Co-adsorption behaviors of asphaltenes and different flow improvers and their impacts on the interfacial viscoelasticity
2022-10-04HaoZhangDaiweiLiuJiangboWenGuangyuSunChuanxianLiXinyaChenHuihuiZhangZeDuan
Hao Zhang ,Daiwei Liu ,Jiangbo Wen ,Guangyu Sun,3, *,Chuanxian Li,3 ,Xinya Chen ,Huihui Zhang,Ze Duan
1 College of Pipeline and Civil Engineering,China University of Petroleum (East China),Qingdao 266580,China
2 School of Petroleum Engineering,Guangdong University of Petrochemical Technology,Maoming 525000,China
3 Shandong Key Laboratory of Oil &Gas Storage and Transportation Safety,Qingdao 266580,China
Keywords:Asphaltene Flow improver Interfacial tension Interfacial rheology Emulsions Adsorption
ABSTRACT Commonly used flow improvers in oilfields,such as ethylene-vinyl acetate copolymer(EVA),poly(octadecyl acrylate)(POA),and polymethylsilsesquioxane(PMSQ)are proven to be effective to enhance the flowability of crude oil.However,the addition of these flow improvers may change the stability of the emulsion and make the crude oil treatment process challenging.In this research,the impacts of different flow improvers on the interfacial properties of the emulsions containing asphaltenes are systematically investigated.The co-adsorption behaviors of the flow improvers and asphaltenes are analyzed through dynamic interfacial tension (DIFT).The rheological properties of the interfacial layer after the adsorption are explored via dilational viscoelasticity.Significant difference is observed in the structural properties of the interface adsorbed by different flow improvers,which is attributed to different interactions between the flow improvers and asphaltenes.To investigate these interactions,conductivity,asphaltenes precipitation,dynamic light scattering (DLS),and contact angle experiments are conducted systematically.Results show that EVA and POA can alter the interfacial properties by changing the asphaltene dispersion state.The interaction between EVA and asphaltenes is stronger than that between POA and asphaltenes due to the difference in molecular structures.Unlike EVA and POA,the change of interfacial property with the addition of PMSQ is attributed to the partial adsorption of asphaltenes on PMSQ.
1.Introduction
During crude oil production,the fluid produced from reservoirs can be easily emulsified by high-speed shearing with wellhead throttle valve and centrifugal pump.However,the formed emulsions bring a series of problems such as energy waste in transportation and additional dewatering treatment.Further,the stability of the emulsions may be enhanced by the interfacial adsorption of asphaltenes and resins,which are usually considered as the natural surfactants contained in crude oil [1-4].It is widely known that asphaltenes are the components with the largest relative molecular weight and strongest polarity in crude oil [5].Asphaltenes usually contain some heteroatoms and metal elements such as O,N,S,Fe,Ni,V [6,7] forming unique molecular structures with polyaromatic rings and aliphatic side chains [8,9].Because of these structural features,asphaltenes have excellent interfacial affinities and are able to form a protective interfacial layer by adsorbing at the oil-water interface,preventing droplets from coalescence [1,10-12].
In order to enhance the flowability of crude oil and mitigate the deposition of paraffin wax during oil production,a variety of new injection additives for the wellbore have been developed.Among them,ethylene-vinyl acetate copolymer (EVA) and acrylate copolymer are the two most commonly used additives because they both can modify the morphology of wax crystals and delay the formation of wax network structures [13-16].Poly(octadecyl acrylate) (POA) is a typical representative of the acrylate copolymer.Both EVA and POA have interfacial affinities because of their polar and nonpolar moieties.In addition,nanomaterials have been gradually used in petroleum industry with the development of nanotechnology [17,18].Polymethylsilsesquioxane (PMSQ) is one of the novel nanocomposites synthetized in our laboratory [19].Experimental results demonstrate that its spatial hindrance effect can improve the flowability of crude oil significantly by participating in the wax precipitation process [19].
Because of the vital role of asphaltenes played in the stability of emulsions,their adsorption behaviors at the interface and subsequently the structural properties of the interfacial layer adsorbed by asphaltenes were widely studied and reported in literature[20,21].The work of Zhanget al.showed that asphaltenes adsorption at the interface between toluene and water is an irreversible process[22].Sztukowskiet al.[23]explored the adsorption process of asphaltenes at the interface by measuring the dynamic interfacial tension (DIFT).They concluded that asphaltene molecules are monomers in the adsorption process at the interface.However,others held the view that asphaltenes can migrate to the interface to form a gel-like interfacial structure by aggregation [24,25].Therefore,a consensus has not been reached on the behavior and mechanism of asphaltenes’ adsorption at the interface.
EVA and POA also have interfacial affinities,but their specific influence on interfacial characteristics lacks systematic research.Some previous studies performed by us and other researchers indicated that both EVA and POA can improve the dispersion state of asphaltenes [26-28].It can be speculated that these interactions may also change the structural characteristics of the oil-water interface and consequently affect the stability of the emulsion.PMSQ microsphere,a newly-developed flow improver,can also adsorb asphaltenes and prevent their precipitation [19].However,the influence of PMSQ on the interfacial properties with asphaltenes has not been widely explored.
As discussed above,a study on the interactions between the asphaltenes and flow improvers as well as their impacts on the co-adsorption behaviors and interfacial properties (e.g.,viscoelasticity) is of significance.In this paper,the co-adsorption behaviors of the asphaltenes and flow improvers were investigated by DIFT experiment and the interfacial viscoelasticity was analyzed by dilational modulus measurement.Conductivity,asphaltene precipitation,and dynamic light scattering (DLS) experiments were conducted systematically to study the effects of the flow improvers on the dispersion state of the asphaltenes.In addition,the influence of asphaltene adsorption on the wettability change of PMSQ was evaluated by contact angle experiment.Finally,the difference in the interactive mechanism between the asphaltenes and different flow improvers was analyzed.
2.Materials and Methods
2.1.Materials
EVA was obtained from Sigma-Aldrich Co.Ltd.n-Heptane,toluene,methylene chloride and sodium chloride (all analytically pure) were purchased from Sinopharm Chemical Reagent.PMSQ microsphere (500 nm) was synthesized with the method of twostep sol-gel route [19] and POA was synthesized with the method of solvent free-radical polymerization in our laboratory [29].The asphaltenes extracted from the Tahe crude oil(Sinopec Tahe Oilfield Company,China)were added into toluene to prepare the model oil.If it is not mentioned below,the concentration of the asphaltenes was controlled at 0.050%(mass)and the concentrations of EVA,POA and PMSQ added to the model oil were all 0.010%(mass).Distilled water and sodium chloride were used to prepare the model water.The concentration of the sodium chloride was 0.05 mol·L-1.
2.2.Asphaltene extraction
Excessiven-heptane was first added to the Tahe crude oil.After blended for 30 min under reflux distillation,the mixture was cooled in dark with vacuum.The mixture was then filtered when its temperature dropped to the room temperature.The residue was washed byn-heptane to remove the soluble components.Then,methylene chloride was used to dissolve the asphaltenes in the residue.Finally,the methylene chloride solution was dried out in the nitrogen atmosphere to obtain the asphaltenes.
2.3.Interfacial experiments
A tensiometer (Tracker H,TECLIS,France) was used to conduct the interfacial experiments.The interfacial tension was measured by analyzing the shape of the pendant droplet.Before the measurement,the model water was added to the quartz vessel and the model oil was loaded into the syringe.Then,one oil droplet was forced to form in the water phase and a charge-coupled device(CCD) camera could capture the shape of the droplet.The interfacial tension could thus be calculated based on the hydrostatic equation and Young-Laplace equation.It should be noted that the densities of the water and oil phase need to be obtained by using the pycnometer first.The measurement was repeated three times for data collection.
2.3.1.Kinetics measurement (DIFT experiment)
The measurement of the DIFT lasted for 3 h because the asphaltenes adsorbed at the interface in a slow way.A water bath(300F,Julabo,Germany) was used to control the temperature at 30°C.The volume of the formed droplet was controlled to be 8 μl.
2.3.2.Dilational modulus experiment
The real-time response of the interfacial tension could be obtained when the interfacial area of the droplet was oscillated sinusoidally.The dilational modulus (E) was achieved according to Eq.(1).

where γ is the interfacial tension andAis the interfacial area of the droplet.
The original volume of the droplet was also controlled at 8 μl and the frequency of the oscillation was fixed at 0.1 Hz.In order to ensure the integrity of the interfacial film structure,the oscillation amplitude was set as 10% of the droplet area.The duration of the measurement was 3 h.
2.4.Conductivity experiment
The asphaltene dispersion state was characterized with a conductivity meter (DDS-307,INESA,China).The extracted asphaltenes were added to the solutions composed ofn-heptane and toluene with different volume ratios.Then the conductivities of the asphaltene solutions with/without the flow improvers were measured at 30 °C.The concentration of asphaltenes was 0.050%(mass) and the concentration of the three flow improvers was 0.010% (mass).Each measurement was repeated three times.
2.5.Precipitation of asphaltenes in toluene solutions
The experiment of asphaltene precipitation was used to evaluate the stability variation of the asphaltenes in the model oil mixed with the flow improvers.First,the asphaltenes were dissolved in toluene to prepare the sample solution.The mass of the sample solution and the concentration of the asphaltenes in the sample solution werew0g and 0.500% (mass),respectively.Then,nheptane was added to the solution to precipitate the asphaltenes.The volume ofn-heptane was 20 times of the solution.After centrifuging the solution containingn-heptane at 10,000 r·min-1for 30 min,the precipitation of the asphaltenes (weighed asw1g)could be obtained by filtration and drying (under nitrogen flow).Consequently,the mass percentage of the precipitated asphaltenes wasw1/w0×100% .The mass percentage of the precipitated asphaltenes in the solutions with different flow improvers could be calculated in the same way.The concentration of the flow improvers was fixed at 0.010% (mass).The temperature of the experiment was 30 °C.Each measurement was repeated three times and the final result was averaged.
2.6.DLS experiment
The particle size distribution of the asphaltenes aggregated in the model oils was measured by DLS at 30 °C.A nanoparticle size analyzer (Zetasizer Nano ZS 90,Malvern,Britain) was used in the experiment.
2.7.Contact angle measurement
The wettability of PMSQ with/without the adsorption of asphaltenes was evaluated by the water contact angle.A contact angle measurement module of the tensiometer(Tracker H,TECLIS,France) was used.The volume of the water droplet used to measure the contact angle was about 5 μl.Each measurement was repeated 3 times.Before the measurement,PMSQ adsorbed with asphaltenes should be prepared.First,the asphaltenes were added into toluene and the concentration was fixed at 5.000% (mass).Then,PMSQ was introduced into the asphaltene solution and its concentration was 1.000% (mass).After that,the solution was placed in dark environment for 24 h to ensure the adsorption of asphaltenes on PMSQ.Finally,the modified PMSQ could be obtained after filtration and drying.
2.8.Molecular dynamic simulation
The interactions between asphaltenes molecules and EVA or POA molecules were investigated by employing the molecular dynamic simulation.The simulation model used was the Visualizer module of the Materials Studio software(Version 8.0).The interactions were calculated with the COMPASS II force field.The molecular structure of the asphaltenes followed the ‘‘continental” type suggested by Kuznickiet al.[30] and the molecular structure of the asphaltenes is displayed in Fig.1.The feasibility of this molecular structure has been verified by other studies[31,32].Dodecane was chosen as the solvent in the simulation model.The molecules of asphaltenes,EVA or POA,and dodecane were randomly packed in a periodic cell with a size of 30 × 10-10m × 30 × 10-10m × 30 × 10-10m.Meanwhile,a vacuum layer of 30 × 10-10m was set to limit the extra free boundaries on the model.After geometry optimization,the Forcite module was used to conduct the simulation.The Van der Waals force and Coulomb force were calculated based on the methods of Ewald and atomic summation,respectively.The NVT mode was applied and a Nose method was used to set the temperature as 30 °C.The step of time was 1 × 10-15s and the duration of simulation was 1 × 10-9s (106steps).Based on the simulation results,the energy of the model reached equilibrium:the balanced state of the system.The interactive energy between the asphaltenes and the flow improvers was calculated by Eq.(2).

whereEtotalis the total energy of the asphaltene and flow improver molecules.EaspandEflowimproverare the energies of the asphaltenes alone and flow improvers alone,respectively.

Fig.1.Molecular structure of the asphaltenes for molecular dynamics simulation.
3.Results and Discussion
3.1.Co-adsorption behaviors of asphaltenes and different flow improvers at the interface
3.1.1.Co-adsorption kinetics
The evolution of the interfacial tension with time was used to indicate the adsorption kinetics.The DIFT of reference interface without the asphaltenes and flow improvers was first measured.As demonstrated in Fig.2,the interfacial tension remains unchanged with time and its value is about 25.8 mN·m-1.Then,the DIFT of the composite system containing both the asphaltenes and the flow improvers was also measured.As indicated in Fig.3(a),the interfacial tension continues to drop with the adsorption of the asphaltenes and POA at the interface.After an initial rapid decrease of the interfacial tension,the declining rate gradually slows down until equilibrium.The resemblance between the DIFT curve of the asphaltenes and that of POA indicates that their adsorption behaviors share some similarities.
To demonstrate the interfacial adsorption of sole asphaltenes and sole POA at the initial process more intuitively,the surface pressure curves are drawn in semi-logarithmic coordinate in Fig.3(a).As expressed in Eq.(3),the surface pressure π refers to the difference between the interfacial tension of the reference interface γ0and that of the interface adsorbed with interfacial agents γ at timet.

Fig.2.DIFT of the blank interface.

Fig.3.DIFT and surface pressure of the single systems with only the flow improvers or only the asphaltenes and the composite systems with both the flow improvers and asphaltenes: (a) POA and asphaltenes;(b) EVA and asphaltenes;(c) PMSQ and asphaltenes.

As presented in Fig.3(a),the surface pressure value of the single system containing only asphaltenes or only POA is close to 0 mN·m-1when the oil-water interface is just formed.This phenomenon indicates that the adsorption of the asphaltenes and POA is not instantaneous but takes a relatively long time.Additionally,the surface pressure of the asphaltenes-POA composite system containing both asphaltenes and POA is also close to 0 mN·m-1at the initial moment.Due to the similar adsorption behaviors of asphaltenes and POA,the evolution of the DIFT and surface pressure of the composite system containing both asphaltenes and POA has no significant difference.According to some studies,POA can interact strongly with asphaltenes [28,33].Apparently,the composite particles formed through this interaction have a similar adsorption behavior with both the asphaltenes and POA.
The DIFTs and surface pressures of EVA and asphaltenes-EVA composite system are presented in Fig.3(b).It can be found that the DIFT curve of EVA is smoother than that of the asphaltenes.Additionally,the initial surface pressure is close to 1.5 mN·m-1when the model oil only contains EVA,but it is close to 0 mN·m-1when the model oil contains asphaltenes alone.This indicates that a certain amount of EVA has already adsorbed at the interface when the droplet is just formed.In other words,the adsorption of EVA is faster than that of asphaltenes.
As is also shown in Fig.3(b),the initial surface pressure of the asphaltenes-EVA composite system is close to 1.0 mN·m-1,which is between those of the two single systems.When an oil contains different interface-active substances,they usually adsorb at the interface in a competitive way [34].Hence,EVA and asphaltenes will adsorb competitively when they co-exist in the oil phase.EVA changes the interfacial tension quickly in the beginning by adsorption,so the initial surface pressure of the composite system is higher than that of the asphaltenes alone.On the other hand,asphaltenes can occupy the adsorption sites at the interface.As a result,the adsorption amount of EVA will decrease,so the initial surface pressure of the composite system is lower than that of EVA alone.
The effects of PMSQ on the DIFT and surface pressure are shown in Fig.3(c).When comparing its DIFT with that of the reference system,we may easily find that PMSQ itself does not affect the interfacial tension.In order to uncover the hydrophilic-lipophilic property of PMSQ,it was added into pure water and pure toluene respectively,at a concentration of 0.100% (mass).It can be observed from Fig.4 that PMSQ is dispersed quite well in toluene but very poorly in water.This also proves that PMSQ has a low interfacial activity and cannot adsorb at the interface.

Fig.4.Photograph of the dispersion state of PMSQ in water (left) and toluene(right).
However,an increase in the interfacial tension is observed in Fig.3(c)when PMSQ is added into the model oil containing asphaltenes.The increase is related to the reduction in the adsorption of interface-active substances.Asphaltenes are the only interfaceactive substances in the oil-water system,so it is the decrease in the amount of asphaltenes at the interface that leads to the increase of the interfacial tension.In other words,the addition of PMSQ causes the decrease of the adsorption amount of asphaltenes.Asphaltenes can be adsorbed on the surface of the nanoparticles because of the interaction between them [35,36].Research has shown that PMSQ can adsorb asphaltenes [19] and the adsorbed asphaltenes can change the wettability of the nanoparticles[37].Because asphaltenes can adsorb on PMSQ,the concentration of asphaltenes in the model oil decreases.This may lead to the decline of their adsorption amount.In addition,PMSQ adsorbed with asphaltenes can adsorb at the interface as well due to the wettability change.Therefore,the original adsorption sites of the asphaltenes may be occupied by the modified PMSQ,thus reducing the adsorption amount of the asphaltenes.
3.1.2.Adsorption equilibria study
As can be observed from Fig.3,the relative changes of the interfacial tension are small at the end of the measurement processes,so it can be reasonably inferred that the adsorption reaches equilibrium approximatively in 3 h.As shown in Fig.3(a),the equilibrium interfacial tensions of the single POA system and single asphaltene system are similar to that of the asphaltene-POA composite system.Because of the similar adsorption behaviors of asphaltenes and POA,the equilibrium interfacial tensions have no significant difference.The effect of the interactions between POA and asphaltenes on the equilibrium interfacial tension is not obvious.
As presented in Fig.3(b),the equilibrium interfacial tension of EVA is obviously higher than that of the asphaltenes.Meanwhile,it should be noted that the equilibrium tension of the composite system is much lower than those of the two single systems.This seems to contradict the principle of competitive adsorption.If the interface is competitively adsorbed by asphaltenes and EVA,the equilibrium value of the composite system should lie between those of the single systems with only the asphaltenes or EVA.In fact,our studies have shown that asphaltenes can interact with EVA [15,26,27].This may be the main reason behind the unusual low interfacial tension and will be further discussed below.
As discussed above,PMSQ can adsorb the asphaltenes and the modified PMSQ can occupy the original adsorption sites of the asphaltenes at the interface.Therefore,the equilibrium interfacial tension of the composite system has significant increase in Fig.3(c).
According to the above discussion,EVA and POA,unlike PMSQ,can decrease the interfacial tension by adsorbing at the interface.However,there are some differences in the adsorption behaviors of EVA and POA.The initial surface pressure of the single EVA system is larger than that of the POA system,indicating that the adsorption rate of EVA is higher than that of POA.As is known to all,EVA and POA are polymeric interface-active substances with similar molecular weights.The difference in their adsorption rates may be attributed to their molecular structures.POA belongs to comb-like polymer while EVA is a linear polymer [16,38,39].The adsorption and arrangement of a linear polymer at the interface are easier than those of a comb-like polymer.Furthermore,the co-adsorption behavior of POA and asphaltenes is also diverse from that of EVA and asphaltenes.The result of the EVA-asphaltene composite system becomes even against the rule of competitive adsorption.The different molecular structures of EVA and POA may be the reason behind the different co-adsorption behaviors due to their different interactions with asphaltenes.By contrast,PMSQ is not an interface-active substance and thus cannot influence the interfacial tension alone.However,it can achieve interfacial activity by adsorbing asphaltenes.
3.1.3.Dilational viscoelasticity of the interfacial layer after the coadsorption
The interfacial dilational moduli of the single systems including only the asphaltenes,EVA and POA were measured respectively.The results are shown in Fig.5.It can be easily found that the interfacial dilational moduli of all three single systems increase gradually during adsorption.Because PMSQ has no effect on the interfacial tension,its dilational modulus should equal to 0 mN·m-1according to Eq.(1).The dilational modulus can indicate the ability of the interfacial layer to resist deformation [40].The equilibrium modulus of the single asphaltene system is larger than those of the single systems containing only EVA and POA,indicating that the ability to resist the deformation of the interface adsorbed by the asphaltenes is stronger than that adsorbed by sole EVA or POA.
The dilational moduli of the composite systems with both the asphaltenes and the flow improvers were also measured.Fig.5(a)and (b) show that the modulus curves of the composite systems are quite similar and close to(slightly lower than)that of the single asphaltene system.This reflects that it is the asphaltenes who determine the deformation-resisting ability of the interfacial layer adsorbed by the composite systems.As mentioned earlier,the flow improvers will compete with the asphaltenes for the adsorption at the interface,which leads to the equilibrium modulus value of the composite system staying between those of the single systems.By contrast,the dilational modulus increases after PMSQ is added into the model oil containing the asphaltenes (Fig.5(c)).This indicates that PMSQ modified by the asphaltenes can take part in the formation of the interfacial layer and enhance its structural strength.
Moreover,the interfacial dilational modulus reflects the adsorption/desorption rate of an interface-active substance when the interface expands or contracts [41].As discussed above,the asphaltenes determine the interfacial property of the composite systems.Therefore,the slightly lower value of the interfacial dilational modulus of the composite systems reflects the increase of the adsorption/desorption rate of the asphaltenes to some extent[42].This is related to the interactions between the flow improvers and asphaltenes,which can affect the dispersion state of the asphaltenes.More dispersed asphaltenes can migrate more easily to or from the interface.In addition,the interfacial dilational modulus of the asphaltene-PMSQ composite system can also reflect the adsorption and desorption rates of the modified PMSQ.The increase of the dilational modulus indicates a decrease of the adsorption/desorption rate.This is because PMSQ particles have a certain size (500 nm).The size will become even larger after the adsorption of asphaltenes.Therefore,the asphaltene-modified particles can only adsorb/desorb slowly and cause the increase of the dilational modulus.

Fig.5.Dilational modulus of the single systems with only the flow improvers or only the asphaltenes and the composite systems containing both the flow improvers and asphaltenes: (a) POA and asphaltenes;(b) EVA and asphaltenes;(c) PMSQ and asphaltenes.
3.2.Interactions between different flow improvers and asphaltenes
3.2.1.Interactions between EVA/POA and asphaltenes and their effect on the dispersion state of asphaltenes
In order to explore the influence of EVA and POA on the asphaltene dispersion state and confirm the conjecture above,conductivity experiment was carried out.The asphaltenes were added in the mixture ofn-heptane and toluene with different volume ratios.Asphaltenes refer to the components in crude oil that can be dissolved in toluene but insoluble inn-alkanes.When the proportion ofn-heptane rises,asphaltenes molecules will aggregate and the asphaltene dispersion state will get deteriorated.The aggregation slows down the migrating rate of asphaltenes.Part of the aggregated asphaltenes will precipitate because of gravity and thus the number of charged particles in the solution will decline.The above two aspects both reduce the conductivity of the solution because the asphaltenes are the only particles with charges in the solution.The conductivities of the solutions added with only EVA and POA were measured and the values are 0 μS·cm-1.This shows that the conductive particles in the solutions are still the asphaltenes after the addition of the flow improvers.As shown in Fig.6,the conductivity of the solutions decreases monotonically with the increase ofn-heptane proportion,proving that the asphaltene dispersion state of is deteriorated.More importantly,the absolute value of the conductivity increases after the EVA and POA are added into the asphaltenes solutions.The absolute conductivity value reflects the migrating speed and the number of charged particles in the solutions.Therefore,it confirms that the asphaltene dispersion state is improved by their interactions with EVA or POA.

Fig.6.Conductivities of the asphaltene solutions with/without the flow improvers and the fitting results.
The conductivity curves are further fitted with the least-square method and the inflection points on the curves are calculated.The solid lines in Fig.6 are the linear fitting curves of the conductivity values and the dashed lines serve as auxiliary lines to mark the inflection points of the solid lines.The inflection is related to the deterioration of the asphaltene dispersion state,so the inflection point can be considered as the initial flocculation point of the asphaltenes [43].Then-heptane proportion corresponding to the initial flocculation point of the asphaltenes increases when EVA and POA are added into the asphaltene solution (Fig.6).In other words,moren-heptane needs to be added into the solution to induce the aggregation of the asphaltenes after added with EVA and POA.In summary,EVA and POA can interact with asphaltenes and improve the dispersion state of them.
The asphaltene precipitation experiment was also performed to evaluate the influence of EVA and POA on the stability of asphaltenes.The asphaltene concentration in the original toluene solution is fixed at 0.500%(mass).As presented in Fig.7,the total mass percentage of the asphaltenes precipitated in the flow-improver-free solution is 0.492%(mass).This means that the addition of excessiven-heptane causes almost all the asphaltenes to precipitate.By contrast,the precipitation mass percentage decreases significantly when EVA and POA are added into the solution.In other words,EVA and POA can disperse over 20% (dividing the difference value of the precipitation mass percentage of the solutions with/without the flow improvers by the precipitation mass percentage of the undoped solution)of the asphaltenes in the solution by interacting with them.Furthermore,EVA has greater influence than POA.This proves from another perspective that EVA and POA can prevent the precipitation of asphaltenes because their interactions can improve the asphaltene dispersion state.
To quantitatively characterize the asphaltene dispersion state in the model oil,the particle size distribution of the asphaltene aggregates was measured using DLS.As presented in Fig.8,the average particle size of the asphaltene aggregates decreases evidently when EVA and POA are added into the oil phase.This offers another proof that the asphaltene dispersion state is improved by EVA and POA and the improvement effect of EVA is better than that of POA.
3.2.2.Interactions between PMSQ and asphaltenes and their effects on the wettability of PMSQ
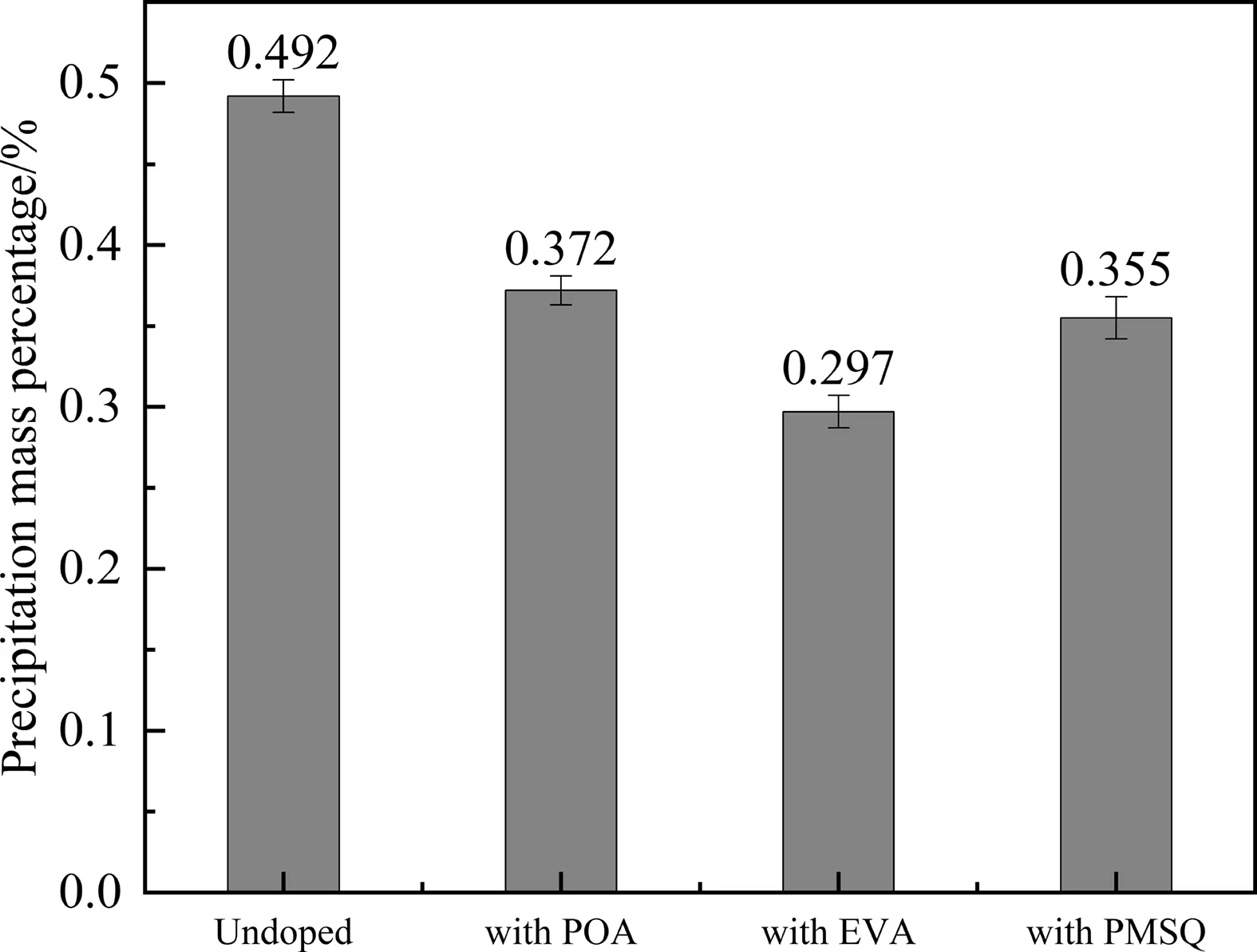
Fig.7.Mass percentage of the asphaltenes precipitated from the solutions with/without the flow improvers.

Fig.8.Particle size distribution and average particle size of the asphaltene aggregates in the solutions with/without the flow improvers.
The effect of PMSQ on the stability of the asphaltenes was also investigated by the asphaltene precipitation experiment.As shown in Fig.7,PMSQ can prevent the precipitation of almost 30%asphaltenes.The mechanism behind the precipitation prevention is absolutely different from that of EVA and POA.The asphaltenes can be adsorbed on the surface of PMSQ.PMSQ is oleophilic and has an excellent monodispersity in the oil.These two aspects can lead to a good stability of the asphaltenes in oil with preventing their precipitation.
Contact angle test verifies that the asphaltenes can adsorb on PMSQ and change its wettability.As exhibited in Fig.9,the contact angle decreases after the asphaltenes adsorb on PMSQ—demonstrating the reduction of lipophilicity.If a particle is expected to adsorb at the interface,it should have the ability to be wetted by both phases [44].Hence,the asphaltenes can modify PMSQ particles and make them amphiphilic.
3.3.Mechanism exploration on the interactions between asphaltenes and flow improvers
EVA and POA are polymeric molecules with polar and nonpolar moieties.Because of the amphipathic structures,they can reduce the interfacial tension through adsorbing at the interface.The competitive adsorption between the flow improvers and asphaltenes can partially impact the interfacial properties.Meanwhile,the interactions between the asphaltenes and EVA or POA can improve the asphaltene dispersion state,which has been confirmed by the conductivity,asphaltenes precipitation,and DLS experiments.Due to the attractions between the polar moieties/groups,EVA and POA molecules can interact with asphaltenes molecules.However,the nonpolar moieties of EVA and POA molecules cannot adhere to asphaltenes and can only stretch in the oil phase.The stretching nonpolar moieties are oleophilic and can enhance the oil compatibility of asphaltenes.They can prevent asphaltenes from aggregating and improve their dispersion state in the oil by steric hindrance effect.
Based on the DLS results,the average particle size of the asphaltene aggregates added with EVA is smaller than that with POA,indicating that the interactions between EVA and asphaltenes are stronger than those between POA and asphaltenes.EVA is a linear polymer with both of its polar and nonpolar moieties situated on the main chain while POA is a comb-like polymer with its polar and nonpolar moieties on the side chains.The interactive energies between asphaltenes molecule and EVA/POA molecule are shown in Table 1.It can be seen that the asphaltenes-EVA interactive energy is higher than that between asphaltenes and POA.Combined with the above experimental observations,it can be inferred that EVA with a linear molecular structure has a stronger binding capacity with the asphaltenes compared to POA with a comb-like structure.Hence,EVA can improve the asphaltene dispersion state better than POA and thus the interfacial properties are also different.

Fig.9.Contact angles of the original PMSQ (a) and asphaltene-modified PMSQ (b) (each measurement was repeated 3 times).

Table 1 Interactive energy between the asphaltene molecule and different flow improver molecules
There is a significant difference between PMSQ and EVA/POA.PMSQ can adsorb asphaltenes and change its own wettability.This makes PMSQ amphiphilic and be able to adsorb at the interface.Hence,the modified PMSQ can also affect the interfacial tension and enhance the structural strength of the interfacial layer.Moreover,PMSQ can prevent the precipitation of asphaltenes due to the adsorption.
4.Conclusions
The interactions between asphaltenes and different flow improvers and their effects on the properties of the interface are systematically studied in this study.The main findings are as follows.
(a) The asphaltene dispersion state can be improved through the interactions with EVA and POA.Because of the different molecular structures of EVA and POA,the effect of the interactions between EVA and asphaltenes is more significant than that of the interactions between POA and asphaltenes.The difference in the interactions between the EVAasphaltenes and POA-asphaltenes is the main reason that causes the difference in the interfacial properties.
(b) PMSQ alone cannot adsorb at the interface and affect the interfacial property.However,it can adsorb asphaltenes and then take part in the formation of the interfacial layer.Therefore,it can enhance the structure of the interface.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work supported by the National Natural Science Foundation of China (51704315).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Pomelo biochar as an electron acceptor to modify graphitic carbon nitride for boosting visible-light-driven photocatalytic degradation of tetracycline
- Tuning alginate-bentonite microcapsule size and structure for the regulated release of P.putida Rs-198
- Molecular reconstruction of vacuum gas oils using a general molecule library through entropy maximization
- High-loading Pt-alloy catalysts for boosted oxygen reduction reaction performance
- Location and size regulation of manganese oxides within mesoporous silica for enhanced antibiotic degradation
- Cross-metathesis of biomass to olefins: Molecular catalysis bridging the gap between fossil and bio-energy
