associated with the development of HF.A short Pdur also improved discrimination of future HF as compared to traditional risk factors.To the best of our knowledge,the correlation between a short Pdur and incident HF has not been reported previously.
The P-wave reflects atrial depolarization and its duration is a measure of right and left atrial conduction times.[24]Nevertheless,the observed robust correlation between a short Pdur and incident HF is difficult to explain although there are some hypothetical mechanisms.The Pdur is influenced by the autonomic nervous system and seems to be significantly shortened by beta-adrenergic stimulation with isoproterenol and significantly prolonged by betablockade.[25]However,both the RR-interval reflecting the autonomic tone and treatment with betablockers were adjusted for,which should have eliminated these possible confounders.Since the association with incident HF was strongest for the shortest Pdur,another hypothetical explanation may be an increased atrial conduction velocity,related to changes in transmembrane ion channels or gap junctions.Relaxin,an emerging biomarker in HF,seems to possess the ability to increase the atrial conduction velocity.[26]Relaxin is a peptide hormone with anti-inflammatory,anti-fibrotic,anti-hypertrophic and anti-apoptotic effects exerted on various organs including the myocardium.In HF,levels of relaxin have been found to be increased with a possible subsequent downregulation of its primary receptors RXFP1.[27]Given that fibrosis is one of the main mechanisms for HF,the enhanced secretion of relaxin may reflect an early pro-fibrotic process in the heart prior to the development of HF.Thus,the enhanced velocity of atrial conduction induced by relaxin may cause the shortening of the Pdur.
A short Pdur has been associated with incidentAF in another study,postulating a triggering (shortterm) effect on AF with a similar U-shaped relationship as found in the present study for incident HF.[28]Nevertheless,the adjustment for AF prior to HF in the present study did not affect the association between a short Pdur and incident HF.
It remains unclear though whether the short Pdur plays a mechanistically causative roll or merely reflects an early,pathological atrial process.
Although lead V1 can be biphasic or more rarely isoelectric,it unlikely affected the results since the same robust correlation was demonstrated for Pdur in lead V3,which rarely displays negative or biphasic P-waves.Intra-and interobserver variability in P-wave measurements was minimized by analyzing ECGs digitally using a validated software.[24]
A prolonged Pdur may be a sign of structural atrial remodeling,often resulting in LA enlargement.[29]Systolic HF or diastolic LV impairment leading to increased LA pressure,remodeling and fibrosis are possible mechanisms of LA enlargement.[30-31]Heart failure caused by abnormal left ventricular (LV) diastolic function alone,i.e.,HF with preserved ejection fraction (HFpEF),is common and thus another plausible cause of a prolonged Pdur.[32]However,in the present study,it was not possible to distinguish between patients with systolic and diastolic HF.
Although a correlation between a prolonged Pdur ≥100 ms and HF has been reported earlier,such correlation could not be confirmed in the present study,which may possibly be related to the low number (6,6%) of subjects with prolonged Pdur.[30]
A prolongation of the PR-interval has been reported to worsen the outcomes in patients with preexisting cardiovascular morbidity,but reports have been inconsistent for other populations.[11]The present study found no significant association between the PR-interval and incident HF which may well be explained by the limited number (6,7%)of individuals with a prolonged PR-interval (≥ 200 msec).
The strength of this study is the long follow-up which enabled the detection of a majority of new HF-cases.The incidence of HF in the present study (9,8/1000 person-years) was comparable to a large epidemiological Swedish study (11,9/1000 person-years,age 70-79 years),even though they used both inhospital records and outpatient diagnoses.[33]
In conclusion,the association between short Pdur and incident HF,as found in the present study,is a novel observation that might facilitate early diagnosis and prevention of HF.The underlying pathophysiological mechanisms behind the association need further clarification.
The amplitude of the second negative component of the P-wave,reflecting the left atrium,was not measured separately which precluded its assessment as a separate risk factor.
The distinction between HF with preserved LVEF versus reduced LVEF was not possible since data on LVEF were not available at the time of diagnosis in most individuals with incident HF.Another limitation is that baseline LVEF was determined from the M-mode recordings.
ACKNOWLEDGMENTS
We would like to thank all the participants from PIVUS cohort.We thank Bo Skallefell,a former employee at AstraZeneca,for providing us with details on the EClysis software.This study was funded by Uppsala University Hospital (Sweden).
杂志排行
Journal of Geriatric Cardiology的其它文章
- In-hospital outcomes and readmission in older adults treated with percutaneous coronary intervention for stable ischemic heart disease
- The interaction effect of grip strength and lung function(especially FVC) on cardiovascular diseases: a prospective cohort study in Jiangsu Province,China
- Association between heart failure severity and mobility in geriatric patients: an in-clinic study with wearable sensors
- Iatrogenic atrial septal defects after transseptal puncture for percutaneous left atrial appendage occlusion and their hemodynamic effects
- Early identification of STEMI patients with emergency chest pain using lipidomics combined with machine learning
- Complement use of Chinese herbal medicine after percutaneous coronary intervention: a prospective observational study
