A Novel Visible-Light-Driven AgI/Ag/BiOBr Photocatalyst with Enhanced Photocatalytic Performance
2022-09-16CAODongdongJIAXuemeiJIANGShanLINHailiCAOJing
CAO Dongdong,JIA Xuemei,JIANG Shan,LIN Haili,CAO Jing
(School of Chemistry and Material Science,Huaibei Normal University,235000,Huaibei,Anhui,China)
Abstract:To improve separation efficiency of the photogenerated electron-hole pairs,constructing a Z-scheme heterojunction using Ag as charge transport bridge is considered to be a promising strategy.A novel AgI/Ag/BiOBr Zscheme system was prepared via deposition-precipitation means assisted by the photo-reduction process.The as-obtained AgI/BiOBr composites with the optimized ratio of Ag/Bi at 0.6 exhibited superior visible-light photocatalytic performance for azo dye methyl orange(MO)and colorless phenol degradation and removal rate reaches 94.8% and 86.2%,respectively.The enhanced performance of AgI/BiOBr heterojunction may be attributed to the fact that the Ag serves as a charge transport bridge in the Z-scheme system to accelerate the separation and migration of the photogenerated carriers.This work not only demonstrates the application of Ag as charge transport bridge but also provides a new way to design and prepare high-efficiency and stable photocatalysts.
Key words:Z-scheme;Ag nanoparticles;cocatalyst;photocatalysis
0 Introduction
Nowadays,with industrial production and rapid development of urbanization,the environment we live has been seriously polluted.As a progressive oxidation technique,semiconductor photocatalysis has been considered as the most desirable ways for sewage treatment,especially for water contaminants caused by antibiotics,synthetic dyes or heavy metal ions[1-5].However,low visible-light utilization efficiencies and high photoinduced carrier recombination in semiconductor limited its practical application.Hence,it is an interesting challenge to develop a facile and efficient method to prepare high photocatalytic property and visible-lightdriven photocatalysts.
Recently,BiOX(X=Cl,Br,I),as multifunctional semiconductors,have aroused extensive concerns due to the favorable structural and band properties.Among them,BiOBr with suitable bandgap(2.7~2.9 eV)enable it to be a visible-light-active photocatalyst,capable of triggering a variety of photocatalytic reactions[6-7].Nevertheless,the photodegradation efficiency of onefold photocatalyst under visible light was still dissatisfactory.Accordingly,numerous strategies have been adopted to overcome the above drawbacks,such as elementdoping[8-10],morphology control[6-7],solid solution design[11-12],heterojunction construction[13-14],noble metal deposition[15],and so on.Among these,the construction of heterojunctions with staggered band structures was conducive to the migration and separation of photoinduced electron-hole(e--h+)pairs,which has been regarded as an efficient and feasible strategy.BiOBr-based type-II heterojunctions such as g-C3N4/BiOBr[16],BiOBr-BiOI[17]and BiOBr/Bi4O5Br2[18-19]have been fabricated to boost the separation efficiency of e--h+pairs.However,these type-II heterojunctions lead to the lower redox ability of carriers,which was disadvantageous to photocatalytic reactions.
On the contrary,as an effective tactic,Z-scheme heterojunctions could conquer above shortcoming to some degree.In this regard,exploiting an efficient electron mediator is indispensable to achieve a practicalZ-scheme system.For example,BU et al.utilized in situ reduction of Ag+into Ag on the surface of Ag3PO4/WO3-xto formZ-scheme heterojunction Ag3PO4/Ag/WO3-x,which enhanced photocatalytic performance[20].LI et al.also successfully prepared a TiO2/Au/CdSZ-scheme system and found out that its excellent photo-electrochemical performance[21].Naturally,some ionic redox couples,such as Fe3+/Fe2+,IO3-/I-,as well as noble metal nanoparticles,have been successfully introduced to construct an all-solid-stateZ-scheme structure,which could facilitate the interfacial photogenerated carriers transfer in aZ-scheme system.In this regard,exploiting a low price,earth-abundant,and efficient electron mediator is indispensable to achieve a practicalZ-scheme system.Metallic Ag is an ideal candidate,owing to the unique advantages of nontoxicity,electrical conductivity,and a surface plasmon resonance(SPR)effect.However,the possible role of Ag nanoparticles as a solid-state electron mediator inZ-scheme hybrid photocatalysts still requires further investigation.
In this regard,exploiting an efficient electron mediator is indispensable to achieve a practicalZ-scheme system.Metallic Ag is an ideal candidate,owing to the unique advantages of electrical conductivity and a surface plasmon resonance(SPR)effect.However,the possible role of Ag nanoparticles as the charge transport bridge inZ-scheme BiOBr-based photocatalysts has not previously been reported.
In this work,binary AgI/BiOBr heterojunction was first prepared by preformed BiOBr nanosheet-mediated chemical deposition of AgI nanoparticles.Subsequently,the AgI/BiOBr composite are partially reduced to form the final ternary AgI/Ag/BiOBr heterojunction.During the reduction process,plasmonic metallic Ag nanoparticles form in-situ and uniformly decorate the AgI/BiOBr.The as-prepared AgI/Ag/BiOBr is acted as an efficient visible-lightZ-scheme system,which not only increases light absorption,but also heightens redox capacity of the photogenerated carriers.Such a bifunctionalZ-scheme AgI/Ag/BiOBr heterojunction can pave the way for further development of a high-efficiency visible light catalyst.
1 Experiment
All reagents of analytical grade were used without further purification.Deionized water was employed during whole experiments.
Firstly,BiOBr was successfully prepared by a facile precipitation method.Secondly,a series of AgI/BiOBr heterojunctions were also fabricated by a facile deposition-precipitation method in the darkroom.Typically,1 mmol the as-prepared BiOBr was dispersed in 40 mL AgNO3solution containing 0.6 mmol AgNO3,and then 0.6 mmol KI was added and stirred for 5 h.The obtained sample was labeled as 60% AgI/BiOBr.Similarly,the other AgI/BiOBr samples(20%,40% and 80%)were obtained as well through adjusting the amount of AgNO3and KI simultaneously.
The phase purity and crystal structure of the as-obtained samples were checked by using an X-ray diffractometer(Bruker,D8 Advance,XRD)with Cu Kα radiation.The micro-morphologies of the materials were observed by scanning electron microscopy(Hitachi,S-4800,SEM)and transmission electron microscopy(JEOL,JEM-2010,TEM).X-ray photoelectron spectroscopy analysis was carried out on an X-ray photo-electron spectrometer(Thermo,ESCALAB 250X,XPS)with Al Kα source.The UV-vis diffuse reflectance spectra of the as-prepared samples were recorded on a UV-vis spectrophotometer(Shimadzu,UV2550,DRS).The specific surface area(BET)and pore size of samples were determined on a Micromeritics ASAP 2010 analyzer.
The target degradation pollutants were azo dye model MO and a typical endocrine-disrupting chemical phenol.A 500 W xenon lamp(EL-NP2000)equipped with a UV cut-off filter(λ>420 nm)used as the light source.50 mg of photocatalyst was uniformly suspended in a 25 mL aqueous solution of MO or phenol(10 mg·L-1),respectively.Prior to irradiation,the mixture was fiercely stirred in the dark for 1 h to reach an adsorption-desorption equilibrium.Afterward,visible light irradiation was carried out and 5 mL of the suspension solution was sampled at each given time intervals and centrifuged.Then,the concentrations of MO and phenol were analyzed by using a UV-1801 UV-vis spectrophotometer.
Transient photocurrent responses and Mott-Schottky plots(M-S)were tested by an electrochemical workstation(CHI Shanghai,CHI 660E)in a standard three-electrode cell.The as-fabricated samples were deposited on a fluorinated-tin-oxide(FTO)conductive glass as the working electrode,Pt electrode and Ag/AgCl(3.0 mol·L-1KCl)were served as the counter and the reference electrodes,respectively.The visible light source was used by a 300 W xenon arc lamp with a cutoff filter(λ>420 nm).A mixed solution consisting of 0.1 mol·L-1Na2HPO4and 0.1 M NaH2PO4aqueous solution was utilized as supporting electrolyte.
2 Results and discussion
The XRD patterns of the as-prepared samples were illustrated in(Fig.1).All diffraction patterns of BiOBr and AgI were in agreement with tetragonal BiOBr(JCPDS:09-0393)and a mixed crystal of AgI including hexagonalβ-AgI(JCPDS:09-0374)and cubicγ-AgI(JCPDS:09-0399),respectively.As to AgI/BiOBr nanocomposites,two sets of diffraction peaks of BiOBr and AgI were observed,confirming the coexistence of these two phases.In addition,the peaks intensity of AgI heightens gradually while the intensity of BiOBr peaks reduces slightly with the increase of AgI content,which could be attributed to the strong interaction between these two phases.

Fig.1 XRD patterns of the sample
The microstructures of the samples were checked by SEM and TEM.As illustrated in(Fig.2a),BiOBr presented microspheres assembled from nanoflakes.For AgI/BiOBr composite(Fig.2c-f),small AgI nanoparticles(Fig.2b)were uniformly dispersed on the BiOBr nanosheets.
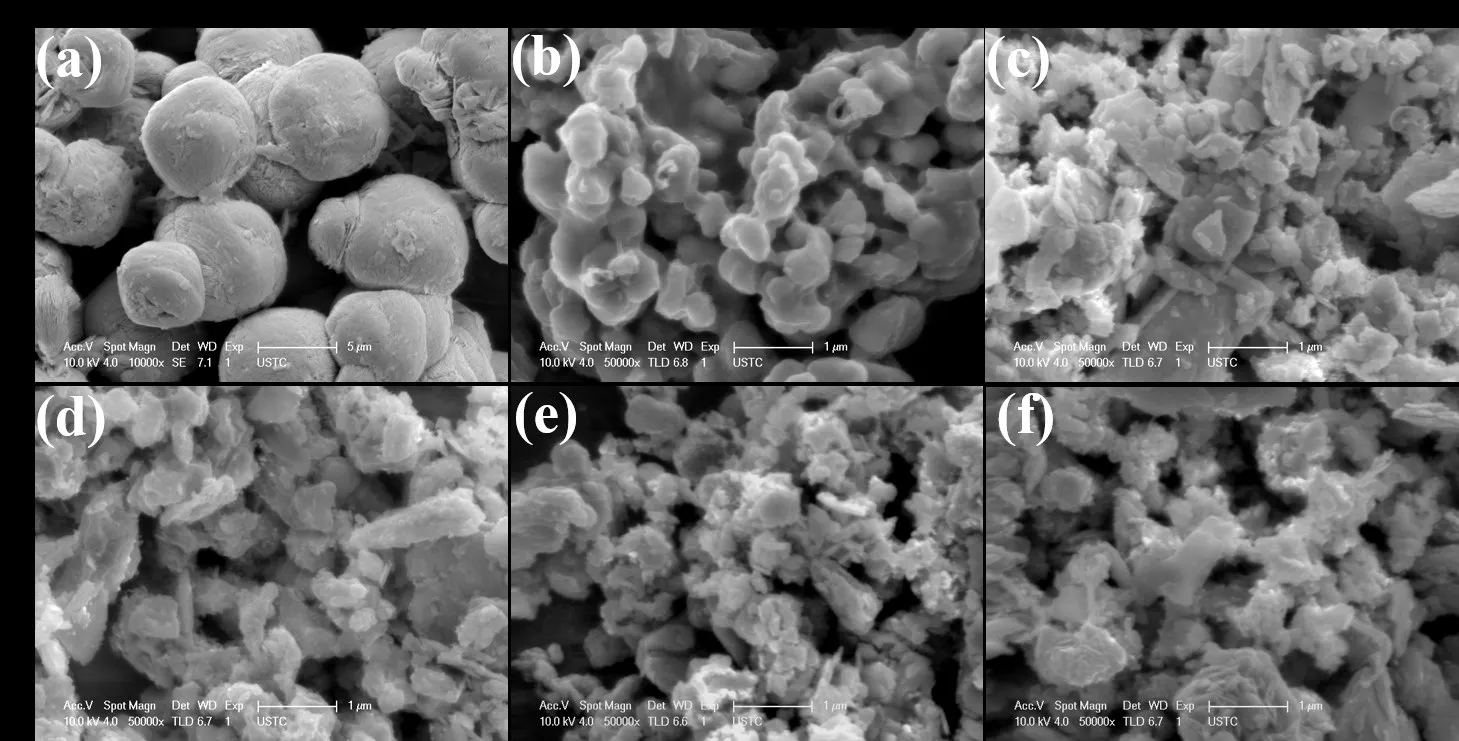
Fig.2 SEM images of as-prepared photocatalysts:(a)BiOBr,(b)AgI,(c)20% AgI/BiOBr,(d)40% AgI/BiOBr,(e)60% AgI/BiOBr and(f)80% AgI/BiOBr
In addition,TEM of 60% AgI/BiOBr further verifies the formation of heterojunction(Fig.3a).Furthermore,HRTEM image of 60% AgI/BiOBr indicates the lattice interlacing between AgI and BiOBr,where the lattice fringe is 0.351 nm and 0.281 nm belonging to theβ-AgI(101)facet and BiOBr(102)plane,respectively(Fig.3b).
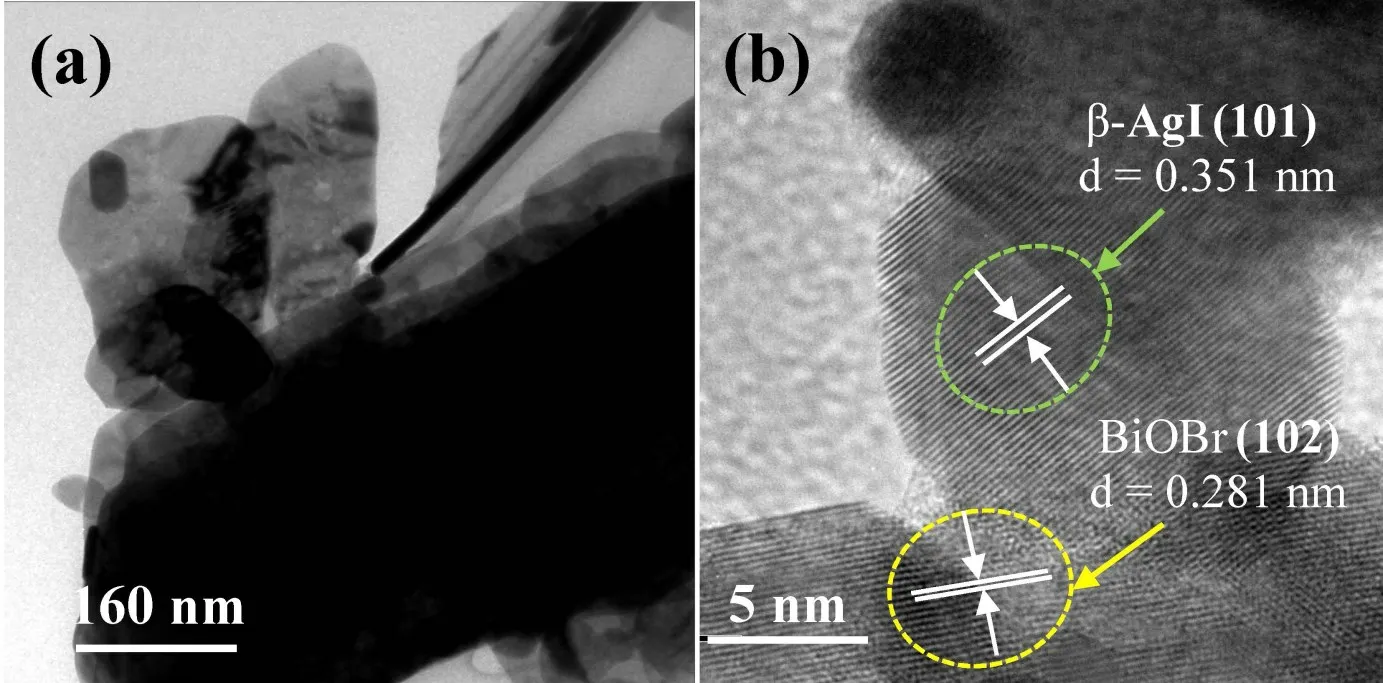
Fig.3(a)TEM and(b)HRTEM images of 60% AgI/BiOBr composite
The XPS survey spectra as depicted in(Fig.4a),manifesting that Bi,O,Ag,Br and I elements were observed in the 60% AgI/BiOBr sample,which further supplied evidence for the coupling of AgI and BiOBr.The two peaks of Bi 4f in 60% AgI/BiOBr located at 164.69 eV(Bi 4f5/2)and 159.37 eV(Bi 4f7/2),which were characteristic of Bi3+(Fig.4b).While,these two peaks for BiOBr shifted to 164.78(Bi 4f5/2)and 159.46 eV(Bi 4f7/2),respectively,manifesting that the local environments of Bi3+had changed in some way[1].The Br 3d core region of AgI/BiOBr showed two peaks at 69.61 eV(Br 3d3/2)and 68.62 eV(Br 3d5/2)(Fig.4c),verifying the presence of Br-.The positive variation of Br peaks in BiOBr(69.31 eV and 68.32 eV)implied lower electron density round Br-[3].An analogous phenomenon was also observed in the I 3d(Fig.4d)and Ag 3d(Fig.4e).The O 1s core region of AgI/BiOBr appeared two peaks(Fig.4f),the fitting peaks at 530.18 eV and 532.02 eV stemming from the Bi-O bond and the surface-adhered hydroxyl groups,respectively[1].Moreover,the binding energy(BE)of Bi-O bond in AgI/BiOBr slight positively shifted relative to BiOBr(529.98 eV),which was probably due to the interaction between AgI and BiOBr in AgI/BiOBr sample.
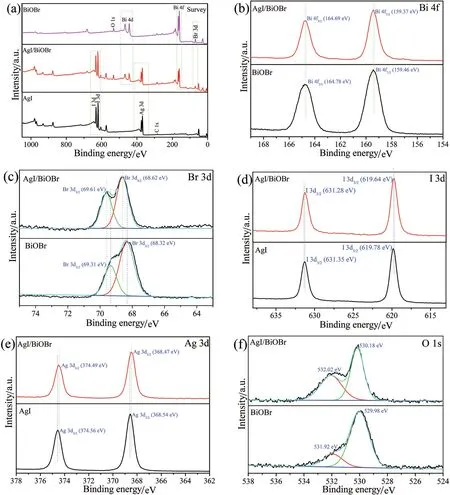
Fig.4(a)XPS survey spectra of BiOBr,AgI,and AgI/BiOBr composites and the high-resolution XPS spectra of(b)Bi 4f,(c)Br 3d,(d)I 3d,(e)Ag 3d and(f)O 1s
The photocatalytic activities of the samples prepared were firstly evaluated by MO decomposition under visible light irradiation(λ>420 nm).As shown in(Fig.5a),for pristine BiOBr and AgI,merely 25.5% and 35.8% of MO was degraded after 2 h of irradiation.But once AgI coupled with BiOBr,the AgI/BiOBr achieved more superior performance for MO removal.Furthermore,the removal rate of AgI/BiOBr gradually increased until the amount of AgI reaches 60%,and the obtained 60% AgI/BiOBr nanocomposite attained optimal degradation efficiency,in which almost all MO was decompose within 2 h.Notably,by further increasing the amounts of AgI,the removal rate of AgI/BiOBr nanocomposites do not boost any longer,but start to decline.This may be ascribed that excess AgI could prevent the light from entering the BiOBr surface and reduce light absorption,leading to the insufficient contacts between active sites and target pollutants.
To further probe the photooxidation ability of the as-obtained samples,phenol was also utilized as target pollutant.Similar to the degradation of MO,the 60% AgI/BiOBr composites presented much superior photocatalytic properties than other samples(Fig.5b),and 86.2% of phenol was degraded in 2 h.(Fig.5c)showed that the 60% AgI/BiOBr also presented the highest rate constant for MO and phenol removal.
60% AgI/BiOBr was also measured by recycling experiment of MO degradation(Fig.5d)and the result verified its high durability.Although no peaks of Ag0could be discovered in the XRD patterns of the used sample(Fig.5e),two peaks at 368.27 eV and 374.3 eV in the Ag 3d XPS spectra are assigned to the Ag 3d3/2and Ag 3d5/2of Ag0(Fig.5f)[14],indicating that the binary AgI/BiOBr composite converts to the AgI/Ag/BiOBr ternary system during photocatalytic reaction.
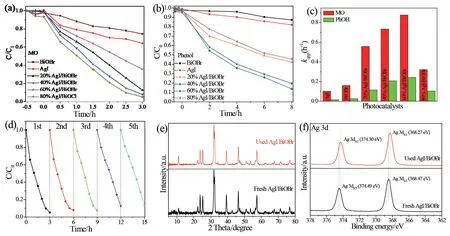
Fig.5 Photocatalytic activities for MO(a)and phenol(b)degradation,and the corresponding reaction rate constants kapp(c)for the asobtained photocatalysts under visible light(λ>400 nm);(d)Cycling runs of 60% AgI/BiOBr for the degradation of MO;(e)XRD patterns and(f)Ag 3d XPS spectra of fresh and used 60% AgI/BiOBr
To further confirm that the presence of in situ Ag bridge accelerated the separation efficiency of carriers,firstly the DRS of the fresh and used AgI/BiOBr was carried out.The DRS intensity of the used AgI/BiOBr increased and presented a red-shift(Fig.6a),indicating that Ag0improved the light absorption ability of the photocatalyst,particularly for visible light.Obviously,a peak appeared at 500~600 nm,which was attributed to the SPR effect of Ag0.To more visually look into the separation efficiency of carriers,the PL intensity(Fig.6b)and EIS signal(Fig.6c)of the used AgI/BiOBr was measured.Moreover,the PL intensity and EIS signal of the used sample were obviously lower than that of the fresh one,indicating that the formation of insitu Ag0bridge accelerated the separation of carriers.
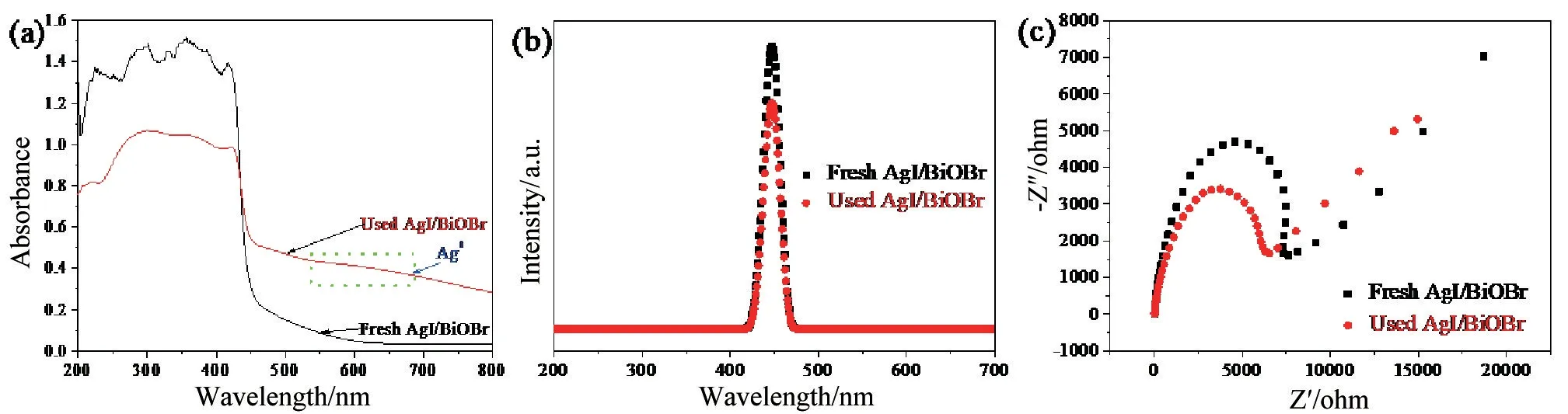
Fig.6(a)DRS spectra,(b)PL spectra and(c)EIS nyquist plots of 60% AgI/BiOBr before and after cycle runs
According to UV-vis analysis(Fig.7a),theEgof BiOBr and AgI were calculated to be about 2.75 and 2.89 eV,respectively.Combining with Mott-Schottky plots(Fig.7b),theECBof BiOBr and AgI were-0.52 and-0.81 V,respectively[13],and the correspondingEVBwere calculated to be 2.23 eV and 2.08 V.
The types of active species were explored by trapping experiments.As illustrated in(Fig.7c),the removal of MO was obviously restrained when the BQ and AO were added to the reaction mixture,but partially reduced after addition of IPA,indicating that·O2-and h+were main active species involved in photocatalyticdegradation[1].
Based on the above mentioned discussion,aZ-scheme charge migration mechanism was proposed in(Fig.7d).Under visible light irradiation,the excited e-on the CB of BiOBr could be quickly recombine with h+on the VB of AgI on the Ag0[22-23],meanwhile retaining the serviceable e-and h+with stronger redox ability on the CB of AgI and VB of BiOBr,respectively.
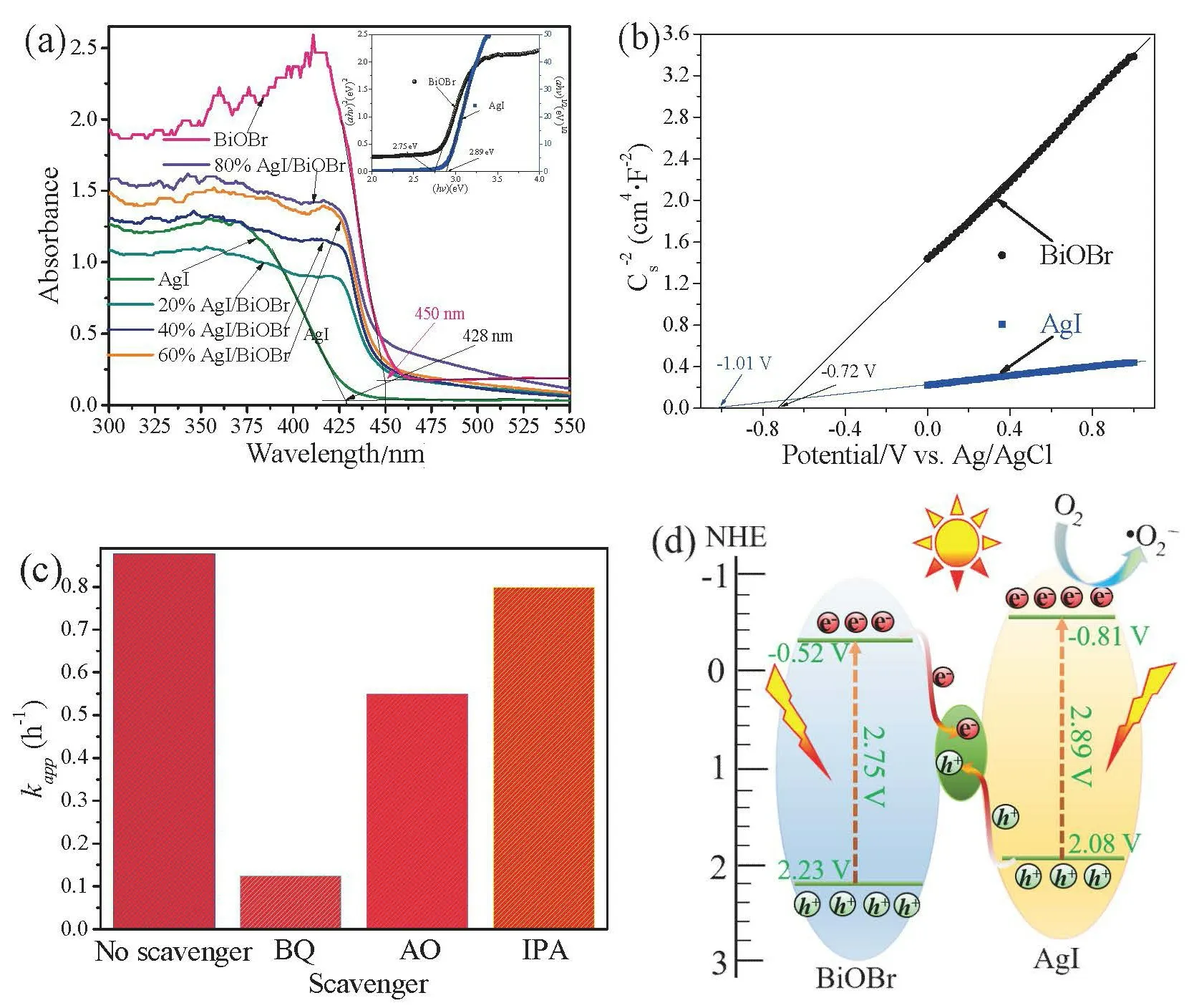
Fig.7(a)UV-vis diffuse reflectance spectra of the as-prepared samples,and the plots of the(ahv)1/2 and(ahv)2 vs.photon energy(hv)for BiOBr and AgI(inset);(b)Mott-Schottky plots of AgI and BiOBr;(c)kapp values of reactive species in the photodegradation process of MO and phenol over 60% AgI/BiOBr;(d)Schematic illustration of the proposed mechanism for photo-generated charge carrier transfer in the AgI/Ag/BiOBr nanocomposite under visible light irradiation
The separation and migration efficiencies of the photoinduced carriers are crucial factors to photocatalytic degradation,which could be characterized by the transient photocurrent response.The transient photocurrent of the as-prepared samples was tested by 20 s on-off visible light with various applied bias potentials for each catalyst to offset potential imparity between two electrodes.As depicted in(Fig.8a),all samples displayed prompt photocurrent response upon illumination,suggesting their sensitivity to visible-light.However,the photocurrent intensity is obviously different to each other.The photocurrent intensity of the AgI/BiOBr heterojunctions is higher than that of pure AgI and BiOBr,illustrating that the formation of the heterojunction could effectively accelerate the separation of hot carriers.Among these AgI/BiOBr heterojunctions,60% AgI/BiOBr heterojunctions displayed the highest photocurrent density,implying that the charges separation and transfer efficiency have been significantly enhanced.In addition,the photocurrent intensity of the 60% AgI/BiOBr heterojunctions was decreased after four cycles′run for MO degradation(Fig.8b),which is in accordance with that of their photocatalytic activity.

Fig.8 Transient photocurrent response of(a)the as-prepared samples and(b)fresh and used AgI/BiOBr
4 Conclusions
Ag/AgI acted as an efficient cocatalyst to enhance the photocatalytic activity of BiOBr photocatalysts.In the AgI/Ag/BiOBr system,Ag0serviced as the charge transmission bridge to capture useless electrons and holes,remaining the strong redox ability of photo-induced carriers and inhibiting the photo-corrosion of AgI/Ag/BiOBr.This work proposes ideas for the application of Ag as charge transport bridge and construction of other efficient photocatalysts,which could be used to remove organic contaminant in wastewater.
