Dendritic cells maturation facilitated by group-adjustable lipopolysaccharide analogues synthesized via RAFT polymerization
2022-09-15XingyuHengRuynFengLijunZhuLiyinYuGojinChenHongChen
Xingyu Heng, Ruyn Feng, Lijun Zhu, Liyin Yu, Gojin Chen, Hong Chen,∗
a College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China
b Center for Soft Condensed Matter Physics and Interdisciplinary Research, Soochow University, Suzhou 215006, China
ABSTRACT Transforming immature DCs into mature state to activate cellular immunity is a critical step in initiating immunoprophylaxis and immunotherapy.Lipopolysaccharides (LPS) can promote DCs maturation by binding receptor on DCs surface, but their clinical application is limited due to biological toxicity.Although many LPS analogues have been developed, complex synthesis and purification hinder their practical application.Here, we propose a novel and simple strategy to synthesize LPS analogues with adjustable structural units.Using monomer units similar to the key functional groups of LPS, we synthesize LPS analogues with different group ratios by RAFT polymerization.The obtained analogues have little negative effect on cell viability.Compared with LPS, the analogues show greater promoting effect on DCs maturation.And the analogues can be applied to different scenarios since the degrees of promoting DCs maturation by LPS analogues with different group ratios are different.This strategy provides a new direction for synthesizing LPS analogues, and it has the potential to produce LPS analogues on a large scale with tunable promoting DCs maturation effect.
Keywords:LPS analogues Glycopolymer RAFT polymerization Dendritic cells (DCs)Immune enhancement
Dendritic cells (DCs) are specialized antigen-presenting cells.In the mature state, they have the ability to activate T cells thereby enabling the body to obtain stronger cellular immunity[1,2].Therefore, mature DCs can be applied in immunoprophylaxis and immunotherapy [3], especially in the development of vaccine adjuvants [4,5] and the treatment of malignant tumors [6,7].At present, there are many substances to induce DCs maturation, such as cytokines (tumor necrosis factor-α(TNF-α), interferon-γ(INFγ)) [8], polypeptide (heat shock protein (HSP70)) [9] and polysaccharide (LPS, dextran) [10,11].Among these, polysaccharide is one of the most studied substances, especially lipopolysaccharide (LPS)and its analogues.
LPS is the main external component of the Gram-negative bacteria cell wall [12,13], which gives rise to the immune effect by promoting DCs maturation by binding to receptors on the surface of DCs [14].However, the structure of LPS is heterogeneous [15],and LPS has strong thermogenesis, which will cause strong immune response and trigger symptoms such as sepsis [16].Therefore, many LPS analogues have been prepared to further optimize biological activity and reduce biological toxicity [17,18].These analogues are usually prepared by the following two methods: (1)Modifying and separating LPS produced by natural strains or genetically modified strains, such as monophosphoryl lipid A (MPLA)and BECC438 [19,20]; (2) Synthesizing new analogues by modification of aliphatic chains and phosphate groups on the sugar ring,such as CRX547, CRX527 and RC-529 [21–23].Although the LPS analogues prepared by the above two methods have increased biological activity and decreased toxicity compared with LPS, and some have even been used as vaccine adjuvants [19,23], these two methods still have limitations.The structure of LPS analogues prepared by bacterial strains is not clear, and there may be bacterial contamination.Although the structure of LPS analogues prepared by the second method is clear, the synthetic steps are complicated and cumbersome [24].In addition, neither of the two methods can regulate the proportion of different structural units of LPS, which leads to the single function of the prepared LPS analogues [25].Therefore, it is necessary to develop a method for synthesizing LPS analogues which can adjust the structure and has simple synthesis steps with free from contamination.
Based on the above research, a simple molecular-mimicking strategy with universality was proposed to fabricate groupadjustable LPS analogues.First, we split the structure of LPS into three parts: sugar units, phosphate groups and aliphatic chains (Scheme 1a), and replaced these three parts with 2-methacrylamido-glucopyranose (MAG), 2-methacryloxyethyl phosphorylcholine (MPC) and dodecyl methacrylate (LMA), respectively.Subsequently, LPS analogues with different group ratios (PMMLn)are synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization (Scheme 1b).The synthesis process of group-adjustable LPS analogues prepared by the method is simple and easily operated which can realize the stable and rapid synthesis of LPS analogues.And by regulating different proportions of functional groups, LPS analogues with different effects on promoting the maturation of DCs can be prepared, thereby adapting to different application scenarios.
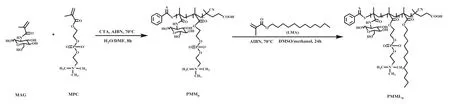
Scheme 2.Synthetic route of PMMLn by RAFT polymerization.
Scheme 2 shows the synthesis route of PMMLn, and the feed ratio of each monomer during the synthesis is shown in Table S1(Supporting information).The successful synthesis of PMMnand PMMLnwas confirmed by1H NMR (Figs.1a and b, Figs.S3–S5 in Supporting information) and infrared spectroscopy (Fig.1c, Figs.S6–S8 in Supporting information).Specifically, from the1H NMR spectra of PMMn, we can observe the characteristic peaks of the sugar ring in MAG and the tertiary amine in MPC, indicating that these two monomers were successfully copolymerized.In addition,the characteristic peak (δ4.0–4.4 ppm) of methylene group in the aliphatic chain of LMA appeared in the1H NMR spectra of PMMLn,which indicated that the LMA was successfully incorporated with PMMnby chain-extension polymerization.The infrared spectrum further confirmed this result, that is, the characteristic peaks of carbonyl (about 1633 cm−1) and amino (about 1532 cm−1) in the amide group and carbonyl (about 1730 cm−1) in the ester group appeared in the spectra of PMMnand PMMLn.The carbonyl signal of the ester group in the spectrum of PMMLnwas much intenser than that of PMMn.In conclusion, we successfully synthesized lipopolysaccharide analogs by RAFT polymerization.
Duo to low cost, rapid analysis, low detection limit, high sensitivity and accuracy, ICP-AES is often used to detect the content of trace elements in natural materials or synthetic materials to get the ratio of components [26,27].By detecting the absorbance of the groups at a specific wavelength, the microplate reader can de-tect the concentration of the groups or the compounds containing the groups [28,29].Therefore, we use ICP-AES in conjunction with the microplate reader to obtain the composition ratio and molecular weight of the polymers (Table 1).In addition, we measured the molecular weight of the polymers by SEC (Table 1 and Fig.S9 in Supporting information).Comparing the molecular weights obtained by the two ways, we find that the molecular weight of PMMnobtained by SEC is larger while the molecular weight of PMMLnis smaller.It can be explained that PMMnis composed of hydrophilic groups, so its state in the aqueous solution is loose leading to a higher molecular weight, while PMMLncontains both hydrophobic fragments and hydrophilic fragments, which make its polymer chain shrinkage in the aqueous environment, resulting in a lower molecular weight.Moreover, all the polymers have narrow polydispersity index, which indicates the polymerization is wellcontrolled.
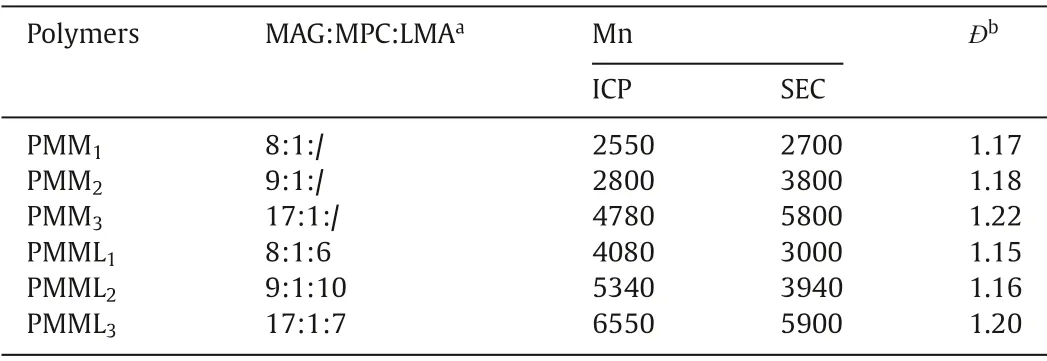
Table 1 Composition, molecular weights and PDI of PMMn and PMMLn.

Fig.1.(a) 1H NMR spectrum of PMMn (D2O).(b) 1H NMR spectrum of LPS mimetics PMMLn (DMSO-d6).(c) The FT-IR spectra of PMMn and PMMLn.
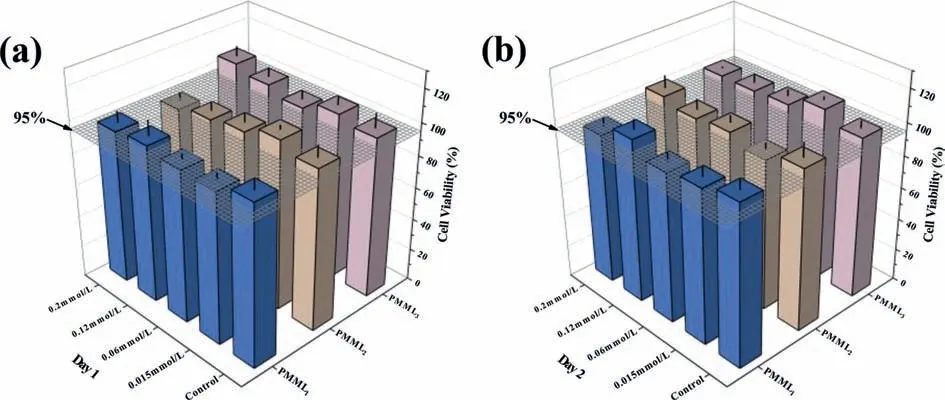
Fig.2.Effect of PMMLn at different concentrations on the viability of L929 cells.(a) Day 1, (b) Day 2.Error bars represent the standard deviation of the mean (n=6).
The viability of cells is easily affected by external substances,such as bacteria, viruses and some synthetic macromolecules [30–32].As a biomacromolecule that directly interacts with DCs, the low toxicity of LPS analogues is necessary for their subsequent experiments and applications.Therefore, we selected L929 cells,which is a commonly used cell model, to detect the effect of analogues on cell viability by CCK8 assay.We can see that the viability of the cells which were co-incubated with PMMLnremained at about 95% on the first day.And the viability of cells increases the next day, with most exceeding 95% and some reaching 100% (Fig.2).It means that the analogues we synthesized have little negative effect on cell viability, and some even promote cell proliferation.
When DCs mature, the proteins expressing on their surface will change.In addition to the existing toll-like receptors and mannose receptors, DCs will express a large number of CD80 and CD86 [33,34].Therefore, the evaluation of DCs maturation is accomplished by detecting the co-expression of these two proteins[35,36].In this study, we selected DC2.4 and used LPS as the reference to detect the co-expression of CD80 and CD86 by flow cytometry, to explore the effect of LPS analogues (PMMLn) on the maturation of DC2.4 (Fig.3a).As shown in Figs.S10–S12 (Supporting information), all analogues can promote the maturation of DC2.4, and the promoting effect is gradually enhanced with the increase of the concentration.To further determine which one has the better effect on promoting the maturation of DC2.4 between LPS and analogues, we measured the relative maturation of DC2.4 after co-cultured with LPS and analogues at the same concentration (120 μg/mL), and the results were shown in Fig.3b.Compared with LPS, the effect of PMML1, PMML2and PMML3are 1.6, 1.3 and 1.2 times that of LPS, respectively.In addition, we further compared the effects of the analogues at several different concentrations.As shown in Fig.3c, PMML1shows the relatively better consequence regardless of the concentration.To sum up, the LPS analogues synthesized by the method of “active unit recombination”have much better performance in promoting DCs maturation than LPS, and illustrate that excess sugar rings and aliphatic chains are not necessary.
In summary, inspired by the concept of "active unit recombination", we have synthesized a series of new LPS analogues by simple RAFT polymerization, of which the composition and molecular weight distribution are well controlled.All these analogues display excellent cytocompatibility and show stronger effect than LPS on promoting DCs maturation.Among the three LPS-mimicking polymers, PMML1(MAG:MPC:LMA=8:1:6), PMML2(MAG:MPC:LMA=9:1:10) and PMML3(MAG:MPC:LMA=17:1:7),PMML1is found to be the most potent analogue in promoting DCs maturation.This means that the excessive number of sugar rings and aliphatic chains can affect the interaction between the polymers and DCs.Therefore, PMML1, as the analogue we obtained with the best effect on fostering DCs maturation, can serve as a reference for the development of LPS analogues in the future.The method of synthesizing the LPS analogues in this paper can be used for preparing the LPS analogues which are suitable for different application scenarios by adjusting the ratio between different functional groups.Furthermore, it can serve as a universal method for the synthesis of other natural substances analogues.
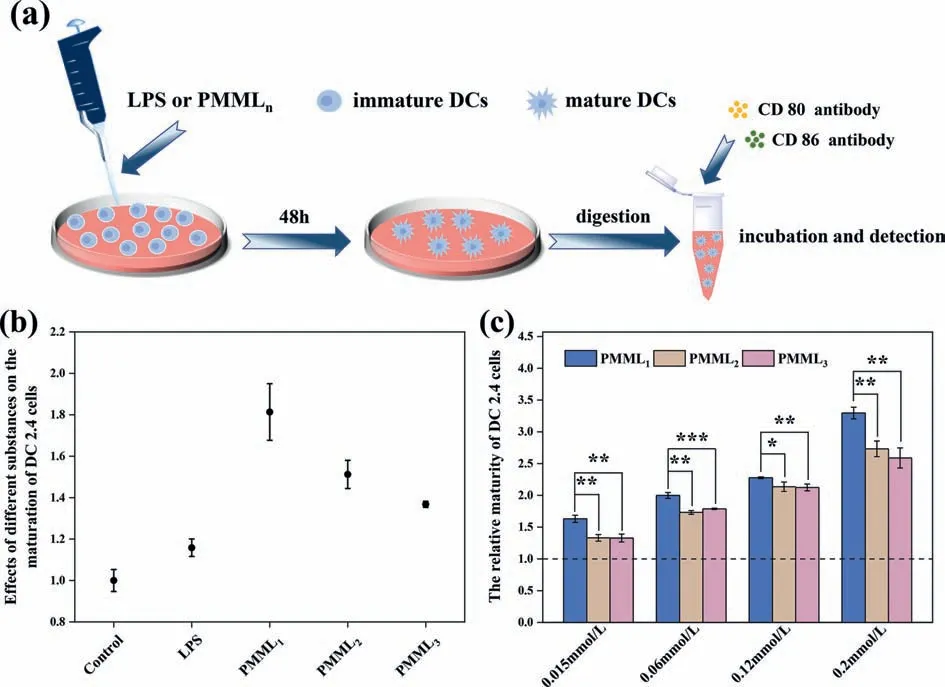
Fig.3.Comparing the difference in maturity of DC2.4 cells after co-incubation with LPS or its analogues by FlowJo.(a) Schematic diagram of immature DCs transformation into mature state.(b) Effects of different LPS analogues on DC2.4 cells maturation (Control is a blank control group, and the concentration of the experimental group is 120 μg/mL).(c) Relative maturity of DC2.4 cells co-cultured with different polymers at different concentration.Error bars represent the standard deviation of the mean (n=4,∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 by t-test).
Declaration of competing interest
The authors declare that there is no conflict of interest in the publication of this paper.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Nos.21935008 and 21774084) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.032.
杂志排行
Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
