Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
2022-09-15YongchaoChuTaoSunChenJiang
Yongchao Chu, Tao Sun, Chen Jiang
Key Laboratory of Smart Drug Delivery (Ministry of Education), Minhang Hospital, State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Institutes of Brain Science, Department of Pharmaceutics, School of Pharmacy, Research Center on Aging and Medicine, Fudan University,Shanghai 201203, China
ABSTRACT The emergence of disseminated metastasis is the leading cause of mortality in patients with malignant tumor.The pre-metastatic microenvironment, including the primary tumor-derived components,pre-metastatic niche (PMN), circulating tumor cells (CTCs), micro-metastases, and tumor immune microenvironment (TIM), are the crucial factors to initiate metastasis and form macro-metastases.It may be a more promising strategy for directly targeting pre-metastatic microenvironment-interrelated factors and cells before they have the chance to form secondary tumors to prevent metastasis.During recent years, a variety of nanosystems, with specific microstructures and functional properties, have been devised to selectively target pre-metastatic cells components and interrelated molecular, and exhibited strong potential on anti-metastatic therapy by absorbing and neutralizing primary tumor-derived components, preventing establishment of the PMN, eliminating the CTCs, eradicating the micro-metastases and modulating the TIM.In this review, we comprehensively review the emerging nanosystems based on the pre-metastatic microenvironments.Hopefully, this review can cast new lights for early preventing and attenuating metastatic progression.
Keywords:Circulating tumor cells Pre-metastatic niche Cancer metastasis Tumor immune microenvironment Micro-metastasis
1.Introduction
Metastasis is one of the most threatening aspects of cancer, accounting for 90% population of cancer-related deaths [1].Among over half of these cases, malignant tumors have developed into metastasis upon diagnosed [2,3].For the rest of cases, metastasis may occur during immediate treatment, or even relapse years after recovery [4,5].Despite advances in recent therapeutic strategies such as chemotherapy, radiotherapy, immune-therapy, and their combination therapy, metastatic tumor remains a great challenge for the high biocomplexity of the processes [6].This condition underlines that the identification of specific molecular pathways and cellular events during metastasis is essential to develop new therapeutic strategies for anti-metastasis.
Invasive-metastatic development involves the dissemination of tumor cells from primary tumor and subsequent colonization in distant organs [7].In the priming phase of metastasis, primary tumors release the various secreted factors, such as extracellular vesicles (EVs) and tumor-derived secreted factors (TDSFs), to establish an immature PMN in distant sites.In the licensing phase,tumor-mobilized bone-marrow-derived cells (BMDCs) and immune cells are constantly recruited into the specific locus under the mobilization of tumor secreted factors and interact with stromal cells here to form a mature PMN with creating conditions for the succeeding survival and colonization of metastatic tumor cells.In the initiation phase, CTCs escape from antitumor immune responses and reach and colonize the PMN, contributing to the metastasis initiation and micro-metastases formation.In the progression phase, the PMN recruits more metastatic tumor cells, promotes the expansion and metastasis of CTCs, and finally forms diagnostically visible macro-metastases [8].Therefore, as a crucial step in early formation of metastasis, the primary tumor-derived components,PMN, CTCs, micro-metastases, and TIM could become valuable direction for successful cancer therapy.It may be a promising strategy for directly targeting or neutralizing the interrelated factors and cellar components in pre-metastatic microenvironment before they have the chance to form secondary tumors to prevent metastasis [9].
Currently, the majority of existing cancer therapies focus on solid tumors (primary and metastatic) rather than the premetastatic microenvironment.Conventional chemotherapy may destroy partial CTCs for treating cancer, but chemo-drugs possess a relatively short blood circulation time, which decrease the effi-ciency to kill CTCs owing to decreased exposure of CTCs to chemical drugs [10].The field of targeting PMN for the detection or prevention of metastasis, just as a newly-emerged field, attracts increasing attentions.The current chemotherapy lacks of selectivity of anticancer drugs towards PMN.Furthermore, their clinical efficacy of targeting PMN needs further validation [11].Therefore, finding effective targeting strategies based on pre-metastatic microenvironment emerges an urgent need in both clinical and academia cancer treatment.
With the rapid advancement of nanotechnology, the multifunctional nanosystems have been developed to recognize and visualize metastatic cells that are hard to be monitored by conventional technology, and specifically release therapeutic agents to eliminate malignant tumor cells and ultimately prolong patient survival [12].Encouragingly, some nanosystems including Abraxane,Genexol-PM, Caelyx and Onivyde have been approved for treating metastatic cancer in the clinic [13].However, these nanosystems enter into the solid tumor through leaky tumor-associated vasculature, and have demonstrated only disappointingly limited improvement of therapeutic effect and survival rate in clinical practice[14].Different from solid tumors, PMN and micro-metastases is relatively un-vascularized, thereby significantly hampering nanosystems delivery to metastasis organ.Moreover, the mobility of CTCs and hemodynamic shear stresses could dramatically influence on the nanosystems efficacy against CTCs [15].Therefore, the nanosystems based on the pre-metastatic microenvironment are more challenging for anti-metastatic therapy.
The development of nanotechnology primarily aiming at the PMN, CTCs, micrometastases and TIM of early cancer metastasis has attracted considerable attention in the last few years.The nanosystems linked with specific targeting ligands can recognize related molecule and cellular of pre-metastatic microenvironment with high affinity, allowing for the direct capture using the multifunctional properties of the nanosystems [16].In addition, some TDSFs, EVs, PMN, CTCs, micrometastases and TIM-related cells have been utilized to carry nanosystems suppress the development and function of metastasis, and inspiring advances have been made to develop novel nanosystems approaches for anti-metastatic therapies [17].
In this review, we focus on the emerging nanosystems based on pre-metastatic microenvironment with particular emphasis on the application for absorbing and neutralizing primary tumorderived components, preventing establishment of the PMN, eliminating the CTCs, eradicating the micrometastases and modulating the TIM.Further, we systematically summarize these novel nanosystems with unique therapeutic strategies for different premetastatic phase, including aptamer-modified nanosystems mediated by unique tumor biomarkers (such as epithelial cell adhesion molecule (EpCAM),N-cadherin, C-X-C chemokine receptor type 1 (CXCR1), E-selectin ligands,αvβ3integrin, carcinoembryonic antigen) and other metastasis-associated cytokines (such as S100A8/A9, P-selection, CXCR4, fibrin-fibronectin complexes, very late antigen-4, epidermal growth factor receptor), biological membrane biomimetic nanosystems and bioengineered cell carriers mediated by high integration with organisms (such as platelet, exosomes, neutrophil cells, myeloid-derived suppressor cells (MDSCs)and tumor cell self).These nanosystems are able to block metastasis in early stage of cancer process.Hopefully this review can cast new lights for preventing successful anti-cancer metastasis in clinic.
2.The key processes in the pre-metastatic microenvironment
Development of pre-metastatic microenvironment is an ordered pathological event which can be separated into four major phases following sequential order (Fig.1).Firstly, primary tumors produce the various primary tumor-derived components, including EVs, TDSFs and related factors, which establish an immature PMN.Then, BMDCs and immune cells are constantly recruited into the specific locus under the mobilization of tumor secreted factors and interact with stromal cells here to form a mature PMN.Next, CTCs escape from antitumor immune responses, reach and colonize the PMN leading to the initiation of metastasis, and finally form micrometastases and expand into macro-metastases [8].
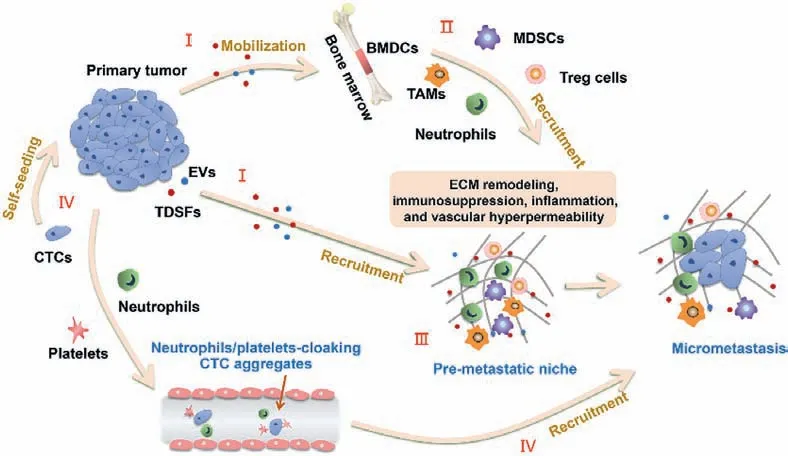
Fig.1.Formation of the pre-metastatic microenvironment.(I) Primary tumor secrete a variety of tumor-derived components, including TDSFs, EVs and other factors, which have impact on various pre-metastatic organs.(II) Regulatory/suppressive immune cells including myeloid-derived suppressor cells (MDSCs), T regulatory (Treg) cells, tumor associated macrophages (TAMs), and neutrophils are mobilized from the bone marrow and recruited to pre-metastatic organs in response to TDSFs and EVs.(III) The action of BMDCs helps to create niches by modulating micro-environment through processes including extracellular matrix remodeling, immunosuppression, inflammation, and vascular hyper-permeability.(IV) CTCs in blood vessel arrive and colonize at the fertile PMN and form micro-metastases.Some of them could come back to the primary tumor according to the tumor self-seeding model.
2.1.Primary tumor-derived components
Within the primary tumor, neoplastic cells struggle against acid environment, hypoxia, nutrient deprivation and high interstitial pressure.Subsequently, tumor cells release more primary tumorderived components, including EVs, TDSFs, and other molecular factor.Among others, TDSFs include tumor necrosis factor (TNF),transforming growth factors (TGF) and vascular endothelial growth factor (VEGF) that upregulate vital factors like lysyl oxidases (LOX),S100A8/9 (calprotectin), fibronectin and matrix metalloproteinases(MMP), all recruiting a vast array of different cell types to the PMN[18].Among them, S100A8/9 can induce the expression of serum amyloid A (SAA) 3 in the pre-metastatic organs, which can act as a positive-feedback modulator for chemoattractant secretion and promote the accumulation of CD11b+ myeloid cells, all of which enhanced invasion and metastasis [19].LOX, arising from hypoxic tumor cells, have been shown to remodel the extracellular matrix(ECM) in PMN.Once secreted from the primary tumors into circulation, LOX localized with fibronectin in pre-metastatic organs where it served to cross-link collagen IV in basement membrane to increase the adherence of MMP2-secreting CD11b+ myeloid cells,which lead to invasion of the metastatic sites and further recruitment of metastasizing tumor cells [20].
Tumor derived EVs, especially exosomes, have been considered as another spearhead of PMN formation.Tumor exosomes that contain nucleic acids and proteins educate PMN through regulating dynamic communication between cancer cells and surrounding factors or through transferring their contents to recipient cells[21].For example, tumor exosomal RNAs promote PMN formation in lung by activating alveolar epithelial toll-like receptor 3 (TLR3),consequently inducing chemokine secretion to recruit neutrophils[22].Exosome integrins can promote the upregulation of proinflammatory S100 at the metastatic organ microenvironment by the activation of Src phosphorylation [23].Some exosome-induced inflammatory signaling pathways are able to suppress anti-tumor functions of the host immune system.Tumor-derived exosomes can suppress IL-2-stimulated natural killer (NK) cells proliferation[24].Macrophage migration inhibitory factor (MIF) overexpressed in pancreatic cancer exosomes can induce the release of TGF-βfrom Kupffer cells in liver, which in turn facilitates the formation of fibronectin and production of TGF-β, subsequently enhances the recruitment of tumor associated macrophages (TAM) and neutrophils into the liver, facilitating the formation of PMN [25].
2.2.Establishment of PMN
BMDCs and interrelated immune cells continuously migrated into secondary organs in response to TDSFs and EVs secreted by primary tumor, which interact with stromal cells here to form a mature PMN with creating conditions for the succeeding survival and colonization of metastatic tumor cells.BMDCs are one of the most critical cell types of the tumor microenvironment, which can be selectively recruited to PMN before tumor cells arrived [26].
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of myeloid cells with immunosuppressive properties in consist of myeloid progenitor cells and immature myeloid cells.MDSCs and immune cells (including Treg cells, TAMs, and tumorassociated neutrophils (TANs)) are recruited into secondary organs together by tumor cell-derived factors and components, and these cells and factors facilitate the establishment of immunosuppressive microenvironment in PMN [27].Increased expression of prometastatic proteins in MDSCs including Bv8, MMP, S100A8/9 promotes more effective tumor cells extravasation, infiltration and proliferation in PMN [28].
2.3.Colonization of CTCs in the PMN
During metastasis, CTCs migrating away from primary tumor must penetrate basement membrane, invade through ECM and then intravasate into the circulation to finally spread into PMN[29].The well-established niches with a suitable microenvironment support the seeding, colonization, and outgrowth of CTCs,leading to initiating metastasis and forming micrometastases and macrometastases.It is understood that NK cells can target and destroy tumor cells in blood.However, tumor cells in blood can induce platelets aggregation and forming a physical “cloak” which defend them from immune elimination.Activated platelets can promote endothelial interaction to enable CTCs extravasation and form metastasis [30].Simultaneously, CTCs can also interact with neutrophils and the association between them drives cell cycle progression within the blood and increases the metastatic potential of CTCs.These forming neutrophils/platelets-cloaking CTCs aggregates protect CTCs in bloodstream from physical stress and host immune attack, and promote cancer metastasis [31].In addition,the pre-recruited neutrophils in PMN can release neutrophil extracellular traps (NETs) to facilitate tumor cells extravasation to metastasis organs [32].
2.4.Formation of micrometastases
Every metastatic tumor starts as a single or small clusters of micrometastatic cells [33].Most CTCs are destroys or intercepted or keep dormant due to the hostile microenvironment and only 0.02% would eventually survive in bloodstream, form detectable metastases [34].These tumor cells exist as disseminated tumor cells (DTCs) or further form micrometastatic foci.The DTCs are followed by a long period of dormancy and are “seeds” that potentially form metastasis.Further studies found that these dormant DTCs are cancer stem cells (CSCs) or keep stem-like characteristics, which would remain a non-proliferative or quiescent state,resist chemotherapy and survive longer in the host tissues until being triggered to re-emerge by suitable “soil” of PMN [35,36].Immunosuppression and chronic inflammation of the PMN support the micrometastases development with contributing to ECM remodeling and tumor angiogenesis due to the secretion of supportive growth factors and MMPs from MDSCs and metastasisassociated macrophages (MAMs) [37].Moreover, some secreting cytokines from primary tumor can act on stromal cells and BMDCs,and provide remote support to the newly established foci.Activated stromal cells, such as fibroblasts, would generate inflammatory inducers and proteases, which are able to facilitate epithelialcell proliferation or stimulate angiogenesis and activate dormant tumor cells [35,38].
3.Strategies targeting pre-metastatic microenvironment
At the primary site, tumors cells produce primary tumorderived components (TDSFs, EVs and related factors, which cause the mobilization of various BMDCs and immune cells in TIM to metastatic organs, and establish PMN.Then, CTCs escape from antitumor immune responses and reach and colonize the PMN,resulting in the initiation of micrometastases.The PMN recruits more metastatic tumor cells and finally forms diagnostically visible macro-metastases [8].Therefore, primary tumor-derived components, PMN, CTCs, micrometastases and TIM appears to be a crucial target in the early stage of metastasis formation.It could be a potential strategy for directly targeting pre-metastatic microenvironment-interrelated factors and cells before they have the chance to form secondary tumors to prevent metastasis.
3.1.Absorbing and neutralizing TDSFs and EVs
Primary tumors secreting TDSFs and EVs is the initial step trigger tumor metastasis.Developing corresponding treatment strategies for this stage would help block metastasis process from the source.Some novel nanosystems able to absorb and neutralize TDSFs and EVs have be developed to interrupt metastatic processes for cancer treatment (Table 1).
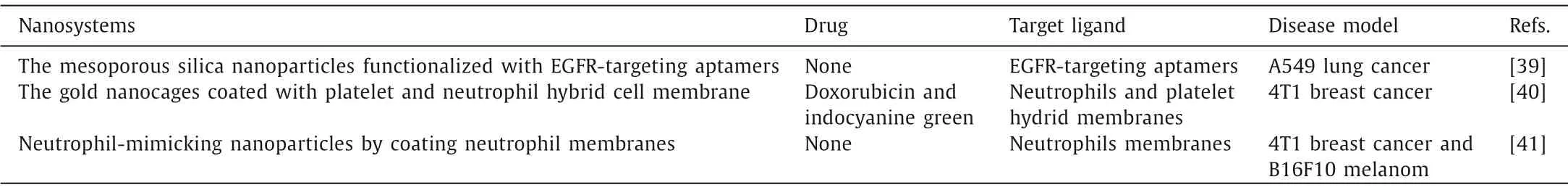
Table 1 The nanosystems for absorbing and neutralizing TDSFs and EVs.
Tumor derived EVs, especially exosomes, have been considered as another spearhead of PMN formation.Capturing oncogenic exosomes in circulation can also prevent PMN establish-ment.Xieet al.prepared specific mesoporous silica nanoparticles(NPs) that identify and bind oncogenic exosomes that overexpress surface epidermal growth factor receptor (EGFR) biomarkers [39].The nanosystems-exosomes conjugates traversed hepatobiliary layers and into bile duct rapidly, thereby eliminated circulating levels of tumor-derived exosomes and subsequently prevent the formation of metastasis.In another study, the platelet-neutrophil hybrid membrane-bionic nanocages were devised to capture and eliminate the migrating oncogenic exosomes completely, thus improving the immunosuppression of tumor microenvironment.In vivoon mice, the multifunctional nanocages showed exosomes capture efficiency of 65.71% and successfully inhibited the tumor metastasis[40].
MDSCs are major immune-responses modulators in cancer.They can be recruited to assisting in the construction of PMN and facilitate tumor immune escape through multiple mechanisms.Therefore, disrupting the myeloid expansion and trafficking into tumor sites help to restore the antitumor immunity in the tumor microenvironment.Based on this tendency, a nano-sponge coated with neutrophil membrane was developed to disrupt the expansion, recruitment, and activation of polymorphonuclear MDSCs.It inherited major membrane receptors from neutrophils which absorb and neutralize key growth factors and chemokines, and subsequently reduce the accumulation of MDSCs in peripheral lymphoid and tumor tissues.Furthermore, combining the nanosystems with the programmed death-1 (PD-1) immune checkpoint blockade improve the infiltration and function of anti-tumor immune cells for improved cancer therapy [41].
3.2.Preventing establishment of PMN
The PMN refers to a supportive and receptive microenvironment through a complex succession of changes to form the future sites of metastasis, or the prepared “soil” for the reception of the CTCs, thus sustaining tumor growth in the distant sites and facilitating metastasis [8].In recent years, the area of targeting PMN for detecting or preventing metastasis has just emerged and attracts increasing attentions.Because targeting the host microenvironment, a less dynamic and more easily predictable system, may offer a superior alternative therapeutic strategy for preventing and delaying cancer progression [42].The PMN is composed of a complicated micro-environment, including ECM proteins, stromal cells, inflammatory immune cells, tumor-derived exosomes, and selective homing molecules.Liu and Cao proposed that the unique characteristics of the PMN enable the niche to support the survival and colonization of metastatic tumor cells, including immunosuppression, inflammation, angiogenesis/vascular leakage, lymph-angiogenesis, organotropism, and reprogramming [8].Thus, targeting the PMN-promoting factors to monitor, remodel and prevent PMN establishment and consequently inhibit metastasis could probably be a more effective approach for cancer diagnostic and therapeutic.Nanosystems based on the characteristics of tumor microenvironment, owing to their special physical and chemical properties, have been widely applied in cancer imaging and targeted therapy [43,44].These functions can be combined with various therapeutic drugs to block key functional factors in the PMN.Currently, some novel nanosystems have be developed to prevent the formation and function of the PMN for anti-metastasis treatment (Table 2).
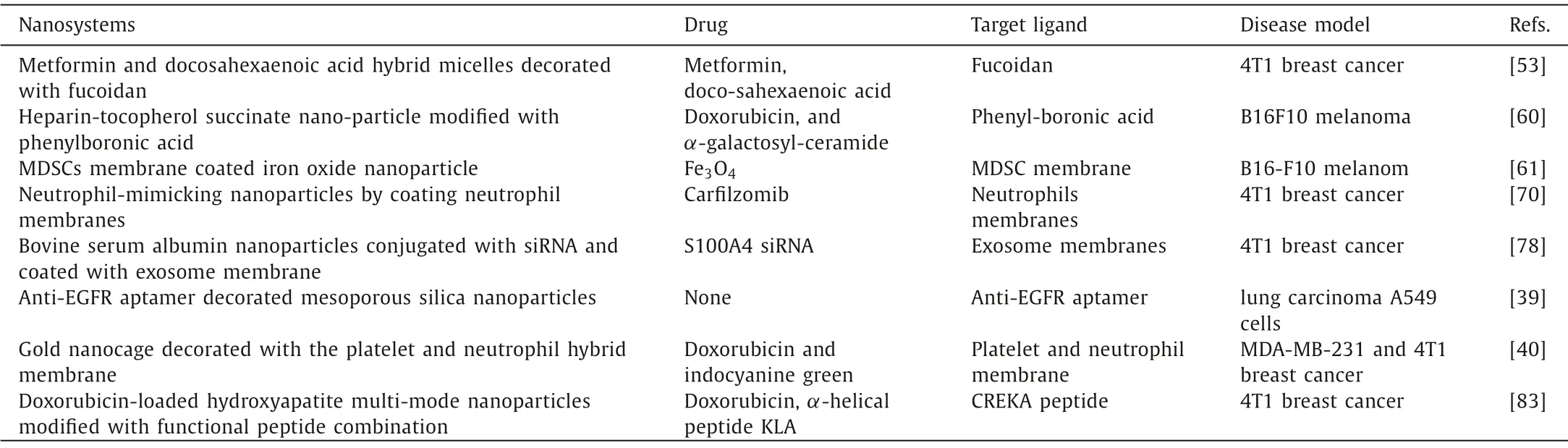
Table 2 The nanosystems for preventing establishment of PMN.
3.2.1.Inflammation microenvironment-based nanosystems
Local inflammatory microenvironment is one of the indispensable drivers for PMN establishment [8].Tumor-derived cytokines and exosomes can promote the release of the pro-inflammatory mediator S100 family of proteins in PMN [45,46].S100 proteins(S100A9) can induce the inflammatory microenvironments through nuclear factor-κB (NF-κB) signaling pathway activation, which facilitates PMN formation in inflamed tissues and accelerate metastasis progress [46,47].Furthermore, other metastasis-associated inflammation mediated by up-regulated interleukin-6 (IL-6) and activated signal transducer and activator of transcription-3 (STAT3)also contributes to the establishment of PMN [48].Therefore, the formation of inflammatory micro-environments is an essential process for the development of PMN and thereby for the survival and proliferation of CTCs [49].
It is verified that the improvement of the PMN with regulating anti-inflammatory components is an effective strategy for tumor metastatic detection and therapies.S100A8/A9 can serve as a reliable marker applied to the non-invasive imaging and reported the purposes of antibody-based single-photon emission computed tomography (SPECT) for S100A8/A9 detectionin vivo[50].Systemic S100A8/A9 SPECT imaging reflected condition of a metastasis-permissive microenvironment and allowed for evaluation of immunosuppressive status in pre-metastatic organ.Moreover, the chemokine receptor CXCR4 has also been used as a prognostic biomarker in facilitating breast cancer metastasis and targeting therapy [51].Zhaoet al.have reported the design and application of CXCR4-targeted gold nanoclusters doped with64Cu and grafted with a targeting ligand AMD3100, which monitor upregulated CXCR4 earlier in premetastatic lung with higher sensitivity, and can act as a potential platform for detection of metastasis at early stage [52].Recently, Jianget al.developed PMNtargeted micelles loaded with two anti-inflammatory drugs (metformin and docosahexaenoic acid) to amend the pre-metastatic inflammatory microenvironment and inhibit cancer metastasis (Fig.2) [53].The functionalized micelles are enveloped with fucoidan which has high affinity P-selection for targeting PMN.The combination of metformin and docosahexaenoic acid can inhibit the expression of MMP9, S100A9 and fibronectin, and modulate multiple inflammatory pathways to powerfully suppress the metastasis of primary tumor in murine models.Notably, the therapeutic strategy that early intervention against the PMN with targeted antiinflammatory drugs might afford a novel direction for metastasis prevention in cancer therapies.
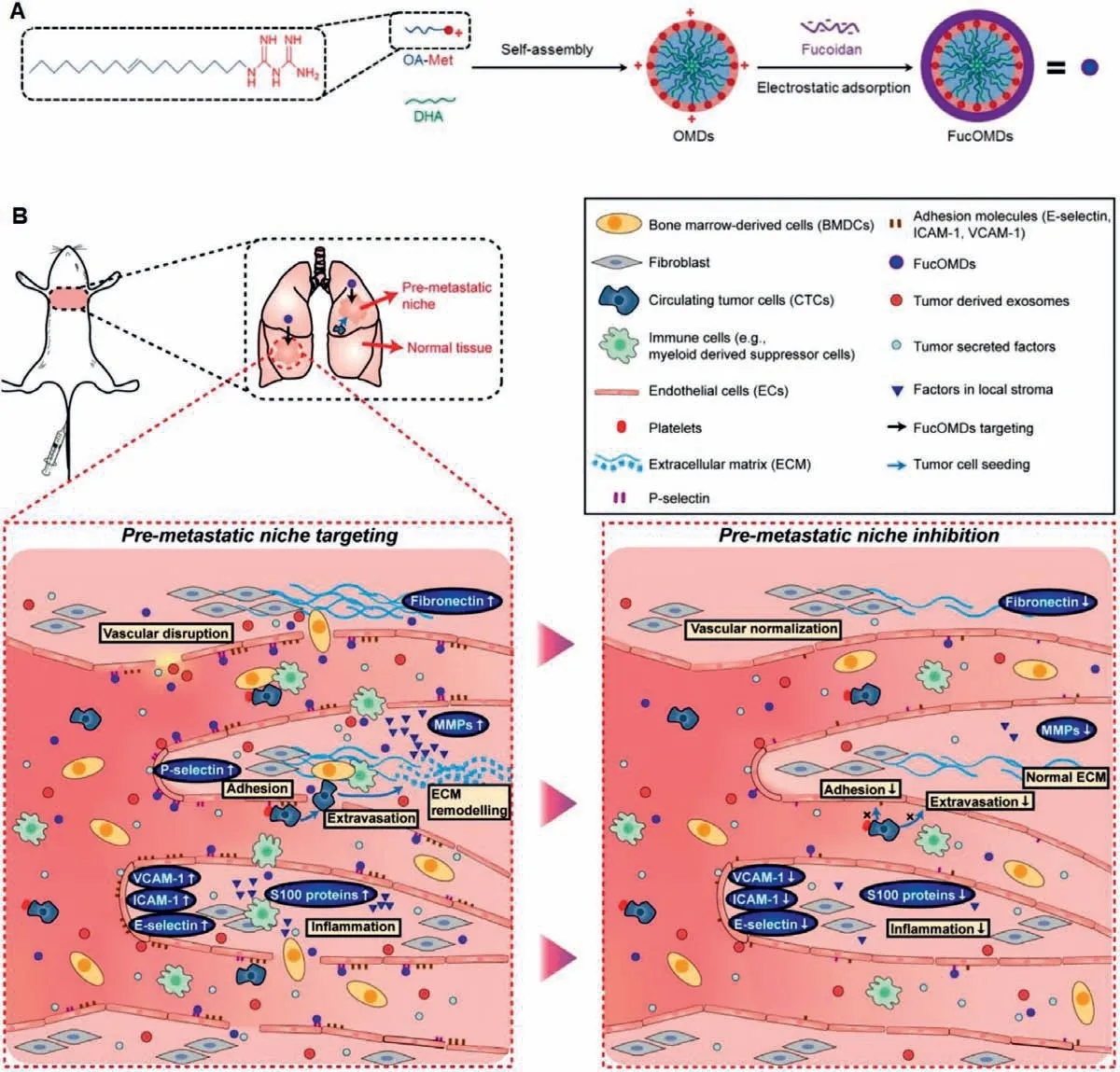
Fig.2.The multifunctional micelles loaded with metformin and DHA (FucOMDs) for PMN-targeting and modification.Copied with permission [53].Copyright 2019, American Chemical Society.
3.2.2.MDSCs-based nanosystems
BMDCs is essential for the development of PMN, which can be selectively recruited to PMN before tumor cells arrived and thereby participate in the establishment of PMN microenvironment as a non-resident cellular component [8].BMDCs expressing vascular endothelial growth factor receptor-1 (VEGFR-1) and very late antigen-4 (VLA-4) reach metastatic sites and establish a suitable microenvironment for tumor cells.VEGFR-1-positive BMDCshematopoietic progenitor cells (HPCs) were found to form clusters at metastatic sites.Penget al.developed a high-affinity peptidomimetic ligand, LLP2A, for the activated conformation of VLA-4 [54].Accordingly, Shokeenet al.developed a64Cu-labeled LLP2A for positron emission tomography (PET) imaging.The PET imaging can visualize BMDCs reorganization and extension noninvasivelyin vivoto detect the progression of the PMN and further provide an effective diagnostic approach that could act on key intervention point in the tumor treatment process [55].
MDSCs are a heterogeneous population of immature myeloid cells with immunosuppressive properties which accumulate in cancer patients and appear at the early stages of PMN and are the critical determinants in the progression of PMN [56].The MDSCs exhibit different biological functions which can induce vascular permeability and alter ECM to the influence of systemic immune that promote metastatic progression [56].Suppressing MDSCs recruitment in early stage is beneficial for PMN elimination.In one study, the C5a/C5aR1 axis was found to contribute to premetastatic angiogenesis through the regulation of MDSCs, and C5aR1 blockade combined with antiangiogenic vaccines reduces lung metastasisviainhibiting the growth of primary breast tumors, reducing lung angiogenesis and increasing lung T cell infiltration [57].In addition, Luet al.found that, after surgical resection of primary tumor, low-dose adjuvant epigenetic therapy improve the premetastatic microenvironment and suppresses both the development of metastatic site through its selective effect on MDSCs [58].Longet al.have devised a heparin-tocopherol succinate nanoparticle that can suppress early pulmonary recruitment of granulocytic myeloid-derived suppressor cells (GMDSCs) through preventing their extravasation by suppressing P-selectin/PSGL-1-mediated adhesion between GMDSCs and vascular endothelial cells[59].Furthermore, the intensive immune response mediated byαgalactosyl-ceramide could be helpful in extended suppression of metastatic cancer and postoperative recurrence.The self-delivery nanoparticle offers a powerful platform to improve anti-metastasis therapies by interfering with establishment of PMN.In postoperative metastasis mouse models, the survival time of mice in the self-delivery nanoparticle-treated group was prolonged by 1.9-fold in comparison to the PBS-treated group, and nearly 40% of mice survived to the 55thday.Yuet al.constructed iron oxide magnetic nanoparticles coated with MDSCs membrane for tumor imaging and photothermal therapy.The bioinspired nanosystems could reduce the uptake rate by murine macrophages and escape host immune clearance.MRI instrument demonstrated that the mouse injected with MDSCs membrane-coated nanoparticles yielded a fortified dark image at the tumor site and have high tumor accumulation.The treatment with them could significantly induce macrophage state switch from M2 to M1, synergize immunogenic cell death, for enhanced antitumor immune response upon laser irradiation in C57BL/6 melanoma mice model [60].We speculate that the MDSCs camouflaged nanosystems might be applied to target metastatic niche due to their capacity to successfully be recruited in premetastatic microenvironment.
3.2.3.Immune cells-based nanosystems
Tumor cell-derived chemokines and cytokines recruit the premetastatic microenvironment cellular components containing MDSCs, macrophages, Treg cells, and TAN into distal metastatic organs,and these immune cells facilitate tumor metastasis by supporting PMN establishment [61,62].Recent research have reported that a large amounts of neutrophils play a vital part in the early phase of the PMN establishment [63].Initially, tumor-secreted components and granulocytic myeloid cells (precursors of neutrophils)are mobilized by hypoxic TDSFs and initiate the PMN formation[64].Then, neutrophils together with CTCs are constantly recruited to the PMN, in response to the inflammatory signals of the niche[65,66].Hijacking neutrophils has become an effective strategy forin vivousing nanoparticles to deliver therapeutics into tumor [67,68].Kanget al.devised a nanosized neutrophil-mimicking nanoplatform coated with neutrophils membranes onto polymer nanoparticle cores [69].They inherited the integral membrane proteins on surface of neutrophils and exhibited the capacity of targeting both CTCs and PMN.The nanosystems loaded with carfilzomib can promote selective CTCs apoptosis in blood, suppressed the formation of pulmonary nodules, and induced growth inhibition and apoptosis in metastatic mice models.Identification of new niche functions will contribute to expand the range of therapeutic options.
3.2.4.Exosomes-based nanosystems
Tumor-derived EVs probably depart away from their original position to serve as underlying regulators for establishing PMN,the EVs that comprise genetic material, proteins and lipids contribute to the formation of PMN by regulating dynamic exchanges between cancer cells with pericellular environment or by horizontally delivering their contents to receptor cells [70].Numerous current researches focus on exosomes, a nanosized vesicles (30–100 nm diameter) that are encapsulated by a lipid bilayer.They contain fibronectin on external surface, which promoted interaction with target cellsviaheparin sulfate [71], and exosomal small nuclear RNA increased the expression of MMP-9 and fibronectin in PMN, thus facilitating neutrophils recruitment [72].Therefore,tumor-secreted exosomes are considered main drivers in the development of PMN and crucial mediators of cancer cell-immune cell communication [73].The exosomes can not only upregulate inflammatory factors, induce immune suppression, enhance angiogenesis and vascular leakage, but also determine organotropism metastasis and facilitate ECM remodeling [74].
Analogous in size and function to synthetic nanoparticles, exosomes display distinct advantages, rendering them the potential candidates for drug carriers.Due to the inherent biological property of exosomes from diverse metastasis-associated cells, they can transport various chemotherapeutic agents or siRNAs to accurately destroy tumor cells [75,76].Zhaoet al.have developed cationic bovine serum albumin (CBSA) conjugated S100A4 siRNA (siS100A4)and exosomal membrane coated nanoparticles that are composed of a CBSA/siS100A4 core and an exosomal membrane shell [77].siS100A4 can silence S100A4 expression and suppress tumorigenesis.The existence of exosomal membranes could improve the delivery of therapeutic siS100A4 to PMN in the lung and enhance the adhesion of nanosystems to the lung in a postoperative lung metastasis mouse model.
3.2.5.Fibrin-fibronectin complexes-based nanosystems
Tumor nets contained a meshwork of fibrin-fibronectin (FBFN) complexes, which were almost undetectable in normal tissues [78,79].However, the overexpression of FB-FN was found in PMN, which demonstrated metastatic formation in distal secondary sites [80,81].Even more importantly, unlike biomarkers that could change over time and space and thereby decrease targeting efficiency, FB-FN has been shown to be overexpressed throughout the cancer progression, from avascular multicellular aggregates to mature tumor tissues.Thus, the peptide which has high affinity for FB-FN was exploited as the active targeting module to decorate the functional nanosystems for targeting therapy [80].Further,Xionget al.developed a peptide-modified hydroxyapatite multimode nanosystem loaded with doxorubicin (Dox) to achieve tumor metastasis therapy [82].Thein vivoimaging in metastatic 4T1 orthotopic tumor model revealed that the nanosystem had effective drug delivery capacity targeting to micro-metastasis and cathepsin B-triggered intracellular mitochondria and nuclei dual-targeted therapy could improve tumor suppression efficacy of Dox.
3.3.Eliminating CTCs
The clinical usefulness and prognostic significance of CTCs has been recognized in a wide variety types of cancer [83].Molecular characterization of CTCs is helpful to monitor and predict the response to ongoing therapy [84].Although CTCs detection have become a proven technique in early screening, monitoring of can-cer progression and treatment, and indication of cancer relapse, effective neutralization of CTCs for preventing metastasis remains a clinical challenge.The key reasons are that high mobility properties of CTCs and shear stresses generated by the flow of blood have a dramatic impact on the nanosystems efficacy against CTCs.Currently, designing nanosystems rationally by virtue of unique markers of CTCs and metastasis-accessory cells have become an effective strategy to target CTCs for anti-metastatic therapy (Table 3).
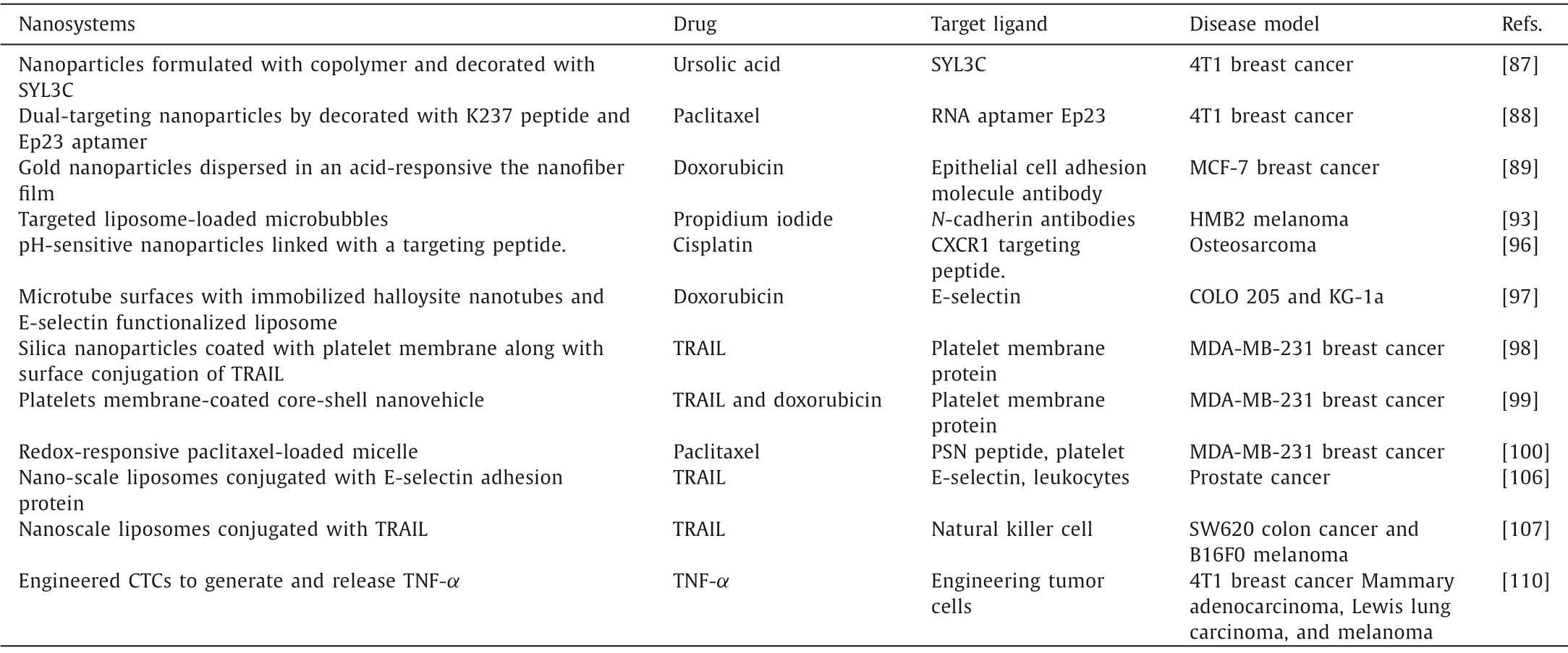
Table 3 The nanosystems for eliminating CTCs.
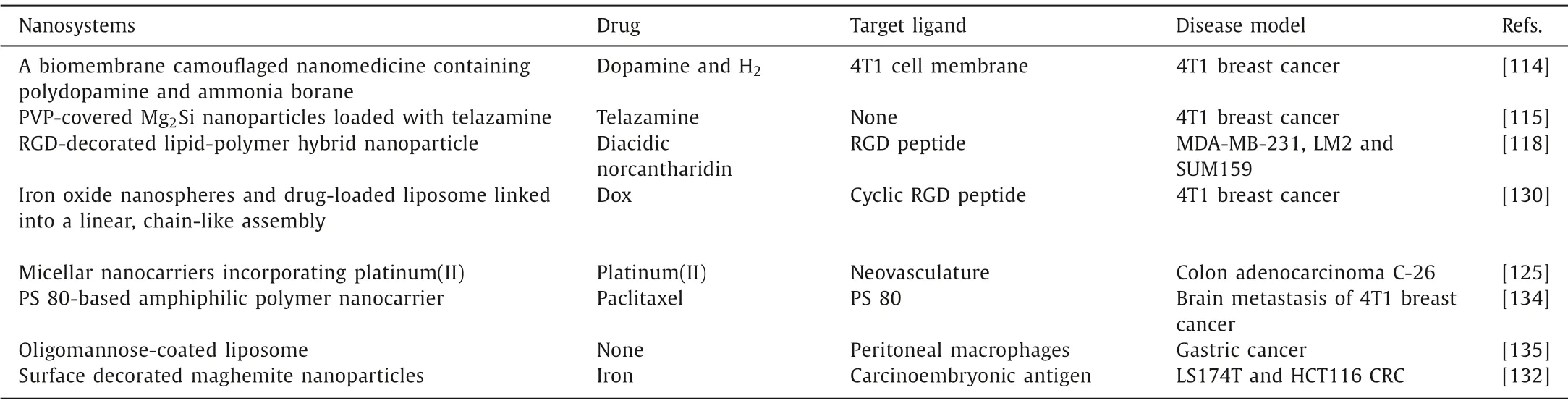
Table 4 The nanosystems for eradicating micrometastatic foci.
3.3.1.Unique CTCs markers-based nanosystems
EpCAM is overexpressed on various cancers including colon,lung, prostate, ovarian, breast and gastric cancer, and is the most favored biomarker exploited for the positive-enrichment of CTCs [85].In the Food and Drug Administration (FDA) approved Cellsearch® system, anti-EpCAM antibodies have been binded onto the magnetic beads’surface to capture CTCs from the peripheral blood [86].Liuet al.have devised nanosystems modified with an aptamer SYL3C which can be bound to the EpCAM for promoting the anti-tumor-metastasis delivery of ursolic acid [87].Furthermore, to combine the functions of CTCs neutralization and primary tumor damage, Yaoet al.have utilized a RNA aptamer Ep23 for binding capability to EpCAM sensitively and devised a neovasculature and CTCs dual-targeting nanosystem.In 4T1 metastasis mice model, the nanosystem displayed the capacity of CTC-targeting and neutralization effect [88].To deal limited encounter between CTCs and nano-delivery system, Wanget al.developed a novel composite nanofiber film with a CTCs enrichment function to prevent the viability [89].In this system, gold nanoparticles functionalized with Dox were mixed in solution of acid-responsive polymeric materials to synthesize the nanofiber film.The composite nanofiber film could capture 36.70% of the fluid tumor cells in 30 min at a fluid rate of 1.00 μL/min, and it is expected to be applied as an implanted device to reduce the viability of CTCs.
Mesenchymal cadherins were one of the vital factors that induce cell motility and invasiveness [90].N-Cadherin and vimentin are both expressed in mesenchymal cells and serve as markers for detecting mesenchymal CTCs [91].In one study, Geerset al.reported liposome-loaded microbubbles which coupleN-cadherin antibodies to liposomes and subsequently load such constructs to microbubbles [92].Such targeted delivery systems can specifically adhere toN-cadherin-expressing HMB2 cells, and deliver propidium iodide into the target cells.It may find application as theranostics devices aimed for eliminating CTCs specifically in blood.
CXCR1, a specific receptor for IL-8, mainly modulated immune inflammatory response [93].Some studies reported that CXCR1 played an essential role in tumor metastasis [94].Hanet al.have found that CXCR1 was overexpressed and correlated with the cancer stem cells (CSCs) and have designed a pH-responsive magnetic nanoparticle modified with CXCR1 targeting peptide [95].The outermost layer of mesoporous silica nanoparticles was coated with polyacrylic acid to alleviate burst release and control cisplatin release in the acidic microenvironment of solid tumors.The nanosystems exhibit synergistic anti-tumor interaction through precise targeting of CXCR1 on CTCs and cisplatin delivery.
CTCs could adhere to selectin proteins along inflamed endothelium during the progression of metastasis through overexpression of E-selectin ligands on their surface.Mitchellet al.assessed the viability of utilizing immobilized selectin proteins as a targeting treatment for CTCs.Further, they developed a combination device of E-selectin functionalized liposomes and a biomimetic microtube to target and capture CTCs under shear flow [96].The nanostructured surfaces consisting of functionalized liposomal Dox could enhance COLO 205 and KG-1a cancer cells recruitment for chemotherapeutic drug delivery, concurrently also suppressing healthy cells adhesion and uptake of therapeutic intended for CTCs.
3.3.2.Platelet-based nanosystems
Biomimetic strategies have been paid increasing attentions in drug delivery applications to increase therapeutic effect on cancer metastasis.The cell-mediated nanocarriers are highly integrated with organisms because they originate from their own cells and possess unique metastasis-targeting capability, they can target a specific location and release the drug specifically, thereby reducing unnecessary stimulation to the body [97].
Inspired by the adhesion effect of platelets to CTCs-associated microthrombi, silica nanoparticles were functionalized with platelet membrane along with surface conjugation of TNF-related apoptosis-inducing ligand cytokine (TRAIL) [98].The bionic nanoparticles incorporated into CTCs-associated microthrombi in lung vasculature and significantly reduced cancer metastasis in lung metastasis model.Similarly, Huet al.devised a platelets membrane-coated nanovehicle for sequential and site-specific delivery of extracellularly active protein and intracellular smallmolecule drugs.The overexpressed P-selection on the platelets membrane is able to specifically recognize CD44 receptors overexpressed in CTCs and subsequently trigger TRAIL/Dox-induced apoptosis signaling pathways [99].
Although platelets membrane-based nanosystems have been used for targeting CTCs, the isolated membrane was not different from internal activated platelets, which may attenuate its interaction with tumor cells.Consequently, we have devised a paclitaxel (PTX)-loaded micelle modified with PSN peptide to target activated platelets (Fig.3) [100].PSN peptide is capable of specifically bind to P-selectin which is a receptor present in plateletαgranules, and PSN-modified nanosystems possessed strong affinity to activated platelets [101].Utilizing platelets as a “bridge”for interaction with tumor cells, the functional micelles easily adhere to activated platelets, then capture and eradicate CTCs in blood circulation to block dissemination and inhibit the progression of metastasis.Recently, Xuet al.proposed a new strategy with block tumor-specific platelet functions to prevent platelet adhesion around CTCs and suppress distant metastasis.In this study, a tumor microenvironment-responsive nanoparticle was devised to release nitric oxide (NO) and was able to specifically co-deliver the NO and the PTX into tumor sites, and simultaneously suppress platelets adhesion around CTCs to prevent distant metastasis [102].
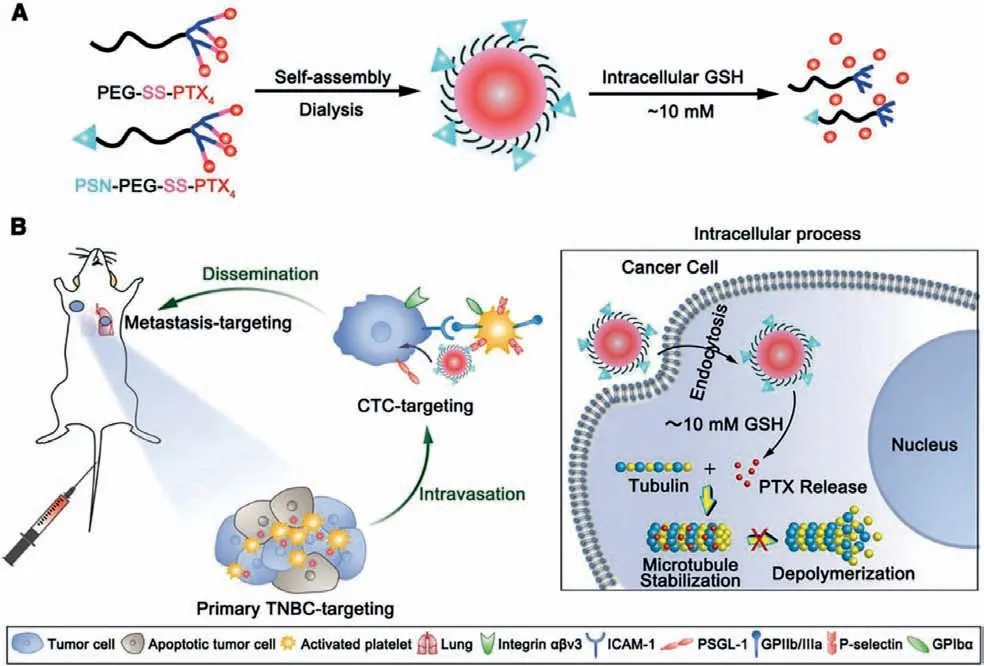
Fig.3.(A) Scheme of the functional micelles preparation and (B) in vivo tumor targeting effect of micelles at different stages (mM: mmol/L).Copied with permission [100].Copyright 2019, Wiley Publishing Group.
3.3.3.Immune cells-based nanosystems
The inflammatory neutrophils show a CTCs adhesive capacity during the multiple processes in the metastatic cascade [103].They can interact with CTCs and increase their metastatic potential.Using this process, Kanget al.have designed a nanosized neutrophilbionic nanosystem to alleviate the metastatic burden by targeting CTCs [104].In another study, the platelet and neutrophil hybrid membrane nanocages were developed to simultaneously capture and clear the CTCs [40].In the breast tumor mouse model, the hybrid membrane nanosystems could completely suppress primary tumor and effectively prevented tumor metastasis.Notably, although the treatment modalities of cell membrane-based nanosystems to target and eliminate invading tumors is extensively studied, their clinical potential cannot be fully achieved without reproducible technologies to reliably manufacture large-scale, highquality and low-cost cellular products.Unlike traditional pharmaceutical manufacturing, these products are living organisms that can change with every process manipulation.
It is another attempt for tumor therapy that liposomes are integrated into the circulating cells and blood components that bind to CTCs, thus inducing the apoptosis of CTCs.Wayneet al.have demonstrated an strategy by liposomes conjugated with E-selectin adhesion protein and Apo2L/TRAIL that attach to the surface of leukocytes and rapidly eliminate CTCs in blood circulation [105].Such an approach could be applied to inhibit the tumor metastasis in prostate cancer model, by highly diminishing the number of CTCs.Similarly, Chandrasekaranet al.described a therapeutic strategy to target and kill tumor cells by functionalizing NK cells with liposomes conjugated with TRAIL, an antibody against NK1.1 antigen expressed on murine NK cells [106].The functional NK in the tumor draining lymph nodes, creating super NK cells with continuous retention time, effectively inhibits the dissemination of cancer cells from primary tumor to lymphatic system.
3.3.4.Tumor self-targeting based nanosystem
A tumor “self-seeding” phenomenon is discovered in multiple experimental models.CTCs can metastasize from primary or metastatic lesions to the peripheral blood and then return to their origin to re-infiltrate tumor by sensing attractive signals from the tumor.The process of “self-seeding” selects a more aggressive cancer cell population and requires little additional adaptation of CTCs to the recipient microenvironment [107,108].According to this phenomenon, Dondossolaet al.used tumor cells as vehicles to directly transmitted TNF-αto primary and metastatic tumors through engineered tumor cells to achieve “tumor selftargeting” [109].They genetically engineered CTCs from mouse mammary adenocarcinoma, Lewis lung carcinoma, and melanoma cells to generate and release TNF-α.The “armed” tumor cells can specifically home to tumors sites through a process of tumor selfseeding and release TNF, induce tumor vasculature damage and subsequently lead to tumor cell apoptosis.The study validates that genetically modified CTCs could serve as targeted delivery of anti-cancer drugs.In a clinical context, the unique paradigm can be translated into applications potentially utilizing patient-derivedCTCs as self-targeted vectors for drug delivery.Notably, the injected tumor cells could give rise to new risks of neoplastic lesions.
3.4.Eradicating micrometastatic foci
Every metastatic tumor starts as a single micro-metastatic cells or small avascular population of cells [33].The awakening of micrometastatic cells is the key step in metastasis, and if we could mitigate the survival of residing dormant DTCs/CSCs or maintain them in a dormant state, the genuine breakthrough in antimetastasis therapy could be achieved.
To date, the unique structures and material properties of nanoscaled vehicles have been developed to exploit the deliver various drugs to solid tumor lesionsvia“size effect” [110].Remarkably, the size effect is restricted to vascularized type of tumors with diameters of more than 4.6 mm [111].Nevertheless, micrometastases are mostly small tumor cell clusters with poor vascularization and angiogenic dormancy at a diameter of 1-2 mm,thereby remarkably hindering nano-delivery to micro-metastases[112].Encouragingly, diverse targeting schemes and ligands have been exploited to direct nanosystems to micrometastases sites for enhanced therapeutic responses (Table 4).
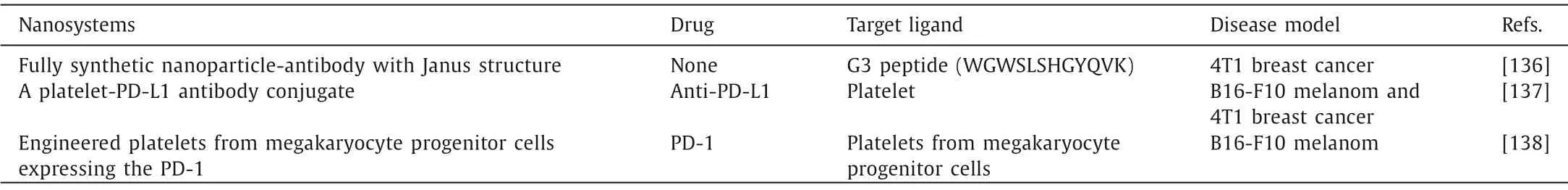
Table 5 The nanosystems for modulating TIM.
3.4.1.Stem-like neoplastic cells-based nanosystems
The cancer cells with stemness features play a substantial role in neoplastic recurrence, metastasis and drug resistance.The tumor cells in distant organs usually exist as DTC, CSC, and/or micrometastases before they develop into overt metastasis.These single cells or small clumps of cells can keep dormant, maintain cell stemness, and have the capability of evading the treatment [113].In order to target such micro-metastatic cells, eradicating these dormant cells before they awaken or keeping them dormant indefinitely could be potential direction for cancer treatment.
Zhanget al.designed a synergistic nanosystems combined polydopamine-mediated photothermal therapy (PTT) and ammonia borane-mediated hydrogen therapy.The hyperthermia during the process of PTT lead to inflammatory responses, which could awake distant dormant tumors, however, the ammonia borane could reduce inflammation to inhibit distant dormant tumot by releasing H2in tumor microenvironment [114].In another study, Chenet al.developed a Mg2Si nanoparticle covered by polyvinyl pyrrolidone(PVP) for regulating cell-dormancy gaseous microenvironment.In mouse 4T1 breast tumor xenograft model, these magnesium silicide nanoparticles could be activated by the mildly acidic microenvironment, create an anaerobic gaseous environmentviaoxygen consumption and further induce anaerobic cell dormancy in solid tumors.Meanwhile, the released tirapazamine kill dormant tumor cells by the bioreductive cytotoxicity [115].
Wnt/β-catenin signaling pathway was shown to play a pivotal role in the maintenance of tissue homeostasis by regulating selfrenewal of normal stem cells.This pathway is involved in malignant transformation, and associated with tumor aggressiveness,metastases and poor clinical outcome [116,117].Liet al.reported an RGD-decorated lipid-polymer hybrid nanoparticles for triple negative breast cancer (TNBC)-targeted delivery [118].This nanosystem is able to recognize and bing integrinα5 overexpressed in TNBC cells andin vivoimaging analysis showed that it can accumulated more significantly and remained much longer than unmodified nanoparticles in experimentally-induced lung metastatic tumor in nude mice.It is loaded diacidic norcantharidin reduced nude mouse orthotopic mammary TNBC tumor growth and metastasisviadown-regulatingβ-catenin.
3.4.2.Tumor vessel-based nanosystems
The metastatic progression is mediated with metastatic cells,their surrounding stroma and the inflammatory microenvironment by promoting angiogenesis [119–121].Due to vascular permeability induced by inflammatory microenvironment which involves both intraendothelial transport and interendothelial leakage of macromolecules, the rapid retention and accumulation of nanosystems in the preangiogenic micrometastases could be promoted by systemically administration.Taking advantage of this unique physiological trait, Wuet al.developed a polymeric micelle incorporating the parent complex of oxaliplatin for targeting micrometastases and vascularized macrometastases [122].The novel delivery systems demonstrated selective accumulation into tumor sites and high anti-tumor activity in diverse cancer models [123–126], and are under evaluation in phase I clinical stage.
Extravascular metastases are often preceded by metastatic tumor cells that reside inside the lumen of vessels [127,128].CTCs can utilize the adhesion molecules of the leukocyte adhesion cascade to adhere to the endothelium in the future metastatic sites.After initial colonization, micrometastases proceed to proliferate by constructing their own microenvironment [127].Therefore, targeting the endothelium associated with micrometastasis is a novel approach used to transport the drug cargo.Peiriset al.devised a vascular-targeting nanoparticle for accurately detection of micrometastases [129].Furthermore, they designed a multicomponent nanochain utilizing a RGD peptide to specifically bind integrinαvβ3receptor, which can get access to and be deposited at 4T1 micrometastatic sites [130].Considering that expression of integrinαvβ3plays central role in the progression of metastasis in melanomas, prostate, pancreatic and cervical cancers [131], targeting integrinαvβ3appears to be a proper candidate for micrometastasis therapy of other types of cancer.
3.4.3.Unique cancer markers-based nanosystems
Monitoring micrometastasis appearance by detecting the biomarkers has been proved to help define the standardized clinical treatment and management protocols of patients.DetectingCTCs which express carcinoembryonic antigen (CEA) is associated with overall survival of the colorectal cancer patients.The developed nanomaterial device consist of surface decorated maghemite NPs, which can target CEA-overexpressed tumor cells and be used as a diagnosis and treatment tool for cancer micrometastasis [132].Moreover the chemokine receptor CXCR4 can also been used as a target which localizes to lung metastatic lesions due to abundant expression on highly motile cancer cells.For example, Zevonet al.devised human serum albumin NPs loaded with rare-earth that were capable of specifically accumulating sub-tissue tumor microlesions in a metastatic model, which have a maximum depth of 10.5 mm [133].
3.4.4.Others
In the different sites of micrometastases, different delivery strategies need to be customized according to the different physiological environment.In brain metastasis of breast cancer, the blood brain barrier (BBB) prevents most chemotherapy drugs entering into the metastatic lesions in brain.Heet al.developed NP coated with polysorbate 80, which can facilitate NP to cross the BBB [134].The result confirmed the nanoparticles extravasated from brain microvessels and delivered successfully the paclitaxel into micrometastasis lesions in the brain.For specifically targeting peritoneal micrometastasis with low dose administration, Matsuiet al.designed a novel nanosystem for specific delivery of antitumor agents to initial metastatic lesions of the peritoneal cavity in the gastric cancer using peritoneal macrophages as cellular vehicles by carrying liposomes coated with oligomannose [135].Oligomannose-coated liposome-incorporated macrophages can selectively accumulate in the micrometastatic foci formed by gastric cancer cells.
3.5.Modulating TIM
The various immune cells and cytokines secreted in the TIM play crucial roles in metastatic progression of tumor.At the primary tumor site, tumor cells evade host anti-tumor immune responses through immune escape mechanisms and remotely initiate the PMN for cell disseminations.TDSFs and EVs could facilitate MDSCs and other immune cells to migrate to potential PMN and remodel immune microenvironment.Further, the CTCs in blood recruit immune cells to facilitate their survival in circulation and help to form micrometastases.Therefore, to achieve the effect of immunotherapy, the nanosystem can be engineered with immune modulatory agents to remodel the TIM, block the immune checkpoints or regulate immune responses to improve the efficacy of anti-tumor therapy (Table 5).
MDSCs are major modulators of immune responses in cancer metastasis.They can be recruited to assisting in the construction of PMN and facilitate tumor immune escape.Therefore, disrupting the myeloid expansion and trafficking into metastatic sites help to restore the antitumor immunity in the tumor microenvironment.In one study, a fully synthetic nano-antibody was devised to replace immunotherapy-based monoclonal antibody.The novel nanoparticle-antibody possesses a Janus structure which is decorated with targeting ligands on one “face” and Fc-mimicking ligands on the opposite “face” [136].Systemic injection of MDSCstargeting nanoparticle-antibody can efficiently target and deplete circulating MDSCs in TNBC mice models and enable T cells and Natural Killer cells infiltration into tumors to enhance anticancer immunity.
The discovery of programmed cell death-ligand 1 (PD-L1)/PD-1 immune-suppressive checkpoint has led to a surge in new investigational therapies for the tumor immunotherapy.The neutralization of the signals can improve the capacity of T cell immune responses to eradicate tumor cells.Guet al.conjugated the antibodies against PD-L1 to the surface of platelets for blocking the immune checkpoints in CTCs and suppressing post-operative tumor recurrence and metastasis [137].The platelet-PD-L1 systems could be activated into platelet-derived microparticles with anti-PD-L1, thereby transporting to the residual micrometastases and CTCs in the blood circulation.Furthermore, these authors genetically engineered platelets from megakaryocyte progenitor cells to produce platelets of PD-1, thereby accumulating within the tumor surgical wound, reinvigorating CD8+T cells, and enhancing cancer immunotherapy after surgery [138].
4.Clinical implications
Although anti-metastasis therapy targeting pre-metastatic microenvironment is still at a relatively early stage, it opens up many exciting novel opportunities for the development of new diagnostic and therapeutic strategies.As discussed earlier in this review, the nanosystems that can directly neutralize primary tumorderived components, prevent the PMN establishment, eliminate the CTCs or micro-metastases, or modulate the TIM may be easier to cure than treating already established metastatic tumors.Currently,some drugs that potentially could target PMN are already available,such as denosumab and volociximab [139].An antiangiogenic drug,TSU68, can prevent the progression of hepatic metastases by inhibiting chemokine (C-X-C motif) ligand 1 (CXCL1) expression in the pre-metastatic sites [140].CellSearchTMtechnology based on antibody-coated magnetic nanoparticles has been approved by FDA for CTCs detection.These studies have all demonstrated that targeting various cellular and molecular components involved in premetastatic micro-environments development may be a powerful direction for inhibiting metastasis progression.
Many encouraging accomplishment have been achieved in the direction of nanosystems for pre-metastatic microenvironment in recent years, however, great challenges remain be resolved prior to their clinical application.Given the clinical translation and largescale production of nanosystems, biomaterials need to be biocompatible, biodegradable and bioabsorbable, and their preparation approaches should also be reliable and well-established.Moreover,the unanticipated off-target toxicity also results in clinical failure.Hence, the mechanisms of identification and interaction between nanosystems and target sites need to be better identified for further effective optimization.We should also pay more attention on the physical characteristics of nanovsystems, including particle sizes, polydispersity index, zeta-potential during their preparation and chemical modifications, which can greatly influence their fatein vivo.Despite progress in animal studies, tremendous efforts are still required to realize the effective targeting and treatment of metastatic tumors with nanotechnology for clinical translation.
5.Conclusions and perspectives
Mortality after progression of cancers to a metastatic disease is mainly caused by the absence of effective therapies.Despite great strides made by nanotechnology in treating primary tumors,targeting tumor metastasis has not been very successful due to the complexity of metastasis [141–143].Because primary tumorderived components (Evs, TDSFs), PMN, CTCs, micrometastases and TIM-interrelated cells and factors play pivotal roles in the progression of metastasis, developing novel strategies based on premetastatic microenvironment for treatment of the cancer metastasis at early stages is realistic.
Nanosystems that specifically bind to exosomes or absorb cell cytokines in blood have shown potential advantages in preventing cancer metastasis.Although the progression of cancer metastasis is blocked to some extent, a combination of chemotherapy or immunotherapy is required if good treatment results are achieved.CTCs have acted as an efficient biomarker and a means of studying the process of metastasis.These unique biomarkers or unique cells that bind specifically to CTCs, have been designed to nanosystems for specific targeting of CTCs.The strategy that neutralizing CTCs in the blood may represent a new paradigm for the intervention of metastases in distant organs.Monitoring and treatment of PMN and micrometastases would promote to optimize the properly timepoint of clinical intervention, prevent metastatic development before overt metastasis formation.Specific cellular components such as S100A8/A9, CXCR4, VLA-4, have bene regarded as potential biomarkers of the formation of PMN.These biomarkersbased nanosystems have been developed and exhibit excellent PMN-binding capacity.In addition, they also provide more optional approaches for preventing and controlling cancer metastasis, such as preventing the production of PMN-facilitating components, disrupting the inflammation niche, inhibiting recruitment of MDSCs,disrupting crosstalk among the local stroma, BMDCs, or immune cells within PMN, and re-activating of anti-tumor immunothetapy.
In this review, we summarize the current status of nanosystems for targeting premetastatic microenvironment irrelated factors and cells components, and reveal that novel premetastasisbased nanosystems can improve current therapies for tumor metastasis.The functionalized nanosystems, including micelles, liposomes, polymer nanoparticles, bioinspired nanoparticles and engineering cells, have been explored for directly neutralizing Evs and TDSFs, eradicating CTCs in the circulation, preventing the formation of the PMN and micrometastases and modulating the TIM.Despite advances in animal studies, substantial efforts are still required to achieve the valid targeted therapy of metastatic tumors with nanosystems for clinical translation.With further exploration of the underlying molecular mechanisms promoting metastatic formation and identification of their function on cancer metastasis,the new therapeutic strategies will be provided for eliminating metastatic disease.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos.92059110 and 81872808), Development Fund for Shanghai Talents (No.2020090), FDU 2025-Excellence Program Fund, National Key R&D Program of China (No.2017YFE0126900),Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZJLab.
杂志排行
Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
- The effect of organic ligand modification on protein corona formation of nanoscale metal organic frameworks
