Boosting electrocatalytic selectivity in carbon dioxide reduction: The fundamental role of dispersing gold nanoparticles on silicon nanowires
2022-09-15FnLioXingFnHuixinShiQingLiMengjieWenxingZhuHipingLinYouyongLiMingwngSho
Fn Lio, Xing Fn, Huixin Shi, Qing Li, Mengjie M, Wenxing Zhu,Hiping Lin,1,∗, Youyong Li, Mingwng Sho,∗
a Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University,Suzhou 215123, China
b School of Physics and Information Technology, Shaanxi Normal University, Xi’an 710062, China
ABSTRACT Carbon dioxide electrochemical reduction (CO2RR) has been recognized as an efficient way to mitigate CO2 emissions and alleviate the pressure on global warming and associated environmental consequences.Gold (Au) is reported as stable and active electrocatalysts to convert CO2 to CO at low overpotential due to its moderate adsorption strength of ∗COOH and ∗CO.The request for improved catalytic performance,however, is motivated by current unsatisfied catalytic selectivity because of the side hydrogen evolution reaction.In this context, the design of Au based binary catalysts that can boost CO selectivity is of great interest.In the present work, we report that Au nanoparticles can be feasibly dispersed and anchored on silicon nanowires to form Au-Si binary nanomaterials.The Au-Si may stably drive CO2RR with a CO Faraday efficiency of 95.6% at −0.6 V vs. RHE in 0.5 mol/L KHCO3 solution.Such selectivity outperforms Au particles by up to 61%.Controlled experiments illustrate that such catalytic enhancement can chiefly be ascribed to electronic effects of binary catalysts.Theoretical calculations reveal that spontaneously produced silicon oxide may not only inhibit hydrogen evolution reaction, but also stabilize the key intermediate ∗COOH in CO formation.
Keywords:Gold nanoparticles Silicon nanowires Electrocatalysis Carbon dioxide reduction Noble metals
There is an ever-increasing demand to reduce atmospheric CO2concentration due to the greenhouse effect resulting from increasing use of fossil fuels [1–4].It is an attractive strategy to convert CO2into value-added fuels through electrochemical reduction because of mild operating conditions and its potential integration with the renewable electricity intermittently generated from solar or wind energy [5–11].
Among various electrocatalysts for CO2electrochemical reduction (CO2RR), gold (Au) shows nice activity in converting CO2into CO, a fundamental chemical building block for other carbon products [12], due to weak binding interactions between Au and CO[13,14].Unfortunately, CO2RR on Au nanoparticles suffer from declining stability and selectivity with increasing overpotential owing to the competing hydrogen evolution reaction (HER).A number of studies demonstrate that better catalytic performances can be achieved by adjusting the size of Au nanoparticles, tuning crystallographic orientations, and producing multicomponent catalysts[15–19].Despite successfully design and synthesis of catalysts, the selectivity issue remains a longstanding challenge that preclude further considerations of Au for practical CO2RR applications.
One strategy to alleviate this challenge is to disperse Au on suitable substrates that can effectively modulate the electronic structures (known as the electronic effect) and consequently the interactions with key reaction intermediates, as we have learned from previous electrocatalysis [20–23].For example, Gonget al.loaded Au on CeOxnanosheets.The Ce3+promotes the activation of CO2and the formation of∗COOH [24].The reduced graphene oxide supported Au nanoparticles delivers high Au-specific mass activities and good Faradaic efficiency (FE) for the CO2-to-CO conversion at moderate overpotentials [25].Despite the encouraging performance gains, the fabrication of these catalysts often needs extra reduction process, which increase the operational complexity and decrease the tightly contact of metal to the substates.
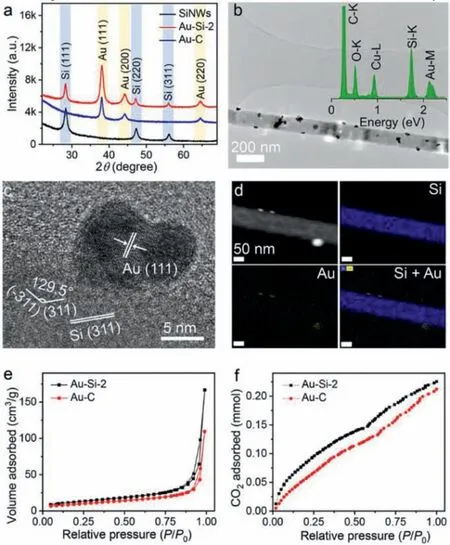
Fig.1.Phase and morphology characterization of Au-Si-2.(a) XRD patterns of Au-Si-2, SiNW and Au-C.(b) TEM image of Au-Si-2, insert is the EDS spectrum.(c)HRTEM image of an Au nanoparticle on SiNW, showing the lattice spacing of Au and Si.(d) HAADF-STEM image of Au-Si-2, and the corresponding EDX mapping of Si (blue), Au (yellow), and overlay.(e) N2 adsorption-desorption isotherms and (f)CO2 adsorption-desorption isotherms for Au-Si-2 and Au-C.
Compared with metal oxides and carbon materials, silicon is an outstanding multifunctional supporting material which offers not only the electronic effect, but also structural engineering of metallic electrocatalysts at the nanoscale, for example, enhancing the electrochemical surface areas or creating abundant undercoordinated sites [26].In our previous works, we observed that silicon can directly fix CO2from the atmosphere [27].When the noble metals such as Pt and Ir are cooperated with silicon nanowires(SiNWs), the nanocomposites are excellent HER electrocatalysts[28,29].The presence of silicon can change thedband center of noble metals, which finally regulate the hydrogen adsorption energy closer to zero and facilitate the production rate of hydrogen[30].To the best of our knowledge, there are very few successful demonstrations of using Si as substrates to design CO2RR catalysts.
In this work, we report the rational synthesis of uniformly dispersed Au nanoparticles on SiNWsviaa feasiblein-situreduction method.A layer of silicon oxide is formed when the Au-Si catalysts are exposed to the atmosphere.Subsequently, electrocatalytic studies show that Au-Si with Au content of about 20 wt% can drive CO2RR with an onset overpotential of 0.15 V in the 0.5 mol/L KHCO3solution.The CO FE reaches 95.6% at −0.6 Vvs.RHE.The promotion of CO production by introducing SiNWs into metal catalysts is also confirmed in other metal catalysts including Ag, Ru and Pd.Mechanism study by density functional theory (DFT) reveals that the silicon oxide on the surface of Si inhibits HER.The enhanced CO2RR performance can be attributed to the increased binding interaction to∗COOH at the interface of the catalyst.
The fabrication processes of the catalysts are schematically shown in Fig.S1 (Supporting information) and the raw materials and metal contents are listed in Table S1 (Supporting information).The Au-Si-2 composite was first investigated by X-ray diffraction (XRD) and compared with the pure SiNWs and Au-C.Besides the peaks of Si at 28.4°, 47.3° and 56.1° (JCPDF Card No.27–1402), other peaks for Au-Si-2 (Fig.1a) are confirmed to be the face-centered cubic structure of Au nanoparticles (JCPDF No.04–0784).Scanning electron microscope (SEM) image showed the overall morphology of Au-Si-2 (Fig.S2 in Supporting Information).The nanowire structure was retained.When the carbon black was added, the nanowires are connected by carbon and form a network structure, which increase the conductivity of the catalyst.Au nanoparticles are not obvious at this enlargement factor due to their small size.Transmission electron microscopy (TEM) image in Fig.1b gives a detail for Au-Si-2.Several Au nanoparticles are uniformly dispersed on a single SiNW.The inserted energy dispersive spectroscopy (EDS) spectrum indicated the existence of Au and Si elements.C and O originated from the surface absorbance or sample oxidation.Cu came from the sample support grid.Highresolution TEM enlargement (HRTEM, Fig.1c) shows Au nanoparticles embedded in the SiNWs with lattice spacing of 0.245 nm corresponding to the (111) interplanar distance of cubic Au.The lattice spacing of 0.164 nm belongs to the (311) plane of Si.The intersection angle for the (311) and (−311) faces is 129.5° Highangle annular dark field scanning TEM (HAADF-STEM) image of Au nanoparticles attached to SiNW (Fig.1d) and the corresponding EDS mapping show the elemental distribution of silicon (blue),Au (yellow) and their overlay, respectively, which is in accordance with the EDS spectrum inserted in Fig.1b.
The X-ray photoelectron spectroscopy (XPS) was applied to research the surface chemical element existence of Au-Si-2.The survey spectrum in Fig.S3a (Supporting information) confirmed that the composite consists of Au and Si elements.C and O elements originated from the surface oxidation and absorption of samples as confirmed by EDS before.The high-resolution Si 2p spectrum was divided into two components (Fig.S3b in Supporting information).The peak located at 99.6 eV was attributed to Si−Si bonds,while the higher binding energy peak at 103.5 eV was assigned to the Si−O peak [31].The high-resolution spectrum of the Au 4f orbital reveals the existence of Au(0) in Fig.S3c (Supporting information) with the binding energy at 84.18 (4f7/2) and 87.88 eV (4f5/2).Two small shoulder peaks located at 85.37 and 87.92 eV belong to the oxidation peak of Au.The strong peak at 533.2 eV in the O 1s spectrum (Fig.S3d in Supporting information) was assigned to the Si−O bond [31].Both oxide peaks are observed in Au and Si elements, which are caused by the oxidation of the sample in the air.
The specific surface areas of the catalysts are evaluated by Brunauer-Emmett-Teller (BET) method.The surface area of Au-Si-2 is 45.18 m2/g (Fig.1e), larger than that of Au-C (34.17 m2/g).CO2adsorption is one of the key steps in CO2RR, which can be evaluated through the CO2adsorption-desorption isotherms (Fig.1f).The Au-Si catalyst adsorbs more CO2than the Au-C catalyst at room temperature.Large surface area and high CO2adsorption capacity are conductive to improve the performance of CO2RR.
Next, the electrochemical CO2RR activities of all catalysts were carried out in a two-compartment cell with a standard threeelectrode configuration.The CO2RR performance of Au-Si-2 catalysts was firstly evaluated by linear sweep voltammetry (LSV)in 0.5 mol/L KHCO3solution with saturated N2and CO2respectively (Fig.2a).It can be seen that the current density normalized to the geometric area of the Au-Si-2 electrode in CO2-saturated 0.5 mol/L KHCO3(pH 7.2) is obviously larger than that in N2-saturated 0.5 mol/L KHCO3.For example, the current density is enhanced from 5.1 mA/cm2in N2to 10.6 mA/cm2in CO2at −0.8 Vvs.RHE.The increased current density is contributed to reduction of CO2.After short time potentiostatic electrolysis, the gas phase products were analyzed by gas chromatography (GC) and the liquid phase products were quantitatively determined by nuclear magnetic resonance (NMR).The main products were only CO and H2while the liquid products are below the detection limits.Trace CO can be detected at −0.26 Vvs.RHE.It indicates that the catalyst has low onset overpotential of 0.15 V.The products were collected from −0.3 Vvs.RHE.Fig.2b presents an intuitive histogram for the calculated FEs of CO and H2for the Au-Si-2 at different applied potentials from −0.3 V to −1.0 Vvs.RHE.It can be seen that the FEs of CO show a rapidly incremental tendency from −0.3 V to −0.6 Vvs.RHE and reach to the maximum value of ∼95.6% at a potential of −0.6 Vvs.RHE.This value is higher than many reported works(see Table S2 in Supporting information).Afterwards, the FEs of CO declined gradually when the applied potential became more negative and the minimum value declines to ∼76.1% at the potential of −1.0 Vvs.RHE.In the potential range from −0.5 V to −1.0 V, the catalytic selectivity of Au-Si-2 for CO2RR is higher than that of water electrolysis, indicating a more favorable electrocatalytic CO2RR activity compared to the competing HER reaction.
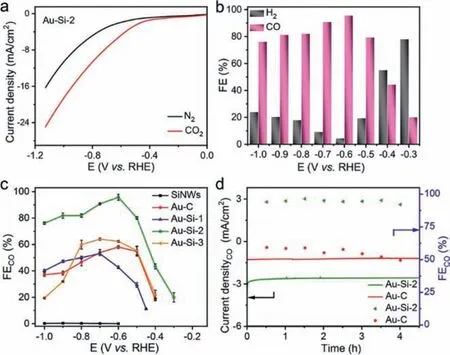
Fig.2.(a) LSV curves of Au-Si-2 measured in CO2-saturated (red) and N2-saturated(black) 0.5 mol/L KHCO3 electrolyte, respectively.(b) FE histograms of CO and H2 vs.the applied potential catalyzed by Au-Si-2.(c) FEs of CO at different applied potentials for SiNWs, Au-C and Au-Si catalysts with different Au concentration.(d) Stability performance of Au-Si-2 and Au-C for CO2 reduction operated at potentiostatic potentials of −0.6 V vs. RHE for 4.5 h.
The FE of CO for Au-Si-2 is also compared with SiNWs, Au-C and Au-Si nanocomposites with different concentration of Au (Fig.2c and Figs.S4 and S5 in Supporting information).The SiNWs exhibited a negligible CO2RR activity (Fig.S4a) and the main products are H2(Fig.S4b).The catalysts that contained Au all can effectively convert CO2to CO, which means the electrocatalytic activity mostly came from Au.However, the FEs of CO for other catalysts are lower than that of Au-Si-2 at the same tested potentials.The Au-Si-1 and Au-Si-3 both showed similar current-voltage profiles with lower current response for CO2RR than Au-Si-2 (Fig.S5).Different content of Au would affect the ratio of CO and H2products.The selection of CO for Au-Si catalysts increased and then decreased with the metal content.With low Au concentration, the active sites were not enough, while with high Au concentration,they tended to agglomeration, which also decrease the catalytic performance.When SiNWs were completely removed from the catalysts and employed only carbon black as substrate (Au-C), the FE of CO decreased obviously (Fig.2c and Fig.S6 in Supporting information).During the catalyst ink preparation process, carbon black was added in Au-Si-2.The effect of carbon black in the catalyst was also investigated.Au-Si-2 catalyst without carbon black (the detailed preparation method is in Supporting information) was also tested for CO2RR.The FEs of CO at different potentials are a little lower than those of the Au-Si-2 sample with carbon black (Fig.S7 in Supporting information).The addition of carbon black may increase the conductivity of the samples, which is beneficial for the increasement of the CO2RR performance.
The electrochemically active surface areas (ECSAs) of Au in Au-Si-2 and Au-C were determined by measuring the charge from the reduction peak in the cyclic voltammetry curves and dividing it by the specific charge of one monolayer of Au (390 μC/cm2) [32].The ECSA of Au-Si-2 is 28.9 m2/gAuand that of Au-C is 21.9 m2/gAu(Fig.S8 in Supporting information), indicating that Au-Si-2 exposed more accessible active sites than Au-C.The electrochemical impedance spectroscopy (EIS) spectra for Au-Si-2 and Au-C were explored to investigate the charge-transfer process in CO2reduction (Fig.S9 in Supporting information).The apparent smaller radius for Au-Si-2 relative to Au-C is observed, which may originate from increased conductivity due to connection through SiNWs between Au and carbon black.
The long-term stability test is one important parameter to evaluate the performance of catalysts.The Au-Si-2 composite was measured at the constant potential of −0.6 V for 4.5 h and maintain the fluctuated FECOof 92.0% (Fig.2d).The FECOof Au-C showed downward trend after a period of time with the FECOlower than 50%, indicating that the addition of SiNWs improved the stability of the catalyst.This is reasonable for Au nanoparticles are closely attached on the surface of SiNWs and not easy to migrate during durability test.
In the following control experiments, the LSV curves in CO2-saturated 0.5 mol/L KHCO3electrolyte for Ag-Si, Pd-Si and Ru-Si catalysts were compared with Au-Si-2 (Fig.S10a in Supporting information).The metal contents of Ag, Pd and Ru in each catalyst are close to 20% (Table S1 in Supporting information).All catalysts possess CO2RR performance, and the main products are CO without any liquid products.From Fig.S10a, Au-Si-2 catalyst exhibits larger current density than others, which is appropriately 5.5-, 1.4- and 2.8-fold compared with Ag-Si, Pd-Si and Ru-Si catalysts at −1.0 V, respectively.The FEs of CO for different metal catalysts (Fig.S10b in Supporting information) showed that the electrocatalytic reduction of CO2is not only related to the ratio of Au in Au-Si but also related to the kind of metals.
The FEs of CO for metal-Si are compared with those of metal-C catalysts (Figs.3a-c).After the addition of Si, the FEs of CO all increased.The largest FEs of CO for Au-Si, Pd-Si, Ru-Si and Ag-Si(Fig.3d) are 95.6% (−0.6 Vvs.RHE), 54.4% (−0.9 Vvs.RHE), 50.1%(−0.8 Vvs.RHE) and 40.1 (−0.8 Vvs.RHE), 61.2%, 28.2%, 24.6% and 13.3% higher than those of the Au-C, Pd-C, Ru-C and Ag-C, respectively.
To better understand the reason for the significant performance improvement of the metal-Si catalysts, Tafel analysis was used to research the CO2reduction kinetics of metal-Si and metal-C composites (Figs.3e and f).Tafel plots were plotted according to the logarithm ofjCOand its overpotential.The values of Tafel slope for metal-Si are all lower than that of the corresponding metal-C, respectively, indicating the addition of Si can accelerate the kinetic rate of CO2RR.When the rate-limiting step of CO2to CO is the adsorbed CO2to generate the surface adsorbed CO2•−through one single electron transfer process, the theoretical Tafel slope is 118 mV/dec [33].For metal-C catalysts, the Tafel slope is consistent with that theoretical value, suggesting that the rate-limiting step of CO2RR is not changed.In comparison, the Tafel slopes for metal-Si are lower than 118 mV/dec.In particular, the Tafel slope value of Au-Si-2 is 82 mV/dec, showing a trend to the theoretical value of 59 mV/dec, demonstrating that the rate-limiting step is likely to undergo a preequilibration change from the first one electron transfer step to the proton transfer chemical step [34–37].The smaller Tafel slope indicates that the overpotential in the catalytic process is lower, and the Au-Si-2 catalytic has the fastest reaction CO2RR kinetics than other catalysts.
The addition of Si in the four metals increases the FECOand kinetics processes of CO2RR.Generally speaking, the conversion of CO2to CO on the surface of Au is divided into the following steps(Eqs.1–3) [38]:
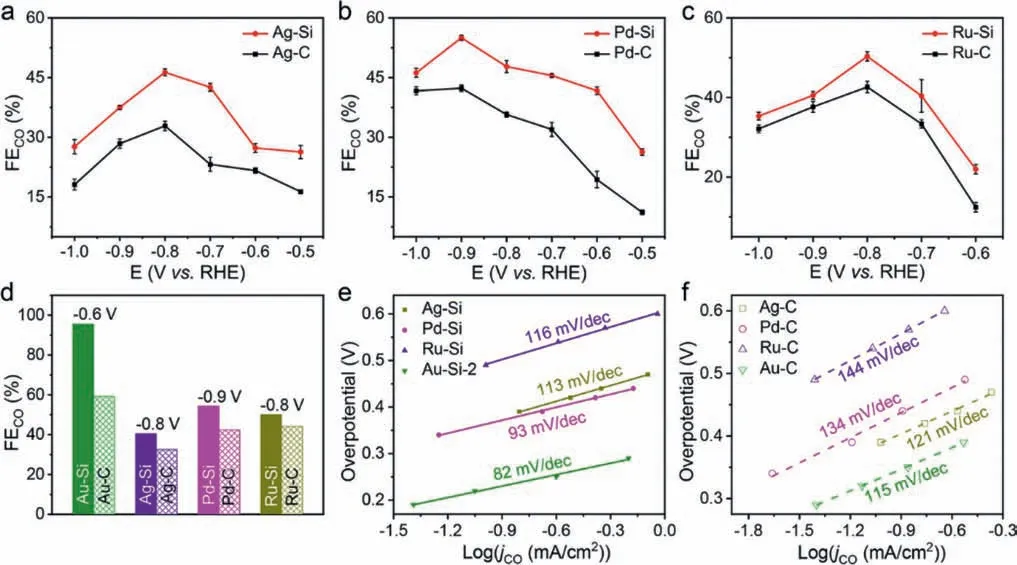
Fig.3.(a-d) FEs of CO comparison of metal-Si and metal-C: (a) Ag-Si and Ag-C; (b) Pd-Si and Pd-C; (c) Ru-Si and Ru-C; (d) summarize of the optimal FEs of CO at optimal applied potentials vs. RHE.(e) Tafel plots of CO2RR by Au-Si-2, Ag-Si, Pd-Si and Ru-Si composites.(f) Tafel plots of CO2 reduction by Au-C, Ag-C, Pd-C and Ru-C composites.
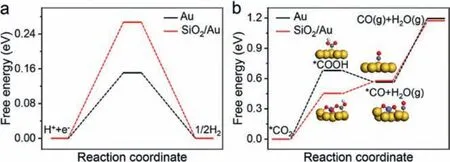
Fig.4.DFT calculation.(a) The free energy diagram of HER on Au(111) and SiO2/Au(111).(b) The free energy diagram of CO2 reduction to CO on Au(111) and SiO2/Au(111).The yellow balls represent Au, the gray balls represent C, the red balls represent O, the white balls represent H, and the purple balls represent Si.
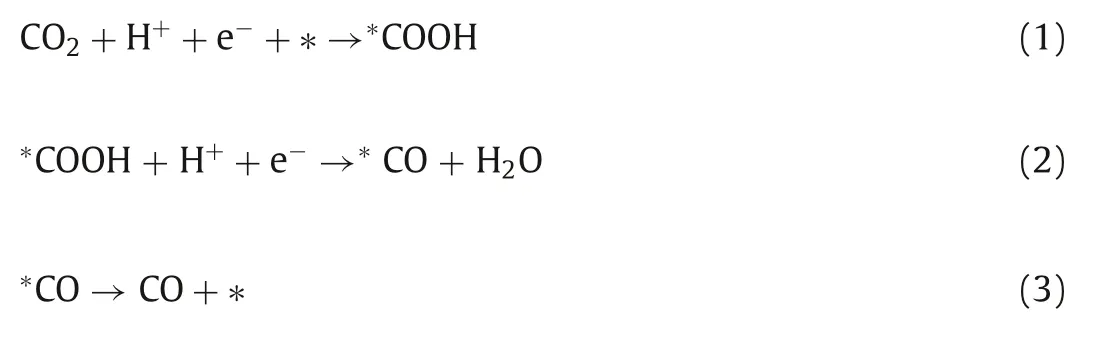
The transition from∗COOH to∗CO is a relatively easy step,while the formation of∗COOH and the final CO desorption have a high energy barrier.To further understand the contribution of SiNWs, the DFT calculation is adopted to analyze the potential barrier that needs to be overcome for the reduction of CO2on Au and Au-Si catalysts [39].As confirmed in the XPS results, the Si surface is covered by an amorphous silicon oxide.In the neutral electrolyte, this oxide is retained.So, the interface is simplified as Au on the surface of SiO2.
The DFT calculations were first conducted to compare the electrocatalytic selectivity and activity for CO2RR on the interfaces of SiO2/Au(111) and Au(111).TheΔGH∗ value on SiO2/Au(111) is far away from zero compared with that on Au(111), as shown in Fig.4a.According to Sabatier’s principle [34], the HER should be much less active on the SiO2/Au(111) surface.
As shown in Fig.4b, when SiO2is introduced, the formation of COOH∗is energetically much more favorable than that on Au(111).The free energetic difference decreased from 0.68 eV to 0.45 eV,suggesting a strong tendency of the conversion from CO2to CO on the interface of SiO2/Au(111).As Fig.S11 (Supporting information) shows, the intermediate of∗COOH forms the adsorption bond on the pure Au(111) surface based on C and Au atoms, while it can form an additional coordination bondviathe oxygen atom in the∗COOH group with the Si atom in the SiO2of the SiO2/Au(111)catalyst, leading to the improved bonding strength and stability of∗COOH on the SiO2/Au(111) surfaces (Figs.S12 and S13 in Supporting information).
In summary, four noble metals, including Au, Ag, Ru and Pd,are modified on the surface of SiNWs by using the reductive properties of Si−H bonds and employed as electrocatalysts for CO2RR.Au-Si catalytic system shows the best CO2RR performance among the four metals.The specific surface area and the adsorption of CO2of Au-Si are higher than those of Au-C.When the content of Au is about 20%, the onset CO2RR potential is 0.15 V and the highest FE of CO reaches 95.6% at −0.6 Vvs.RHE, which is superior to the FE for Au-C (59.3% at −0.6 Vvs.RHE).The presence of Si can improve the selectivity of the catalysts to CO.The conversion efficiency from CO2to CO for other three precious metals are all improved after the introduction of SiNWs, indicating the universality of this Si-assistant method.By DFT calculation for the Au catalytic system,ΔGH∗on SiO2/Au(111) is higher than that on Au(111).The∗COOH intermediate is stable on SiO2/Au(111), in favor of the formation of CO.SiNWs make the noble metals uniformly dispersed, increase the surface areas and CO2adsorption capacity,and improve the stability of the catalysts.It also plays a role of cocatalysts, which suppresses HER and promotes CO2RR at the same time.Such a multi-functional support design strategy can be generalized to other catalytic applications.
Declaration of competing interest
The authors declare that they have no known competing finan
cial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by the National Key Research and Development Program of China (No.2020YFA0406103), National Natural Science Foundation of China (Nos.51902217 and 21771134),National Key Research and Development Program of China(No.2017YFA0204800), National MCF Energy R&D Program (No.2018YFE0306105), the Suzhou Key Laboratory of Functional Nano& Soft Materials, Collaborative Innovation Center of Suzhou Nano Science & Technology, the 111 Project, Joint International Research Laboratory of Carbon-Based Functional Materials and Devices.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.034.
杂志排行
Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
