A novel corneal adhesive based on functionally coupled PEG-lysozyme hydrogel for wound closure after surgical eye surgery
2022-09-15ShohuZhngHngZhouChngHungJinguoSunXueQuYiLu
Shohu Zhng, Hng Zhou, Chng Hung, Jinguo Sun,∗, Xue Qu, Yi Lu,∗
a Eye Institute and Department of Ophthalmology, NHC Key Laboratory of Myopia (Fudan University), Key Laboratory of Myopia, Chinese Academy of Medical Sciences, Shanghai Key Laboratory of Visual Impairment and Restoration, Eye & ENT Hospital, Fudan University, Shanghai 200031, China
b Key Laboratory for Ultrafine Materials of Ministry of Education, School of Material Science and Engineering, East China University of Science and Technology, Shanghai 200237, China
ABSTRACT Corneal wound closure for surgical eye surgeries or accidents is typically performed to prevent pathogens from the sterile intraocular environment and avoid potential postoperative complications.Tissue adhesives are increasingly employed for corneal wound closure with superior treatment efficiency and less adverse effects.In this study, we successfully develop a novel corneal adhesive based on functionally coupled PEG-lysozyme (PEG-LZ) hydrogels for wound closure after surgical eye surgeries.PEG-LZ hydrogels have plenty of micropores and gradually decreased pore size with increasing concentration from 10%,15% to 20% (w/v), in which PEG-LZ (15%) represents the suitable pH value, gelation time and elastic modulus.PEG-LZ hydrogels have no in vitro cytotoxicity and excellent ex vivo wound closure effectiveness in porcine eyes.The in vivo wound sealant in rabbit eyes by PEG-LZ hydrogels presents a superior therapeutic effect compared with the conventional methods of stromal hydration and suture, in terms of the wound closure percent, mean corneal thickness, percent of wound gaping, and the Descemet membrane detachment.PEG-LZ hydrogels do not induce obvious histological pathology changes.The PEG-LZ corneal adhesive is considered as a tissue adhesive alternative for wound closure after surgical eye surgeries.
Keywords:Corneal adhesive Hydrogel Wound closure Polyethylene glycol (PEG)Lysozyme
Corneal wound closure for surgical eye surgeries or accidents is typically performed to prevent pathogens from the sterile intraocular environment and reduce potential postoperative complications.The complications include inflammation, neovascularization, secondary infection, and endophthalmitis which is a catastrophic event to threaten sight [1].As the most common method to perform surgical eye surgeries, clear corneal incisions (CCIs) are increasingly preferred by surgeons owing to ease of construction,minimal astigmatism and faster visual rehabilitation [2].The conventional methods used to close CCIs include sutures, stromal hydration and sealants [3].Sutures are routinely used after surgery completion.Loose or broken sutures are more susceptible to leaks and infections, whereas tight sutures distort wound, increase astigmatism, and reduce visual acuity [4].Suture-assisted wound closures have advantages of better wound apposition and reduced risk of endophthalmitis [5,6], however, they possibly cause irritation,inflammation, and neovascularization [7].Stromal hydration is another routinely used method for corneal incision closure, which involves hydration of the lateral walls and roof of the main corneal incision to achieve wound apposition and self-sealing [8].Stromal hydration-assisted wound closures have advantages of convenience, reduced postoperative irritation and foreign body sensation, but they possibly result in postoperative intraocular pressure(IOP) fluctuations, decreased visual acuity and increased incidence of endophthalmitis [3,9].As a promising substitute, corneal adhesives are thus being developed and increasingly employed to overcome the shortcomings of sutures and stromal hydration for the closure of CCIs with superior treatment efficiency and less adverse effects than the conventional techniques.
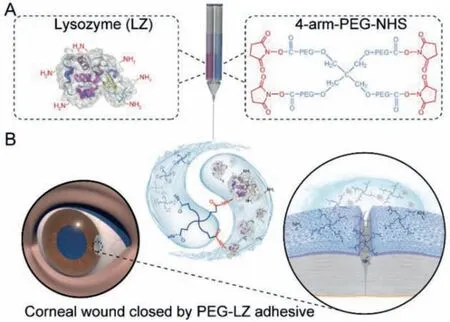
Fig.1.Schematic illustration of PEG-LZ hydrogel preparation by a specific chemical reaction of -NHS groups in PEG-NHS and -NH2 groups in lysozyme (A), and its clinical application as a corneal adhesive for incision closure after a surgical eye surgery(B).
The human eye has a highly complex architecture in which several layers of tissue are precisely organized to allow light passage.Thus, an ideal ocular adhesive should be biocompatible and comfortable for patients, which can be applied to seal the injured area easily and rapidly.Besides, after solidification, it should be transparent and refractive, permeable to nutrients and gases,but impermeable to microbials [10].Recently, many ocular adhesives have been developed based on cyanoacrylates, polyethylene glycol (PEG), proteins,etc.[11].Cyanoacrylate-based adhesives are synthetic, multipurpose hydrogels which offer a quick, effective and easy treatment of ocular wounds.However, they have several drawbacks including discomfort to patients, cytotoxicity, opaque and inflexible [12,13].PEG-based adhesives are frequently used for wound closure due to their good solubility in both non-polar and polar solvents, antifouling properties, nontoxicity, and low immunogenicity [14].As a PEG-based ocular adhesive, ReSure® is approved by FDA and specifically used to seal CCIs for the preclusion of fluid egress after cataract surgery [15].ReSure® adhesive is a hydrogel comprisingN-hydroxysuccinimide (NHS)-terminated 4-arm PEG prepolymer, trilysine amine crosslinker, buffering salts,and more than 89% water [16].Protein-based adhesives to repair ocular tissues have recently gained significant attention, such as fibrin and collagen (gelatin) [11,17].Fibrin-based sealants or glues,together with cyanoacrylate-based adhesives, remain the most commonly used suture substitutes in ophthalmology [11]; however, fibrin-based glues are poor in adhering to wet surfaces.Collagen (gelatin)-based adhesives exhibit appropriate adhesive effectiveness for corneal closure [18].As a naturally present protein in the corneal stroma, collagen is one of the most appropriate materials to produce ophthalmic sealants.As another common protein on the cornea surface, lysozyme also has excellent biology and chemical properties.For example, lysozyme can rapidly crosslink with NHS-based macromolecules by its reactive amine groups and form adhesive hydrogels [19].
In this study, we developed a novel corneal adhesive by functionally coupling 4-arm-PEG-NHS and lysozyme (LZ) to form PEGLZ hydrogels for wound closure after surgical eye surgeries.The gelation time, rheological property, micromorphology, and optical properties were investigated.Thein vitrocytotoxicity of PEG-LZ hydrogels was evaluated by human corneal epithelial cells (HCEC),human lens epithelial cells (HLEC), primary human Tenon’s fibroblasts (HTF) and human conjunctival epithelial cells (HConEpi).The corneal wound closure effectiveness of PEG-LZ hydrogels was investigated by theex vivowound sealant in porcine eyes andin vivowound sealant in rabbit eyes.The workflow is schematically presented in Fig.1.
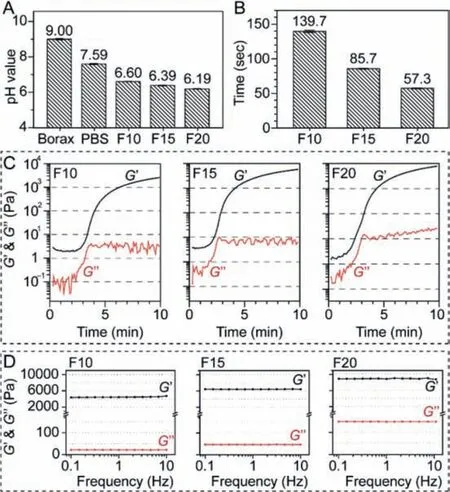
Fig.2.Properties of PEG-LZ hydrogels with increasing concentration from 10%, 15%to 20%.(A) pH values of borax buffer solution (Borax), phosphate buffer solution(PB) and PEG-LZ hydrogels (F10, F15 and F20); (B) Gelation times of PEG-LZ hydrogel precursors; (C) Time-dependent elastic and viscosity modulus of PEG-LZ hydrogels; (D) Dynamic frequency sweep curves of PEG-LZ hydrogels (F10: 10% w/v; F15:15% w/v; F20: 20% w/v).
In order to obtain a safe pH environment and a suitable gelation time for surgical eye surgeries, we combined phosphate buffer solution (PB, pH 7.59) and borax buffer solution (Borax, pH 9.00) to adjust the properties of PEG-LZ hydrogels [20].The purified LZ (Solarbio, China) was dissolved in Borax solution at a concentration of 10%, 15% and 20% (w/v).The 4-arm-PEG-NHS (SINOPEG, China) was dissolved in PB at a concentration of 10%, 15% and 20% (w/v).The two solutions were mixed at a 1:1 (v/v) to obtain three kinds of PEG-LZ hydrogels (F10: 10%, F15: 15%, F20: 20%).As a critical issue in the actual application of disease treatment, the final pH value of the PEG-LZ hydrogels was measured, and the results are shown in Fig.2A.The final pH values of F10, F15 and F20 were 6.60, 6.39 and 6.19, respectively, compared with those of Borax (pH 9.00) and PB (pH 7.59).In the crosslinking reaction of -NHS groups in the 4-arm-PEG-NHS and -NH2groups in the LZ, slight acid NHS compounds were sustainably released, which caused a decreasing pH value in the PEG-LZ hydrogels.However, the mean pH value on the ocular surface was 7.11, and any exposure to an acidic (pH<4) or an alkali (pH>10) solution can cause chemical eye injury [21].The-NH2groups are easily protonated and show more reactivity at a lower pH, while -NHS groups are easily hydrolyzed at a higher pH[22].Thus, weakly basic borax buffer (pH 9.00) and neutral PB (pH 7.59) were used to dissolute LZ and 4-arm-PEG-NHS, and neutralize the slight acid NHS compounds produced during the gelation process to obtain a safe pH value (4–10).Thus, F10, F15 and F20 formulations were relatively safe and comfortable when applied on the ocular surface.
As shown in Fig.2B, the gelation time of F10, F15 and F20 was 139.7, 85.7 and 57.3 s, respectively.The gelation time was directly associated with the concentration of 4-arm-PEG-NHS and LZ solutions.Rheological measurements demonstrated that PEGLZM hydrogels presented a time-dependent increase of elastic and viscosity modulus in F10, F15 and F20, which further confirmed the gelation process and time of PEG-LZ hydrogels (Fig.2C).The gelation time gradually shortened with increasing solution concentration from 10%, 15% to 20%.After solidification, the dynamic strain sweep of PEG-LZ hydrogels was investigated and the results showed that the linear viscoelastic region was 0.6%−3% (Fig.S1 in Supporting information).The dynamic frequency sweep of PEG-LZ hydrogels was investigated at the linear viscoelastic region (1%),and the results demonstrated that PEG-LZ hydrogels had almost constant elastic and viscosity modulus with the increasing shear frequency in F10, F15 and F20 (Fig.2D), which was attributed to typical elastic materials [19].PEG-LZ hydrogels represented an increasing elastic modulus from 4000, 6200 to 8500 Pa in F10, F15 and F20.The viscosity modulus also followed the same trend.It might be due to the continuously increased crosslinking density in the PEG-LZ hydrogelsviaconstructing abundant covalent and noncovalent bonds.A 4-arm-PEG-NHS molecular (Mw = 10,000 Da)has 4 -NHS groups, while a LZ molecular (Mw = 14,400 Da) has 18 free -NH2groups [23].The molar ratio of -NHS groups in the 4-arm-PEG-NHS and -NH2groups in the LZ used in this study was all set as 4:12.5 in the F10 (10%:10%), F15 (15%:15%) and F20(20%:20%).However, according to the results determined by the MALDI-TOF MS measurements (Fig.S2 in Supporting information),only 6 free -NH2groups per LZ molecular could react with -NHS groups in the linear PEG-NHS molecules.Thus, the possible molar ratio of -NHS and -NH2groups was 4:6.Furthermore, considering the steric hindrance effect of 4-arm-PEG-NHS molecules, the actually reacted -NH2groups per LZ molecular might be less than 6,and the actual molar ratio of -NHS and -NH2groups coupled in the PEG-NHS hydrogels might be close to 1:1, which is the theoretical ratio of functional groups in the reaction of -NHS and -NH2.
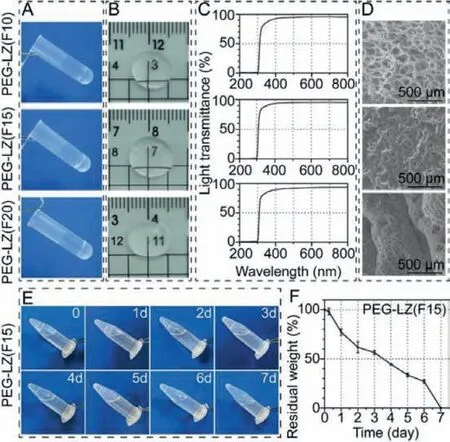
Fig.3.Gelation process, transparency, micromorphology and in vitro degradation of PEG-LZ hydrogels.(A) Photographic images of non-flowing hydrogels after crosslinking reaction; (B) Transparency; (C) Light transmittance curves; (D) SEM images; (E)Photographic images and (F) residual weight of the degraded PEG-LZ hydrogels.
After crosslinking reaction of 4-arm-NHS-PEG and LZ, nonflowing PEG-LZ hydrogels were obtained in the centrifuge tube(Fig.3A).As corneal adhesives, PEG-LZ hydrogels presented essential transparency which allowed suitable light transmission(Fig.3B).As shown in Fig.3C, three kinds of PEG-LZ hydrogels (F10,F15 and F20) provided approximately 97% relative light transmittance at a wavelength scale of 400 nm–800 nm, and then a sharp drop from 400 nm to 300 nm, until<1% at wavelength range below 300 nm.To be brief, PEG-LZ hydrogels exhibited high transmittance in the visible range (400–800 nm) and powerful blocking function in the UV range (200–300 nm).The micromorphology of PEG-LZ hydrogels was also observed by SEM and the results are shown in Fig.3D.The PEG-LZ hydrogels had plenty of micropores, and the average pore sizes presented a gradually-decreased trend with increasing polymer concentration from 10%, 15% to 20%.Totally, among the three kinds of PEG-LZ hydrogels (F10, F15 and F20) in terms of the final pH values, gelation times, elastic modulus and micromorphology, PEG-LZ (F15) hydrogels represented the most suitable pH value and gelation time, especially elastic modulus which was consistent with the reported wound sealant and the corneal stiffness [11].Furthermore, thein vitrodegradation of PEG-LZ (F15) hydrogels was investigated by incubating hydrogels(500 μL for each sample) in 250 μg/mL proteinase K solution with a shaker (37 °C, 80 rpm).As shown in Figs.3E and F, the PEGLZ (F15) hydrogels degraded gradually by 43.5% (residual weight,56.5%) at day 3 and diminished completely at day 7 after incubation treatment.
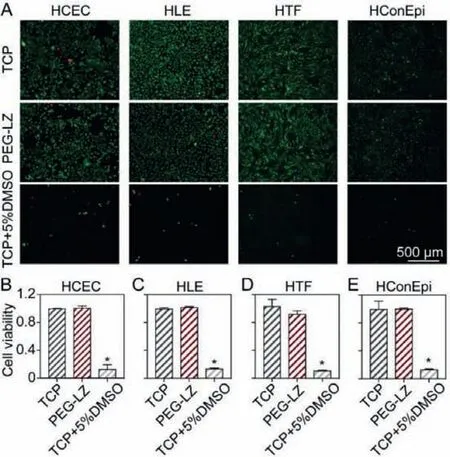
Fig.4.The live/dead cell staining images (A) and cell viability (B-E) of human corneal epithelial cells (HCEC), human lens epithelial cells (HLE), human Tenon’s fibroblasts (HTF) and human conjunctival epithelial cells (HConEpi) after incubation with PEG-LZ (F15) hydrogels for 24 h.Tissue culture plate (TCP) and 5% DMSO were set as negative and positive controls.The cell viability in TCP was thought as 100%(data were expressed as mean ± SD, n = 3).PEG-LZ (F15) hydrogels were placed in the transwell insert.F15: 15% (w/v).
Experimentations on human tissues adhere to the tenets of the Declaration of Helsinki, and the consent is obtained from the eye banks (No.0710020190002, Eye & ENT Hospital of Fudan University, China).Thein vitrocytotoxicity of PEG-LZ hydrogels was evaluated by co-culturing PEG-LZ (F15) hydrogels with HCEC, HLE, HTF and HConEpi cells, and observing live/dead cells and measuring cell viability by CCK-8 assay.The tissue culture plate (TCP) and 5% dimethyl sulfoxide (DMSO) were set as negative and positive controls.The results of live/dead cell staining and their cell viability are shown in Fig.4.No significant difference in the live/dead cell staining images was observed in the PEG-LZ (F15) hydrogels compared with the negative control after 24 h incubation, which showed noin vitrocytotoxicity on HCEC (Figs.4A and B).On the contrary, the positive control presented the most cell death and obviousin vitrocytotoxicity.The results of cell viability also confirmed the above trend, in which PEG-LZ (F15) hydrogels showed good biocompatibility (>95% cell viability), similar to the negative control.In comparison, the positive control produced obviousin vitrocytotoxicity (<5% cell viability).Moreover, these trends were also obtained when HLE, HTF and HConEpi cells were co-cultured with the PEG-LZ hydrogels to investigate thein vitrocytotoxicity (Figs.4A and C-E).Hence, the PEG-LZ hydrogels had apparent biosafety and were convenient for applications as a kind of wound sealants for corneal incision closures.
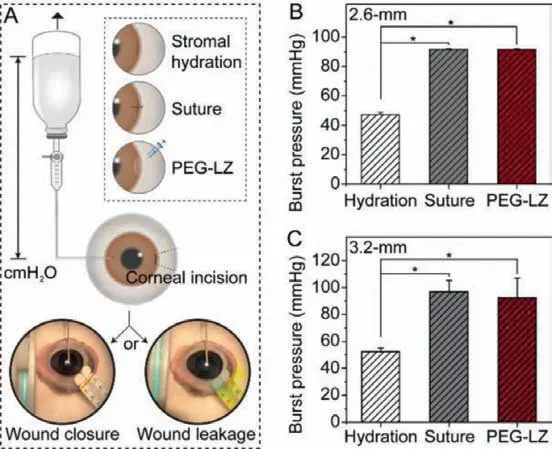
Fig.5.Preliminary ex vivo burst pressure evaluation.(A) Schematic description of the ex vivo procedures carried out in cadaveric porcine eyes, wound closure (left)or wound leakage (right); (B) Eye burst pressure of 2.6-mm-length corneal incision closed by stromal hydration, one-suture or PEG-LZ (F15) hydrogels; (C) Eye burst pressure of 3.2-mm-length corneal incision closed by stromal hydration, twosutures or PEG-LZ (F15) hydrogels.Wound leakage was confirmed by Seidel’s test in which fluorescein (2%) solution was applied to the incision areas and observed using a cobalt blue light.∗P<0.05 compared with the stromal hydration.
In this study, the adhesion strength of PEG-LZ (F15) hydrogels was confirmed by evaluating theex vivoburst pressure point in cadaveric porcine eyes with a corneal incision, at which the closed wound opened again.Human normal intraocular pressure (IOP)is below 21 mmHg (2.8 kPa), while high IOP (>21 mm Hg) is thought as ocular hypertension (TonoPen XL tonometer).The sustained high IOP causes glaucoma, even blindness.Two kinds of corneal incisions (length: 2.6-mm or 3.2-mm) were operated as eye injury models, which simulated the cornea trauma in the most common surgical eye surgeries, such as phacoemulsification, phakic intraocular lenses and implantable collamer lens [24,25].Two conventional methods for corneal wound closures, stromal hydration and suture, were used as controls.One-suture and two-sutures were performed for 2.6-mm and 3.2-mm incisions, respectively(Fig.5A).As far as the corneal incision with 2.6-mm and 3.2-mm length, the wound closure with stromal hydration only maintained IOP to 48 and 53 mmHg (Figs.5B and C), while the wound closure with PEG-LZ (F15) hydrogels maintained IOP to 92 mmHg,which provided almost same adhesion strength compared with the one-suture (91 mmHg) and two-sutures (97 mmHg).In general, the mean IOP increased significantly during the first 20–24 h after corneal incision surgeries, and 70% of patients had an IOP spike to 30 mmHg, even 52 mmHg [26].In the clinic, stromal hydration is the preferred method to close the corneal incision, while one-suture, even two-sutures are the enhanced wound closure method.Therefore, we thought the adhesion strength of PEG-LZ (F15) hydrogels was enough (same as the enhanced wound closure method) to meet the need for wound closures, in which the corneal incision was no more than 3.2 mm in length.
The preliminaryin vivowound sealant effectiveness of PEG-LZ hydrogels was assessed by administrating F15 hydrogels on the surgical corneal incision in rabbit eyes, and two kinds of common closure methods: stromal hydration and suture were used as the controls.Twelve rabbit eyes were classified into three groups(n= 4), and a single 2.8-mm linear incision was created in the limbus of rabbit eyes.Then, a 14-day follow-up was performed to validate the wound sealant effectiveness of PEG-LZ hydrogels.The corneal wound sealant effectiveness of PEG-LZ (F15) hydrogels was confirmed by IOP restoration (Fig.6A).There was no significant difference between these groups as baseline IOP values (P= 0.114).Immediately after intraocular irrigation and corneal incision closures, the IOP values in all groups increased and then decreased until steady state at day 14, which was similar to the physiological values preoperatively.However, the IOP changes in the three groups presented different trends after a provoke procedure.The IOP noticeably decreased after provoking procedure in the stromal hydration group, significantly lower than the physiological IOP value preoperatively.However, the IOP was basically stable in the PEG-LZ and suture groups, similar to the physiological IOP value postoperatively.The decreasing IOP value indicated sealing failure and continuously leaking from the corneal incision in the stromal hydration group, confirmed by the Seidel test (Fig.5A).The IOP values in the PEG-LZ and suture groups showed significantly higher than the stromal hydration group across all time points beyond day 1 after the surgery procedure (P <0.05).The wound closure percent in the stromal hydration group was 75% immediately postoperatively, and they were both 100% in the suture and PEGLZ (F15) groups.When provokes were performed on the treated wounds, all wounds in the stromal hydration group became leaked(wound closure percent: 0), while all wounds were kept closed in the suture and PEG-LZ (F15) groups (wound closure percent: 100%)(Fig.6B).
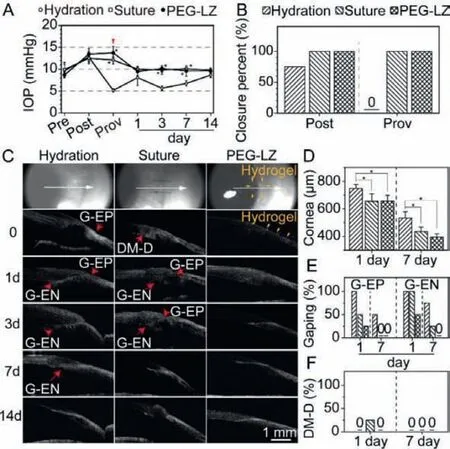
Fig.6.Corneal wound sealant effectiveness of PEG-LZ hydrogels in rabbit eyes with clear corneal incisions, compared with the common closure methods: stromal hydration and sutures.(A) IOP fluctuation preoperatively (Pre), immediately postoperatively (Post), after provokes (Prov), and during follow-up; (B) Wound closure percent immediately Post and after Prov; (C) Two-dimensional AS-OCT images of the corneal incisions during follow-up; Corneal thickness (D), percent of G-EP incidence and G-EN incidence (E), percent of DM-D incidence (F) in rabbit eyes at day 1 and 7 postoperatively.∗Represent P<0.05 versus stromal hydration.AS-OCT: anterior segment optical coherence tomography; IOP, intraocular pressure; G-EP, gaping at the epithelial side; G-EN, gaping at the endothelial side; DM-D, Descemet membrane detachment.
The architectural features of the CCIs in rabbit eyes were assessed according to the previous studies [27], including wound gaping at the epithelial side (G-EP) and endothelial side (G-EN),local detachment of the Descemet membrane (DM-D).The cornea images obtained by an anterior segment optical coherence tomography (AS-OCT) during the 14 days follow-up were shown in Fig.6C.The PEG-LZ (F15) hydrogel was clearly noticeable on the cornea surface immediately postoperatively, which disappeared at day 1, mainly due to the action of blinking and eyelid rubbing.Fortunately, the PEG-LZ (F15) hydrogels between the incision roof and floor still functioned to maintain the incision closure until wound recovery, with the gradual degradation of PEG-LZM hydrogels catalyzed by protease in the cornea surface [28].In the rabbit eyes, the mean cornea thicknesses in the stromal hydration, suture and PEG-LZ groups were 747.7 ± 29.6 μm, 655.0 ± 53.9 μm and 657.4 ± 41.4 μm at day 1, while 533.6 ± 46.0 μm, 434.8 ± 33.3 μm and 395.7 ± 22.4 μm at day 7 after the surgery operation (Fig.6D).The mean cornea thickness in the stromal hydration group was significantly higher than those in the suture and PEG-LZ groups(P<0.05).Moreover, the stromal hydration group tended to show a greater percent of G-EP incidence (100%), compared with those in the suture (50%) and PEG-LZ (25%) at day 1 after surgery operation, respectively (Fig.6E).On day 7 after the surgery operation, there were still 50% G-EP incidence in the stromal hydration group, while no G-EP incidence was found in the suture and PEGLZ groups.On the other side, both stromal hydration and suture groups showed a higher percent of G-EN incidence on day 1 and 7 after the surgery operation.PEG-LZ (F15) presented a relatively lower percent of G-EP and G-EN incidences, even no wound gaping was found on day 7 after the surgery operation (Fig.6E).Only one eye in the suture group showed local DM-D on day 1 after the surgery operation (Fig.6F).Totally, the experimental results demonstrated that the PEG-LZ hydrogel had a superior therapeutic effect for surgical wound closures and recovery compared with the common closure methods: stromal hydration and sutures.
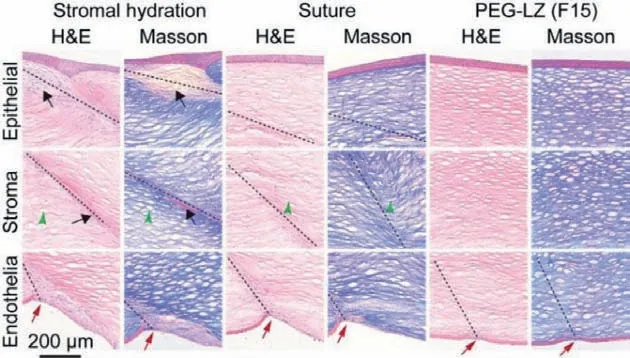
Fig.7.Representative images of histological cross-sections stained by hematoxylin and eosin (H&E) and Masson’s trichrome in the stromal hydration, suture and PEGLZ groups.Black arrows point to fibrotic cells.Green triangles show distorted collagen fibers.Red arrows indicate Descemet membrane discontinuities.PEG-LZM hydrogel disappears because of its biodegradation catalyzed by proteases in the cornea surface.
Thein vivobiosafety of PEG-LZ hydrogel was performed by histological analysis at week 2 after surgery operation to investigate the potential adverse events.The obtained images of H&E and Masson staining are shown in Fig.7.The stromal hydration group presented more epithelial and endothelial cell proliferation than suture and PEG-LZ groups, which showed more multilayered morphology.Besides, many fibrotic cells (black arrows) in the wound area in the stromal hydration group formed hypercellular interwoven tissues under the epithelium layer.In the suture group, the epithelial side showed normal recovery, but the stroma area sprouted some distorted collagen fiber layers (green triangles).The disorganized Descemet’s membrane (red arrows) with abnormal corneal endothelial cells was observed in the stromal hydration and suture groups.The Descemet’s membrane was discontinuous in the PEG-LZ group but showed normal epithelial side and stroma layer recovery.These findings suggest that PEG-LZ hydrogel did not induce distinct tissue toxicity and presented goodin vivobiosafety.
Wound adhesives have emerged as attractive alternatives to sutures due to excellent properties, including fast, atraumatic and painless wound treatment, efficient prevention of body fluid leakage or hemostasis, strong tissue bonding, minimal scarring,etc.[17,29].Although fibrin-based, PEG-based and cyanoacrylate-based adhesives have already been widely used in clinic to reduce complications and improve outcomes [16], they still have some problems or weaknesses, such as weak adhesion strength or potential safety concerns.Thus, those commercialized products are still far from satisfactory.With further studies on adhesion mechanisms and synthesis methods [30,31], it is completely believed that more powerful and effective adhesives will be developed in future,which will revolutionize surgical processes
In this study, a novel corneal adhesive based on functionally coupled PEG-LZ hydrogels was successfully developed for wound closure after surgical eye surgeries.PEG-LZ hydrogels had plenty of micropores and gradually decreased pore size with increasing concentration of PEG and lysozyme, and represented the suitable pH value, gelation time and elastic modulus.PEG-LZ hydrogels had noin vitrocytotoxicity on human corneal epithelial cells, human lens epithelial cells, human Tenon’s fibroblasts and human conjunctival epithelial cells.The treatment effectiveness of the PEG-LZ hydrogels was confirmed by theex vivowound closure in porcine eyes andin vivowound sealant in rabbit eyes.The PEG-LZ hydrogels presented a superior therapeutic effect compared with the conventional methods of stromal hydration and suture, in terms of the wound closure percent, mean corneal thickness, percent of wound gaping and Descemet membrane detachment.PEG-LZ hydrogels did not cause obvious histological pathology changes.It is believed that the PEG-lysozyme hydrogel is a novel corneal adhesive alternative for wound closure after surgical eye surgeries.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the support from the National Natural Science Foundation of China (Nos.81970780, 81670835 and 31922041), the Scientific and Innovative Action Plan of Shanghai(No.19441900600), and the Natural Science Foundation of Shanghai (Nos.15ZR1405900, 19ZR1408300).The sponsor or funding organization had no role in the design or conduct of this research.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.024.
杂志排行
Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
