Tuning alginate-bentonite microcapsule size and structure for the regulated release of P.putida Rs-198
2022-10-04JiaweiDongYanhuiHeJunfenZhangZhanshengWu
Jiawei Dong,Yanhui He,Junfen Zhang,Zhansheng Wu,*
1 School of Chemistry and Chemical Engineering,Shihezi University,Shihezi 832003,China
2 Xi’an Key Laboratory of Textile Chemical Engineering Auxiliaries,School of Environmental and Chemical Engineering,Xi’an Polytechnic University,Xi’an 710000,China
Keywords:Microcapsules sizes Alginate-bentonite Release model Swelling degree
ABSTRACT In this study,different sizes of microcapsules with alginate and bentonite as natural macromolecular materials were prepared to investigate the release property of Pseudomonas putida Rs-198.The characteristics of three microcapsules were evaluated by SEM,FTIR,TG-DSC,XRD and wall thickness.The sizes of three microcapsules(MA,MB,and MC)were 1270.50,831.79 and 42.52 μm,respectively.First,the encapsulation efficiency of three MA,MB,and MC microcapsules were 82.20%,90.41%,and 85.84%,respectively.Second,the contact angles of MA and MB samples were similar,while smaller microcapsules MC have higher contact angle (85.05°),indicating poor hydrophilia and decreasing the swelling degrees.Third,the release cumulant of Rs-198 and macromolecule BSA linear stage was fitted to self-established mathematic model.Results show that the microcapsule size had a considerably positive effect on release detail.The large microcapsule possessed strong leak-tightness for Rs-198 as a slow-release microbial agent.Furthermore,the porosity of microcapsules determined their swelling and release and may affect bacterial growth and survival.In conclusion,the Rs-198 microcapsule with different sizes will be pertinently selected based on the characteristics of agricultural production requirements.
1.Introduction
Microencapsulation is an effective method to improve the biological activity and stability of plant growth-promoting rhizobacteria through extrusion,emulsification,complex condensation,or spray drying [1-4].Carbohydrate polymers have attracted much attention in the preparation of microcapsules,hydrogels,or films as high-biocompatibility,non-toxic,and biodegradable materials,such as sodium alginate (NaAlg) and chitosan [5],which are suitable for the agriculture and food industry for enhancing storage quality and slowing release property [6-9].The size of microcapsules is important and is an easily adjustable parameter for further application.Liet al.[10]found that the size of microcapsules has an important influence on drug loading,aggregation,drug release,and tissue retention in medical applications.In addition,the internal distribution of the microspheres and the interaction with cells are restricted by the diameter of the microspheres [11].Luo et al.reported that the diffusion and release behavior of pesticide microcapsules in the field could be regulated by simply adjusting particle size,and this property is valuable for agricultural applications[12].Thus,the release of bacteria from microcapsules could also be affected by the microcapsule size.
The particle size of microcapsule is greatly influenced by operating conditions,which play an important role in the structural formation and core material release.The large-diameter microcapsules by extrusion showed homogeneous and monodisperse and were used as slow-release fertilizer,thus providing plants with sufficient bacteria in a regular and continuous manner to fit the plant growth without multiple applications [13].Regular the diameter of alginate beads to desirable size for drip irrigation was expected to improve its application efficiency [14].
In recent years,the diffusion behavior of different microcapsules in solution had been extensively studied[15,16].These works mainly focused on the determination of the release kinetics and permeability of microcapsules [17,18].Some differences were observed in the kinetic fit of thermosensitive liposome drug delivery systems with different compositions and sizes,such as driving force and efflux rate[19].The permeability of gelatin acacia microcapsules containing olive oil decreases with decreasing capsule size [20].Large-size aromatic microcapsules have better leaktightness than small microcapsules,while small microcapsules have faster sustained release rate [21].Limited reports are available on the diffusion of bacteria in hydrogels with different particle sizes through effective mathematic models to predict the mobility of bacteria in the microcapsule polymer network.The release property of bacteria should be determined at different microcapsules particle sizes to adapt with diversified application conditions.
To broaden the applications of the microencapsulated Rs-198,the researchers aimed to evaluate the release characteristics with different sizes.The different sizes (millimeter and micron scales)of microcapsules were prepared by extrusion and emulsification.First,the swelling properties of microcapsules altered by salt ion and pH of aqueous solution were compared.The variation of the survival and growth characteristics of different particle sizes was calculated.The diffusion mechanism of microcapsules was divided into three stages to discuss the release profiles.Finally,a release model that reflects the relationship between particle diameter and the release percentage in linear release phase was established,and this model regulated the release program of Rs-198 and macromolecule BSA in NaAlg-bent microcapsules.The use of release model provides a possible theoretical support of the compound suitable for further use in the development of controlledrelease agricultural bacterial fertilizer.
2.Materials and Methods
2.1.Materials
Sodium alginate (NaAlg) was purchased from Guangdong Xilong Chemical Reagent Co.,Ltd.(China).The raw bentonite(Bent)samples were collected from Xinjiang China Non-metal Xiazijie Bentonite Co.,Ltd.The resultant Bent has a composition (%,by mass) of Al2O313.06,SiO264.62,Na2O 2.66,K2O 2.43,CaO 1.92,MgO 2.38,Fe2O34.93,TiO20.59,MnO 0.26,and P2O50.18,and an ignition loss of 6.20.CaCl2(calcium chloride anhydrous),span 80(sorbitane monooleate),NaH2PO4·2H2O (Sodium phosphate monobasic dihydrate),Na2HPO4·12H2O(Disodium hydrogen phosphate dodecahydrate) and BSA (Bovine serum albumin) were purchased from Tianjin Fuchen Chemical Reagent Co.,Ltd.(China).The paraffin liquid which catalog number is 8002-74-2 was from Tianjin Yongsheng Chemical Reagent Co.,Ltd.in China.The strainPseudomonas putidaRs-198 was isolated from the healthy cotton grown rhizosphere soil in a salinization field in Xinjiang,China (GenBank accession no.FJ788425) and propagated in nutrient agar liquid medium consisting 10 g·L-1of peptone,5 g·L-1of beef extract and 5 g·L-1of NaCl while shaking at 170 r·min-1at 30°C for 36 h.
2.2.Preparation of alginate microcapsules
Crossing-linked NaAlg-bent microcapsules were prepared via external ionic gelation.2 % (mass) NaAlg dispersions were prepared by homogeneous sodium alginate in purified water and stirred at 300 r·min-1for 6 h.At the same time 2 % (mass) bentonite was added to the homogeneous dispersions as compound wall material.The culture cells(1.79×108cells·ml-1)were mixed with double sterile NaAlg-bent solution.The mixture was then extruded through 1.30 mm and 0.70 mm needle at dropping rate of 2.0 ml·min-1into 2 % (mass) CaCl2solution at room temperature.Microcapsules (MA,MB) formed instantaneously and then were left in the cross-linking solution for 30 min.Microcapsules (MC)were prepared by external emulsion.The above-mentioned homogeneous dispersions were dropped into mixture solutioncontaining 1 ml span 80 and 100 ml paraffin liquid and stirred at 1800 r·min-1for 20 min.The hardening aqueous solution were added quickly and stirred at 200 r·min-1for 30 min.Finally,the emulsion was centrifuged for 10 min at 8000 r·min-1to get the microcapsules in precipitate.All the samples were washed repeatedly with deionized water to obtain the Rs-198 microcapsule.The formed beads were stored at 4 °C.
2.3.Characterization
The surface morphologies of the microcapsules were examined by scanning electron microscopy (SEM,JSM-6700F,Japan).The samples were anchored onto an SEM sample holder using carbon conductive double-sided adhesive discs.The chemical compositions of microcapsules were characterized by Fourier transform infrared (FTIR,Avatar 360,USA) analysis.The sample were mixed with KBr crystal powder and compressed under hydraulic pressure.The spectral range was 4000-400 cm-1.The specific surface area analyzer (BET,ASAP 2460,Micromeritics,USA) was used to perform the N2adsorption-desorption experiment to obtain the average pore size of the microcapsule surface.The X-ray diffraction results were recorded on an X-ray diffractometer (XRD,D8 Advance,Bruker,Germany)operated at 40 kV and 40 mA.The analysis for thermal stability of different microcapsules was carried out by using a thermogravimetry differential scanning calorimetry(TG-DSC) thermal analyzer (NETZSCH STA 449F3,Germany).The samples were heated from room temperature to 500 °C,under an N2atmosphere flow rate of 10°C·min-1.The particle size of microcapsules was measured by a laser particle size analyzer(McS3500,USA).The samples were dispersed into deionized water and each set of experiments was repeated in triplicate.
The surface wettability of alginate microcapsules was evaluated for static water contact angle measurements.A distilled water droplet of 1 μl was applied on each dry sample and the water contact angle was determined using the sessile drop method with automatic drop shape analysis (VCA optima,USA).Each sample was measured three times and expressed as the mean±standard deviation (SD).
The encapsulation efficiency of thePseudomonas putidaRs-198 was determined according to a procedure reported in the literature[22].The total number of bacteria added to the microcapsules was defined asE0and the number of the unwrapped bacteria was denoted asEu.These values could be obtainedviathe plate count method in nutrient agar medium (NA).All the experiments were performed in triplicate.Then,the encapsulation efficiency of bacteria was calculated as follows:

2.4.Swelling kinetics and cell density
The degree of swelling of the microcapsules was evaluated using a modified method [18].Exactly 1 g dry microbeads and microcapsule particle was immersed in 10 ml of water or different concentrations of NaCl at different pH solutions to equilibrium swell for 12 h at room temperature.Swelling ratio was defined as the weight ratio of the swollen microcapsules to the initial microcapsules.The coating porosity of the swelling process was determined using Eq.(2) as follows:

TheWwandWdwere defined as the mass of wet and dry particle,ρwand ρpas the densities of water and polymer.
The effect of saline ions in the swelling degree(SD)of microcapsules can be evaluated using Eq.(3) as follows:

The SDsalineand SDwaterwere the equilibrium swelling degrees for saline solutions and pure water,respectively.As the value offfor microcapsules in a certain saline solution becomes more close to zero,the ionic effect of such solution to the swelling degree is the lower.Whenfwas close to unity,the stronger the saline effect was.
The water absorption time of the newly formed microcapsules was evaluated at the time when 1 g microcapsule particles was immersed in 9 ml of water to saturation absorption.
2.5.Release behavior of microcapsules
Release assays were carried out as previously described with slight modification.[23].Exactly 1 g microcapsules encapsulated Rs-198 (1.79 × 108cfu·ml-1) and BSA (0.1 mg·ml-1) were added into 9 ml of phosphate buffer saline (pH=7).The bacteria were obtainedviathe plate count method at various time intervals after mixing.The BSA concentration was measured using UV-752 N spectrometer at 595 nm by using Coomassie brilliant blue G250.All the experiments were performed in triplicate.
The diffusion coefficient(D)of alginate microcapsules from BSA and bacterial solution was estimated [24].The BSA and bacteria were added to the wall material of microcapsules because of their large molecular weight.Exactly 10 g microcapsules were diffused in 100 ml of medium environment.The diffusion concentration was measured periodically.For the calculation of the diffusion coefficient,according to Fick’s law,theDvalue of the microcapsules was calculated using Eq.(4) as follows:

Ci,Ct,Cfrepresented the initial,intermediary (at timet) and final concentration of embedded objects,VsandVmwere the volumes of the environmental solution and the microcapsules,Awas the total surface area of the microcapsules andtwas the time.
Since ln[(Cf-Ci)/(Cf-Ct)]had a linear relationship with timet,the formula could be simplified as:

was the average particle size of the microcapsules,Kwas the slope of the straight line obtained by plotting ln[(Cf-Ci)/(Cf-Ct)]versus t.
2.6.Microcapsules release model
To effectively evaluate the release process of the three different microcapsules,we considered the diffusion release of bacteria from the polymer shell into aqueous solution.The influential factors of the release process in microcapsules were explored by constructing mathematical models.
2.7.Microcapsules storage and cell density
The active time of bacteria in microcapsules was closely related to the application of microbial fertilizer.Exactly 100 mg Rs-198 microcapsules were obtained every five days and soaked in 10 ml of 0.3 mol·L-1sterile sodium citrate solution.After complete dissolution,the number of viable bacteria contained therein was measured via plate counting to evaluate the storage performance of the microcapsules.
The swelling may cause onset and continual leakage of bacteria by diffusion.The cell density was the cell-laden alginate microbeads and microcapsules in a nutrient medium(NA)at 37°C for 48 h.The cell density was measured in the beads by adding 0.3 mol·L-1citric acid to crush the microcapsules,and the number of bacteria was counted via plate coating.Moreover,the leakage and growth of bacteria from microcapsules was measured in the medium.
2.8.Statistics analysis
The above experiments were performed in triplicate and the results were mean value ± standard deviation.Statistical analysis was performed using Origin 9.0,significant differences at the level ofp<0.05.
3.Results and Discussion
3.1.Microcapsules preparation and characterization
3.1.1.SEM
This section aims to evaluate the morphology and size of microcapsules.Accordingly,the microcapsule samples were subjected to scanning electron microscopy(Fig.1).SEM images showed that the three microcapsules were spherical particles with a defined size(Fig.1(a)-(c).The surface of MA and MB were rough and irregularly folded(Fig.1(a1)and(b1)),whereas the MC microparticle demonstrated a smooth and compact gel structure(Fig.1(c)).No obvious pores were observed on the microcapsule surface,and this finding is conducive to avoiding the cell rapid leakage.A large number of bacteria was found inside the capsule (Fig.1(a2) and (c2)),and the incorporation of cell did not affect the integrity and shape of beads.The wall thickness of the MA,MB and MC microcapsules were 200.45,62.86,and 28.54 μm (Fig.1(a3)-(c3)),respectively,and the large capsules have high wall thickness.These results were consistent with the findings of Bagaria,wherein the shell thickness of microcapsules increased linearly with increasing diameter [25].
3.1.2.Size distribution
The MC microcapsules had a high percentage in the range of(42.52 ± 5.36)μm with a narrower size distribution,as shown as Fig.S1 (Supplementary Material).The particle sizes of MA and MB microcapsules were (1270.50 ± 29.55) and (831.79 ± 63.16)μm (Table 1),respectively,which were measured using a Vernier caliper.BET was employed to further measure the pore diameters and surface areas of these microcapsules.Results showed that larger microcapsules(MA)had large surface area,pore size,and pore volume (Table1).These observations were consistent with bulky capsules possessing a larger contact area in the release medium.

Table 1 The physical characteristics of three microcapsules

Fig.1.Scanning electron microscopes and particle size distribution of bacteria Rs-198 microcapsules (a-c respectively represent the overall electron microscope images of MA,MB and MC microcapsules;a1-c1 represent the external surface of three microcapsules;a2-c2 represent internal microcapsules are loaded with Rs-198;a3-c3 represent the determination of wall thickness microcapsules).
3.1.3.Encapsulation efficiency
The highest encapsulation efficiency of 90.41% was achieved at MB.The encapsulation efficiency of MA and MC microcapsules were 82.20% and 85.84% (Table 1).Rs-198 was well encapsulated in the wall material for later applications.For the MB microcapsules,the wall material formed smaller droplets by extrusion,causing shorter solidification mass transfer time to avoid a large loss of bacteria.For the MC microcapsules,cleaning oil on the surface of microcapsules caused the decrease of encapsulation efficiency.Perez found that larger capsules have more wrinkles,thus allowing more bacteria to be loaded [26].
3.1.4.FTIR
FTIR spectroscopy was used to analyze the formation of NaAlgbent microcapsules and their interactions(Fig.2(a)).In general,the three microcapsules had one relatively large difference at approximately 2956 cm-1.The stretching vibration of -CH2was weakened in MC capsule at 2929 cm-1,and MB microcapsules had stretching vibration of -CH3at 2925 cm-1.However,the MA microcapsules had no stretching vibration.Except for this instance,all three microcapsules showed no obvious difference.The strong and broad absorption peaks of NaAlg represent the stretching vibrations of O-H in water molecules at 3438 cm-1and -CH2stretching vibrations at 2920 cm-1,which were the characteristic of polysaccharides.The stretching vibration of -C=O asymmetric carboxylate and COO-symmetric carboxylate was observed at 1626 and 1417 cm-1.The C-O stretching vibration in CH2-OH was also observed at 1029 cm-1[27].The characteristic absorption peak of bentonite at 3436 cm-1is associated with the-OH stretch vibration of interlayer water molecules.The sharp peak at 3622 cm-1is associated with the stretching vibration of Al-OH and Mg-OH in the octahedron of montmorillonite.The peak at 1639 cm-1is associated with the -OH bending vibration of the water molecules between the bentonite layers [28].
3.1.5.XRD

Fig.2.The characterizations of three microcapsules.(a)FTIR spectra of NaAlg,Bent,MA,MB and MC,(b)XRD spectra of NaAlg,Bent,MA,MB and MC,(c)TGA spectra of NaAlg,Bent,MA,MB and MC,(d) DSC spectra of MA,MB and MC.
The crystal structures of NaAlg,bent,MA,MB,and MC were analyzed by XRD,as shown in Fig.2b.Alginate only had a broad diffraction peak because of its non-crystalline structure [29].The diffraction pattern of bentonite had nine main reflections at 19.84°,28.38°,36.58°,and 61.87°,which characterized the samples mainly as montmorillonite (Mt).The XRD peaks at 20.89°,26.68°,and 45.52° of the bentonite can be attribute to the quartz (Qtz)structure [30].The raw material also consisted of cristobalite(Cri,peak at 31.76°).By comparing the three microcapsules,MA showed no Cri characteristic peak spectrum at 31.52°,while it had a peak at 29.48°.The MB microcapsules showed two sharp peaks at 45.48° and 50.23°,which may be associated with the quartz structure.
3.1.6.TGA-DSC
The thermal behavior of alginate,bentonite,and the MA,MB,and MC microcapsules indicated different degrees of degradation in Fig.2(c) and (d).As shown in Fig.2(c),the loss of moisture and other volatiles of alginate were first observed in the temperature range of 30-150°C.The second event occurred in the temperature range of 200-320 °C and was in connection with the decomposition of the alginate carbon chains and the formation of sodium carbonate (Na2CO3),which decomposed as the temperature increased [31].Bentonite mass was lost because of water absorption,interlaminar water,water in hydroxyl group,and organic matter loss [30].
By comparison,the TGA curves of three microcapsules also had three stages of heat loss.Notably,the large microcapsules had higher mass loss than smaller microcapsules at the first stage with values of 19.76%,16.42%,and 13.84%.This phenomenon was mainly caused by the lack of free water,and the water content of MA microcapsules was greater than that of MB and MC microcapsules in Table 1.The same phenomenon occurred at the third stage,and the larger microcapsules had higher mass loss.Moreover,MC microcapsules had slower decomposition rate and less total mass loss,possibly because small microcapsules have a tighter network structure.
The thermal behavior of the three microcapsules was observed to discuss and compare the DSC curves in Fig.2(d).The exothermic peaks with a maximum at 87.11,85.16,and 79.98°C were for MA,MB,and MC microcapsules,respectively,corresponding to the loss of water molecules or the evaporation temperature of the water.Based on the TGA curve,the exothermic peaks of disintegration of three microcapsules polymer chain were 287.43,295.27,and 279.04 °C.The pyrolysis temperature required for MA and MB microcapsules was higher than that for MC,possibly because the wall material prepared by extrusion method was thick and rough.
3.2.Swelling kinetics
The swelling process of different microcapsules in water is presented in Fig.3.The dry microcapsules reached swelling saturation after approximately 5 days,as shown in Fig.3(a),and the swelling degrees of MA,MB,and MC capsules were 144.67%,133.00%,and 120.14%,respectively.The large microcapsules (MA) showed high swelling property with many irregular folds of external surface,providing more positions for water absorption.The swelling speed of the MB and MC microcapsules was higher at the initial stage,but the water absorption capacity was not high enough,because the thin wall hindered the water swelling process.The swelling performance of the microcapsules was strongly related to the release of the core material,which also represented the membrane stability and related to cell leakage [32].
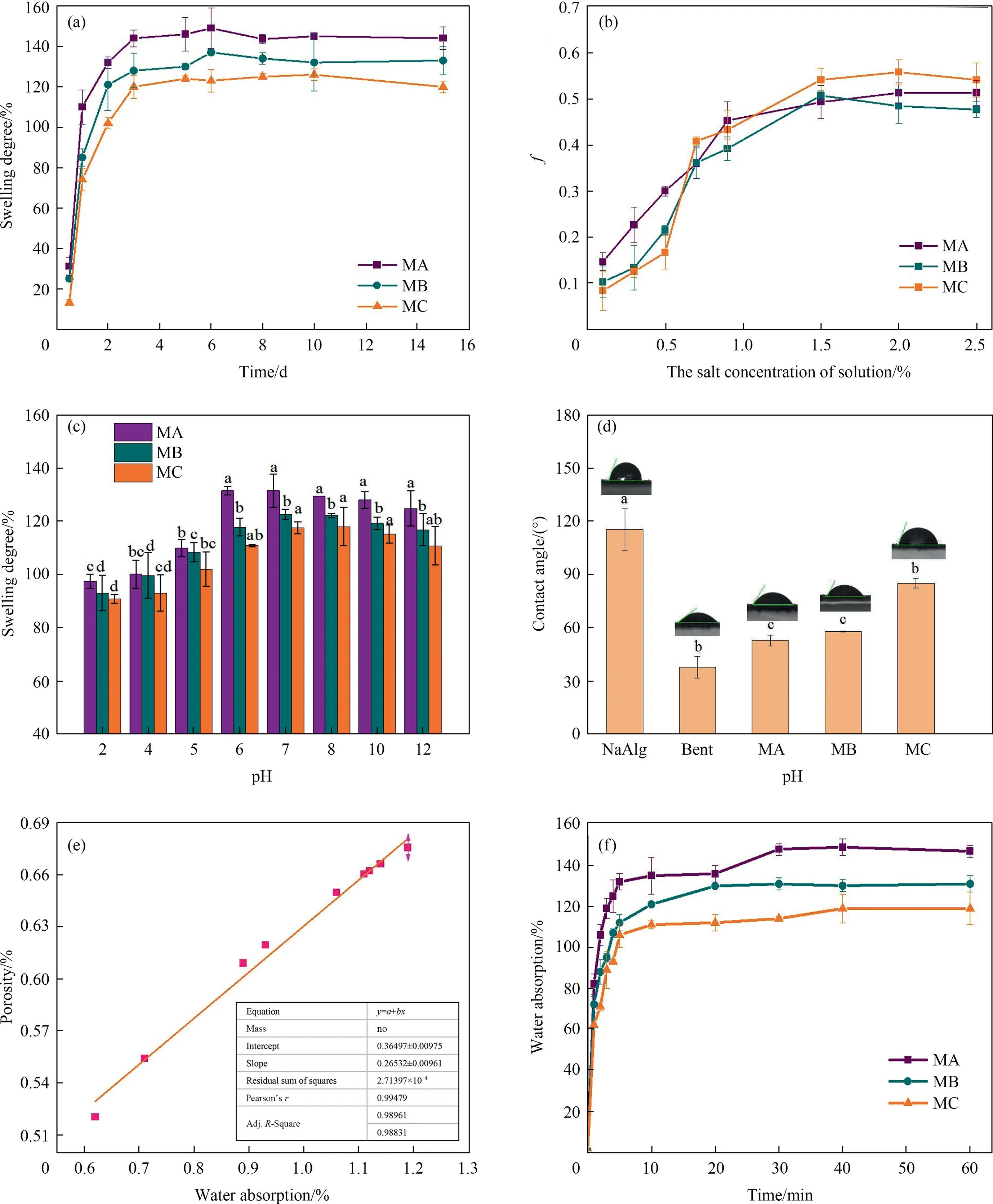
Fig.3.The swelling characteristics of three microcapsules.(a) the swelling kinetics of microcapsules;(b) the effects of different salt concentrations on f values of three microcapsules;(c) the effects of pH on swelling degree of three microcapsules;(d) the contact angles of three microcapsules and wall materials;(e) the linear relation between porosity and water absorption of the MC microcapsule.ρp=1.75 g·cm-3;(f) the water absorption of new microcapsules.
3.2.1.Swelling performance of microcapsules in different salt concentrations
To study the osmolality effect of salts solution on the swelling behavior of different microcapsules,we calculated thefvalues,as shown in Fig.3(b).As thefvalue became close to zero,the microcapsules had the same swelling degree with water.Thefvalues of three microcapsules increased slightly with the increase of salt solution concentration,indicating that the swelling property declined with the increase in salt.The swelling degree of the MA,MB,MC microcapsules decreased by 47.69%,51.33%,and 54.17%,respectively at 2.5 % (mass) salt concentration compared with water.Moreover,the MA capsule was more sensitive to salt ion concentration,and the network structure expanded first to achieve swelling equilibrium.However,the osmotic pressure increased the swelling level.Hence,the initialfvalue was high,and the multiplication rate offvalue decreased thereafter.With the continuous increase of salt ion concentration,the expansion degrees of capsules tend to balance.The effect of sodium ion on swelling degree of MC microcapsules was strengthened,as thefvalue became close to 0.55.The movable ions of MC microcapsules were reduced because of their low thickness,and the outer oil layer impeded the expansion of the network structure.
3.2.2.Swelling performance in different pH solutions
The swelling performances of microcapsules at different pH of solutions were measured and present in Fig.3(c).Similar to the swelling process in water,the large microcapsules (MA) showed high swelling degree of 131.50% at pH 7.At the same time,the microcapsules expanded easily in alkaline solution than in acidic condition,especially for MA microcapsule at pH 10,in which the swelling rate increased by 27.87% compared with that in acidic environment (pH=4).The swelling rate of MB and MC microcapsules also been increased by 23.87% and 19.68%,respectively.The equilibrium swelling occurred in a really short time under low pH.This condition restrained the electrostatic repulsion of anions on the same chain or different chains,causing the microcapsules to shrink slightly.Hence,the swelling degree of MA,MB,and MC microcapsules decreased by 25.86%,24.15%,and 22.72% respectively,compared with that in neutral solution.
3.2.3.Contact angles
The expansion of the network structure of the microcapsules was restricted by their hydrophily in aqueous solution.Hence,the contact angles of the three microcapsules with water relative to the wall material were observed and illustrated in Fig.3(d).The contact angles of MA and MB samples were similar (respectively 52.52° and 57.79°),but smaller microcapsules such as MC have higher contact angle (85.05°),indicating poor hydrophilia.This condition may be associated with the residual oil on the surface of the microcapsules.
The swelling process of microcapsule was accompanied by the expansion of network structure in water and pores opening.The correlation analysis results depicted that water adsorption has a positive relationship with the porosity of MC microcapsules(Fig.3(e)).Considering that bacteria are released from controlled release microcapsules after saturation with water,the swelling saturation time of different wet microcapsules should be determined.The newly formed microcapsules reached the water adsorption balance in approximately 30 min (Fig.3(f)).By comparing the swelling degree of the three wet microcapsules,the larger microcapsules had higher water adsorption amount.
3.3.Microcapsules release properties and kinetics
3.3.1.Release properties
The Rs-198 and BSA were encapsulated in microcapsules with three different sizes,and the release process curves are shown in Fig.4.The three microcapsules exhibited similar release profiles,including an initial fast diffusion,followed by a relatively slowrelease stage (Fig.4(a)).The microcapsule size had a significant effect on release detail.The MC microcapsules had the fastest release rate in PBS solution,and the cumulative release amounts of Rs-198 were 31.83 and 17.48% higher than those of MA and MB microcapsules in the first six hours.These variations in release behavior may be largely caused by the position of core bacteria closer to the external solution.The thickness of the membrane was also closely related to the mechanical strength,and the thicker membrane had stronger ability to resist the surroundings [33].In addition,the Rs-198 maximum release value of MC microcapsules reached 37 × 107cfu·g-1in 42 h,compared with the thick shell,and the thin shell was more favorable to release core bacteria.However,the order of releasing percentage changed in the late stage,and the release degree of MA was the greatest,followed by MB and MC.The release amount of MC microcapsule was 83.69% in the initial burst stage,causing a low Rs-198 content in the later period.Thus,the large microcapsules showed higher resistance to leak,and the small microcapsules exhibited a faster sustained release rate,indicating that the microcapsules with different particle sizes will have different application potentials.
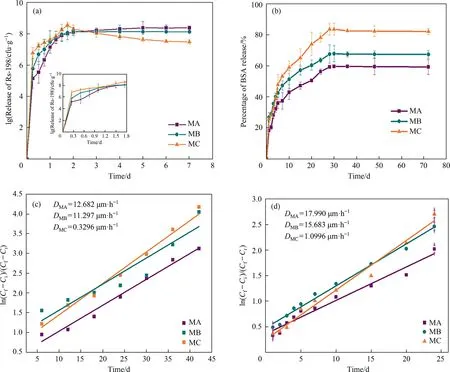
Fig.4.The release properties of the three microcapsules in aqueous solution:The release curve of Rs-198(a)and BSA(b)in PBS buffer solution at pH 7.0;Plot of ln[(Cf-Ci)/(Cf -Ct)] versus t of the Rs-198 (c) and the BSA (d).
For the release of the BSA,the release curve is shown in Fig.4(b).In comparison with the bacteria,BSA had smaller molecular weight,more stable substance,and shorter release time.Approximately 83.75%of BSA were released from MC microcapsules within 28 h,and the release rate slowly increased in the next few hours.The MC microcapsules had the fastest release rate and highest cumulative release proportion in the whole stage.Although the pore diameter of MA capsule was large,the porosity had no obvious effect on the free smaller molecules.The longer diffusion distance blocked the diffusion of the core material.
3.3.2.Diffusion coefficient
BSA and Rs-198 were used as test probes to simulate the diffusion behavior of alginate microcapsules.Their diffusion amount was fitted withtin Fig.4(c) and (d),showing that the ln[(Cf-Ci)/(Cf-Ct)] had obvious linear relationship witht.The linear slope of MC microcapsules for the two markers was larger than that of MA and MB microcapsules,indicating a higher release rate,especially for BSA.The results are consistent with Ma’s research,where the permeability decreased in the wake of amplifying membrane thickness [34].Furthermore,the diffusion process of Rs-198 from the three microcapsules was very slow,indicating that the complex diffusion process of Rs-198 subject to several influencing factors.
The diffusion coefficient (D) of BSA and Rs-198 was calculated using Eq.(5),and theDvalues were proportional to the microcapsule particle diameter.The MA microcapsules had thicker outer shell and longer diffusion distance,thus requiring a larger diffusion coefficient than thin layer microcapsule at the same diffusion time.These observations are consistent with the results reported by Wu,where theDvalues are related to the surface aperture of microcapsules.Moreover,for the same size of microcapsule,theDvalue of BSA was far greater than that of Rs-198,because the slope of the line decreases with the increase of molecular weight of the marker.
3.4.Model theoretical analysis
Three different diameter microcapsules were carefully chosen to verify the influence of size on release.The release process was divided into three stages as follows [35]: (i) initial stage no occur release (lag time),(ii) linear release stage,and (iii) slow-release stage.The initial stage of microcapsules with Rs-198 had nearly no release referring to the absorption water process when microcapsules were placed in aqueous solution.The third stage involved a gradual decay process of the release rate,wherein the number of bacteria in the microcapsule continuously decreased.Hence,the release cumulant of Rs-198 linear stage was fitted to establish a mathematic model.To simplify the release model,we idealized the microcapsule particles with a core-shell sphere structure.The concentration in the polymeric wall was a function of both timetand the position variabler,and was determined by transient diffusion according to Fick’s second law as follows [36]:

whereDis the diffusion coefficient,andCis the concentration of bacteria in the polymeric wall.Cell growth in microcapsules was ignored.Numerical solution and Fourier series expansion were used to determine the percentage of microcapsule release in terms of timetand positionr[37]:
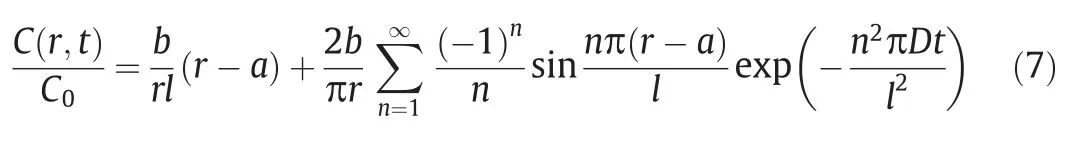
whereC(t) is the concentration of diffused cells at timet,bis the sphere radius,ais the nuclear radius,andlis the wall thickness of the microcapsules,namelyb-a.The following equation represents the condition when the diffusing substance emerges at the external interface:

Fig.5.The release amount and diffusion model linear simulation of Rs-198 cell (a) and BSA (b) from three microcapsules.
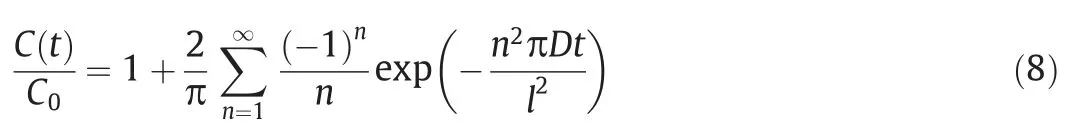
Eq.(8) represents the release percentage of microcapsules in the aqueous solution,but the blocking effect of other factors on the solute during diffusion was not considered.Therefore,the blocking coefficient β should be considered to obtain the following Eq.(9):

The release percentage was related to the diffusion coefficientD,wall thicknesslof the microcapsules,and the time variablet.The difference of microcapsule radius was extended to the distinction of wall thickness and diffusion coefficient.Therefore,this model could predict the concentration profiles of bacteria within the microcapsules in the linear release stage.
To evaluate the validity of the model,the actual measured value and the estimated value of the model were fitted.The matching effect of the Rs-198 release cumulant with the predicted value was slightly reduced because of the complexity of bacterial activity(Fig.5(a)),especially MB capsules,(R2=0.934).Fig.5(b)shows that the release percentage of Rs-198 had an obvious linear relationship with timetin microcapsules and had a high fitting with mathematical prediction degree (R2>0.98).The model could assess the release rate of different diameters of alginate microcapsules,indicating that the MC capsules maintained high release rate in BSA and Rs-198 release with higher slope considering the closer distance to the external medium,which was critical to supply sufficient nutrients and oxygen for cell growth.Hence,the release could be controlled by regulating the microcapsule diameter to fit the desired need.
3.5.Microcapsules storage and cell density
The storage and cell density of the three microcapsules were measured,as shown in Fig.6.The MA microcapsule maintained high storage capacity with 0.87 × 107cfu·g-1after nearly two months (Fig.6(a)),and the survival rates of stored microcapsules decreased with time.The membrane thickness of the microcapsules was closely related to the permeability,and the thicker membrane increased the density and reduced the mass transfer characteristics,resulting in a good protection for barrier [25].

Fig.6.The storage performance (a) and the growth property (b) of cell in three microcapsules.
The Rs-198 growth and density properties of microcapsules with different particle sizes for 48 h are shown in Fig.6(b).The solid line represents the number of Rs-198 from the microcapsules growing in the medium,and the dotted line represents the change of internal Rs-198 number from microcapsules.For the growth curve of encapsulated Rs-198,the MA and MB capsules from the same preparation method were the same,while Rs-198 encapsulated in MC microcapsules grew faster,and the cumulative growth quantity immediately reached the maximum at 17.78 × 107cfu·g-1.These observations were consistent with previous reports,where thin membrane augments membrane mass transfer rate for nutrients and facilitates cell growth[34].Meanwhile,for the internal encapsulated Rs-198,the bacterial activity of three microcapsules was similar and stable at 14.45 × 107cfu·g-1,accounting for the wall material of microcapsules that could provide protection against the bacteria.
4.Conclusions
The characteristics determined by the different sizes of microcapsules were analyzed in this work.The results showed that both the wall thickness and the swelling property increased with the sizes of microcapsules.The relationship between the size,release time,and cumulant release amount of Rs-198 were also analyzed based on the release curves and well-fitted to self-established mathematic model.Furthermore,the size of microcapsules can regulate their growth and survival characteristics.Therefore,the Rs-198 microcapsule will have broad utility in agricultural field because of its controlled release and adjustable porosity through size control.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China(21566035,U1803332),Key Research and Development Program of Shaanxi Province (2020NY-132).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.03.056.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Pomelo biochar as an electron acceptor to modify graphitic carbon nitride for boosting visible-light-driven photocatalytic degradation of tetracycline
- Molecular reconstruction of vacuum gas oils using a general molecule library through entropy maximization
- High-loading Pt-alloy catalysts for boosted oxygen reduction reaction performance
- Location and size regulation of manganese oxides within mesoporous silica for enhanced antibiotic degradation
- Cross-metathesis of biomass to olefins: Molecular catalysis bridging the gap between fossil and bio-energy
- Liquid-phase epoxidation of propylene with molecular oxygen by chloride manganese meso-tetraphenylporphyrins
