Access to 3-alkylselenindoles by multicomponent reaction of indoles,selenium powder and unactivated alkyl halides under transition-metal-free conditions
2022-09-15HuanLiuZhongJianCaiShunJunJi
Huan Liu, Zhong-Jian Cai, Shun-Jun Ji
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, China
ABSTRACT Herein, we reported a convenient and efficient multicomponent reaction of indoles, selenium powder and unactivated alkyl halides.This protocol provides a practical, and facile approach for the synthesis of 3-alkylselenindole derivatives.The advantages of this strategy include mild and transition-metal-free conditions, broad functional group tolerance, the use of simple and easily accessible seleniium powder and alkyl halides as coupling partners.More importantly, the reaction proceeded smoothly with a large scale (>10 g, >90% yield), which further highlighted the potential application of this selenation strategy.
Keywords:Selenium 3-Alkylselenindole Indole Selenization
Organoselenium compounds have attracted enormous attention over the past decades due to their unique biological and medicinal activities [1–5].For example, organoselenium compound Ebselen shows excellent anti-inflammatory, anti-oxidant and cytoprotective activities [6–10], and the latest research shows that Ebselen can effectively inhibit the COVID-19 protease, which made it receive an increasing attention [11].On the other hand, the 3-selenylindoles also exhibited unique bioactivities, such as antiproliferative activity [12], anti-inflammatory activity [13] and act as an inhibitor of tubulin polymerization [14].Therefore, the development of a convenient and efficient method for the synthesis of 3-selenylindoles would be of important synthetic value.
In 2014, Braga and co-workers reported a microwave irradiation-assisted I2catalyzed selenation of indoles using diselenides as selenium source (Scheme 1a) [15].One year later,they modified this reaction by using a catalytic amount of K2CO3to give an alternative and greener protocol for the synthesis of 3-selenylindoles [16].Unfortunately, these methods are limited to aryl diselenides, and the synthesis of 3-alkylselenindole derivatives are still challenging.Soon after, the Liu’s group reported a highly efficient, visible-light-mediated aerobic selenation of indoles with diselenides (Scheme 1b) [17].Although some dialkyl diselenides could also be successfully applied to this reaction, an expensive iridium catalyst and the pre-prepared diselenides are required for this transformation.In 2016, Wu’s group reported a powerful copper-catalyzed C3aryl selenization of indoles using elemental selenium as the selenium source (Scheme 1c) [18].However, a transition-metal catalyst and high temperature are required, and this method is limited to the synthesis of 3-arylselenindoles.
After that, many other protocols also provide efficient access to a wide variety of 3-selenindoles arising from different combinations of indoles and coupling partners [19–26].However, most of the present methods are mainly focused on the synthesis of 3-arylselenindoles and the selenium source are typically limited to some pre-prepared selenating reagents.Therefore, the development of convenient and efficient approach for C3alkylselenization of indoles, employing easily accessible and eco-friendly selenium powder as selenium source, is highly desirable.Given our continuous interest in the divergent synthesis of biologically-relevant organoselenium compounds [27–32], herein, we reported a convenient and efficient multicomponent reaction of indoles, selenium powder and unactivated alkyl halides, which provides a practical,and facile approach for the synthesis of 3-alkylselenindole derivatives (Scheme 1d).
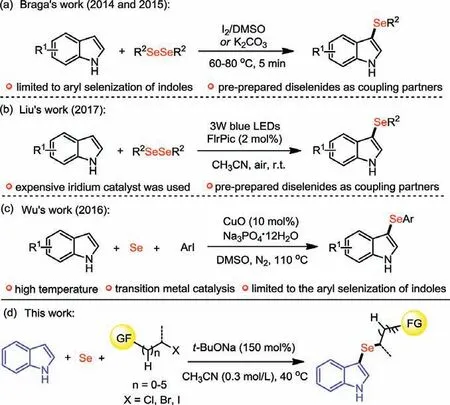
Scheme 1.Synthetic strategies for 3-selenylindoles.
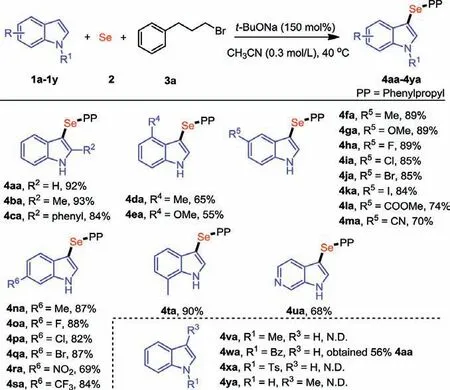
Scheme 2.Substrate scope of indoles.Reaction conditions: 1a (0.36 mmol), 2(0.36 mmol), 3a (0.30 mmol), t-BuONa (0.45 mmol), CH3CN (1.0 mL) at 40 °C for 6 h.Isolated yields.
We initiated our studies by examining the reaction of 1a, 2 and 3a under basic conditions.Gratifyingly, the desired product 4aa was formed in 65% isolated yield in the presence of Cs2CO3.After extensive screening of a series of base and solvents (see Supporting information for details), the highest isolated yield (92%) was obtained under the optimal conditions.
With the optimized reaction conditions in hand, we firstly examined the scope and limitations of indoles 1 (Scheme 2).Satisfactorily, both substrates bearing electron-donating (4ba−4ea, 4fa,4na and 4ta) and electron-withdrawing groups (4ha−4ma, 4oa-4sa) could react smoothly to give the desired products in moderate to excellent yields.We investigated the reaction efficiency by changing the substituted positions of the indoles.The expected products were observed in 87%−93% yields when a methyl substituent located on the C2, C5, C6 or C7 position of indole (4ba,4fa, 4na and 4ta) were applied to the reaction.The yields were reduced slightly when 4-methylindole and 4-methoxyindole were subjected to the reaction (4da and 4ea, 65% and 55%, respectively),which indicated that, compared to C2 position, C4 position had a more significant effect on C3 position from steric hindrance.Other substrates bearing electron-withdrawing groups, such as halogen(4ha-4ka, 4oa-4qa), ester (4la), cyano (4ma), nitro (4ra) and trifluoromethyl (4sa) moieties, were also compatible with the reaction, affording the corresponding products in 69%−89% yields.To our delight, the reaction of 1H-pyrrolo[2,3-c]pyridine succeeded to furnish the desired product 4ua in 68% yield.It is noteworthy thatN-methyl, benzoyl, and Ts substituted indoles (1v-1x) failed to give the desired selenylindoles, which indicated free NH group plays a crucial role in this transformation.Among them, a deprotection product 4aa was obtained whenN-benzoyl indole was applied to the reaction.
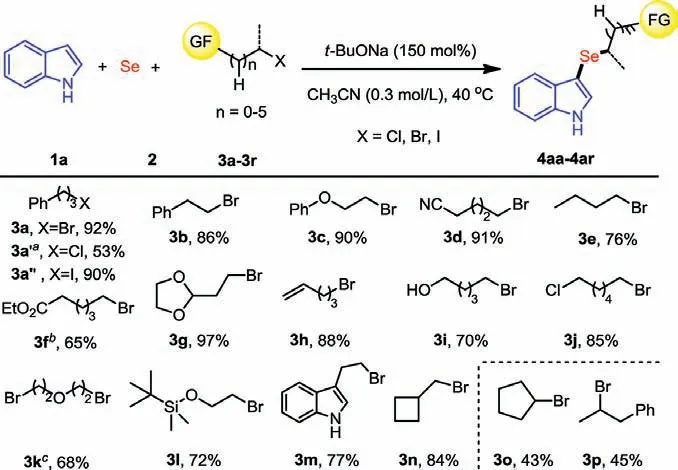
Scheme 3.Substrate scope of alkyl halides.Reaction conditions: 1a (0.36 mmol), 2(0.36 mmol), 3 (0.30 mmol), t-BuONa (0.45 mmol), CH3CN (1.0 mL) at 40 °C for 6 h.Isolated yields. a12 h. b150 mol% DBU were instead of t-BuONa. c1a (0.72 mmol), 2(0.72 mmol), 3k (0.30 mmol), t-BuONa (0.90 mmol), CH3CN (2.0 mL) at 40 °C for 6 h.
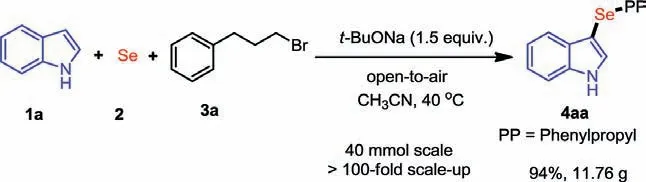
Scheme 4.Scale-up synthesis of 4aa.
To further evaluate the generality of this strategy, we next detected the tolerance of unactivated alkyl halides (Scheme 3).In generally, a wide variety of alkyl halides bearing different functional groups worked well under standard conditions.It was found that a similar good result was obtained when alkyl iodide 3a’’was used to instead of bromide 3a, and alkyl chloride 3a’ also performed well under standard conditions, albeit a lower yield(53%) was observed.The primary alkyl bromides containing functional groups such as ether (3c), cyano (3d), ester (3f), acetal(3g), alkene (3h), alcohol (3i), chloride (3j), silyl ether (3l) and cyclobutene (3n), were all well-tolerated under these mild reaction conditions.In addition, alkyl dibromide was also successfully employed to provide the diselenide product 4ak in 68% yield.Notably, 3-(2-bromoethyl)-1H-indole was also tolerated under the identical reaction conditions, affording the corresponding product 4am in 77% yield.Moreover, it was found that the secondary alkyl bromides, including cyclic and chain secondary bromides,provided the desired products 4ao and 4ap in lower yields (43%and 45%, respectively) alone with ∼50% starting material 1a recovered.Furthermore, tertiary alkyl bromides, such astert–butyl bromide and adamantyl bromide failed to give the selenization product.
To further investigate the potential application of this multicomponent reaction, we tried to increase the reaction scale from 0.3 mmol to 40 mmol (>130-fold).To our delight, the selenization product 4aa was still be isolated in 94% yield (11.76 g) under simple and mild conditions (Scheme 4).
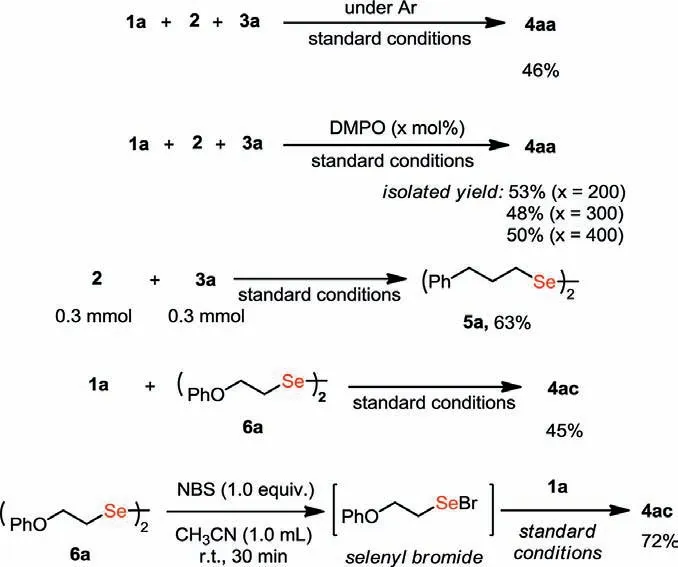
Scheme 5.Preliminary mechanism investigation.
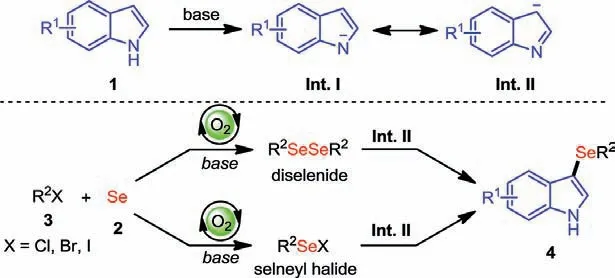
Scheme 6.A plausible mechanism.
To gain a deep insight into the reaction mechanism, several control experiments were carried out.Firstly, it was found that the isolated yield of 4aa was drastically reduced from 92% to 46%, when the model reaction was conducted under an argon atmosphere (Scheme 5a).This result proved that the presence of oxygen was important for this transformation.When the radical scavenger DMPO (5,5-dimethyl-1-pyrrolineN-oxide) was added to the system, 4aa could still be produced smoothly.Even when DMPO was increased to 400 mol%, 4aa could still be isolated in 50% yield (Scheme 5b).This experiment indicated that the reaction might not proceed through a radical pathway.A diselenide 5a was obtained in 63% yield when the reaction was carried out in the absence of indole (Scheme 5c).In addition, the selenization product 4ac was obtained in 45% yield by treating a diselenide 6a with indole under standard conditions (Scheme 5d).Finally, it was found that the corresponding product 4ac was obtained in 72% yield when the in-situ generated selenyl bromide species [33] was treated with indole 1a under standard conditions (Scheme 5e).These results indicated that the diselenide or selenyl bromide specie was likely to be a key intermediate in this transformation.
On the basis of the above experimental results and previous literatures [34,35], a plausible reaction pathway is proposed to elucidate the reaction mechanism (Scheme 6).The cleavage of the acidic N-H of indole usually requires basic conditions to enable the nucleophilicity of C3 position, which generates a negative charge on the indole (Int.II).Probably, a diselenide intermediate or a reactive selenyl halide intermediate is generated when the alkyl halide is treated with selenium powder under basic and aerobic conditions, which would react with Int.II to give the corresponding 3-alkylselenindole derivatives.
In summary, we have developed an efficient selenium-transfer approach [36] for the synthesis of 3-alkylselenindole derivatives through a multi-component “one pot” reaction.Mechanistic study demonstrated that selenium powder and alkyl halides might undergo an “oxidation-addition” process to produce selenyl bromide species.This strategy efficiently synthesized various functional 3-alkylselenoindoles that have been rarely reported before under mild conditions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge the National Natural Science Foundation of China (No.21672157), PAPD, the project of scientific and technologic infrastructure of Suzhou (No.SZS201708), Natural Science Foundation of Jiangsu Province (No.BK20200874) and Natural Science Foundation for colleges and universities in Jiangsu Province(No.20KJD150001).We thank Zhi-Peng Han in this group for reproducing the results of 4aa, 4ba, 4ta, 4ag and 4ah.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.046.
杂志排行
Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
