Chlospicenes A and B, cyclopropane cracked lindenane sesquiterpenoid dimers with anti-nonalcoholic steatohepatitis activity from Chloranthus henryi
2022-09-15JixinLiZhirongCuiYongyiLiChunhuHnYnqiuZhngPengfeiTngLetinCuiHoZhngJunLuoLingyiKong
Jixin Li, Zhirong Cui, Yongyi Li, Chunhu Hn, Ynqiu Zhng, Pengfei Tng,Letin Cui, Ho Zhng, Jun Luo,∗, Lingyi Kong,∗
a Jiangsu Key Laboratory of Bioactive Natural Product Research and State Key Laboratory of Natural Medicines, School of Traditional Chinese Pharmacy,China Pharmaceutical University, Nanjing 210009, China
b School of Pharmacy, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, China
ABSTRACT Guided by MS/MS molecular networks strategy, chlospicenes A and B (1 and 2), the first example of cyclopropane moiety cracked lindenane sesquiterpene Michael addition dimers, along with their biogenetic analogues (3 and 4), were targetedly discovered from the roots of Chloranthus henryi.Their structures including absolute configurations were characterized by NMR, ECD and X-ray diffraction analysis.The plausible biogenic pathway speculation indicated that cyclopropylcarbinyl rearrangement may dominate the key crack of cyclopropane moiety.In addition, compounds 1 and 2 showed significant anti-nonalcoholic steatohepatitis (NASH) activity in free fatty acid (FFA)-induced HepG2 cells by decreasing intracellular lipid accumulation.
Keywords:Chloranthus henryi.Lindenane sesquiterpenoid dimer Molecular networks Cyclopropylcarbinyl rearrangement Anti-nonalcoholic steatohepatitis
In China, many plants of the genusChloranthusare used as folk herbal medicines for the treatment of rheumatic arthralgia, traumatic injuries, fractures, neurasthenia, and pulmonary tuberculosis[1].Lindenane sesquiterpenoids and their polymers, recognized as the characteristic taxonomic symbol of the Chloranthaceae family[2], were the research hotspot of natural products discovery and synthesis [3–6].An overview of the molecular structures of lindenane sesquiterpenoid dimers (LSDs) reveals that the intermolecular Diels-Alder reaction betweenΔ15(4),5(6)andΔ9′(8′)was the main polymerization mode, and other [4+2] cycloaddition, [2+2]cycloaddition, [6+6] Michael addition or acetalization also lead the diverse carbon skeletons [7].The highly conjugated lindenane sesquiterpenoids moiety and unique polycyclic structural skeletons of polymers lead to various physiological activities, such as inhibition of tyrosinase [8], inhibition of the expression of cell adhesion molecules [9], rectifier K+current [10].Our recent experience in the field of LSDs [11–14], discovering unique structures and significant biological activity, indicated that plants from the Chloranthaceae family were the treasury for molecules with unprecedented architectures and interesting biological properties, but must be under the guidance of certain strategies.
With the growing technological advances, molecular networks(MNs) have been becoming a useful strategy that can facilitate the isolation of low-abundant bioactive natural products with specific structural features [15,16].Under the guidance of this strategy, the efficient discovery of four lindenane-monoterpene heterodimers fromSarcandra glabrawere achieved in our group [13].Thus, the searching of novel LSDs inChloranthus henryi[17], was targeted conducted based on MNs.Consequently, chlospicenes A (1) and B(2), two novel lindenane sesquiterpenoid Michael addition dimers with 2,3-secolindenane moiety, were distinguished from an MNs cluster and targetedly discovered (Figs.1 and 2).Biogenetically, cyclopropylcarbinyl rearrangement may dominate the key crack of the between C-2 and C-3 bond of cyclopropane moiety.Herein, we report the targeted isolation by MNs, structural elucidation, possible biogenetic pathway, and anti-nonalcoholic steatohepatitis activity of these two novel LSDs.
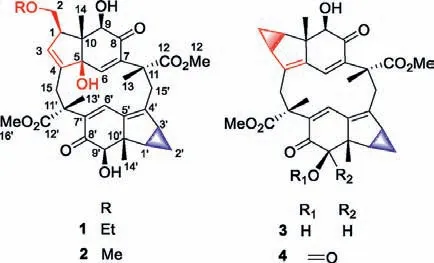
Fig.1.The structures of compounds 1–4.
An MS/MS molecular networking strategy was used to find the potential novel LSDs as our previous research [13,14].The portion of CH2Cl2of air-dried roots ofC.henryi(4.9 kg) was subjected to silica gel column chromatography to yield six fractions (Fr.A–F), respectively.Then all fractions were profiled by HPLC-HRESI-MS/MS (positive-ion mode) with a full scan and processed on the Global Natural Product Social Molecular Networking (GNPS; www.gnps.ucsd.edu) platform [15,16].The metabolites of two nodes of a cluster in fraction B (Fig.2 and Fig.S5 in Supporting information) were identified as LSDs with Michael addition skeleton cycloshizukaol A (3) and japonicone B (4) by using our isolated references [18,19].The above analysis indicated that another two nodes with higherm/z(611.285 and 597.270) may be with attractive structures.Then, the first example of cyclopropane cracked LSDs, chlospicenes A and B (1 and 2), were targeted purified and identified from fraction B.
Chlospicene A (1) was obtained as a triclinic crystal.Its molecular formula of C34H42O10was established by positive HRESIMS atm/z633.2675 [M+Na]+(calcd.for 633.2670) and indicated 14 indices of hydrogen deficiency (IHDs).From 1D NMR data (Table 1),two obvious methoxyl signals atδH3.36 and 3.62, key carbonyl signals atδC176.0 and 175.4 and keto-carbonyl signals atδC198.9 and 198.5, along with two oxymethylenes atδH3.70 and 3.58 and two olefin proton atδH7.04 and 6.73 were observed.Combing the above NMR evidence and MS/MS molecular networks implied that compound 1 was possibly an LSD dimer as that of cycloshizukaol A and japonicone B [18–20].
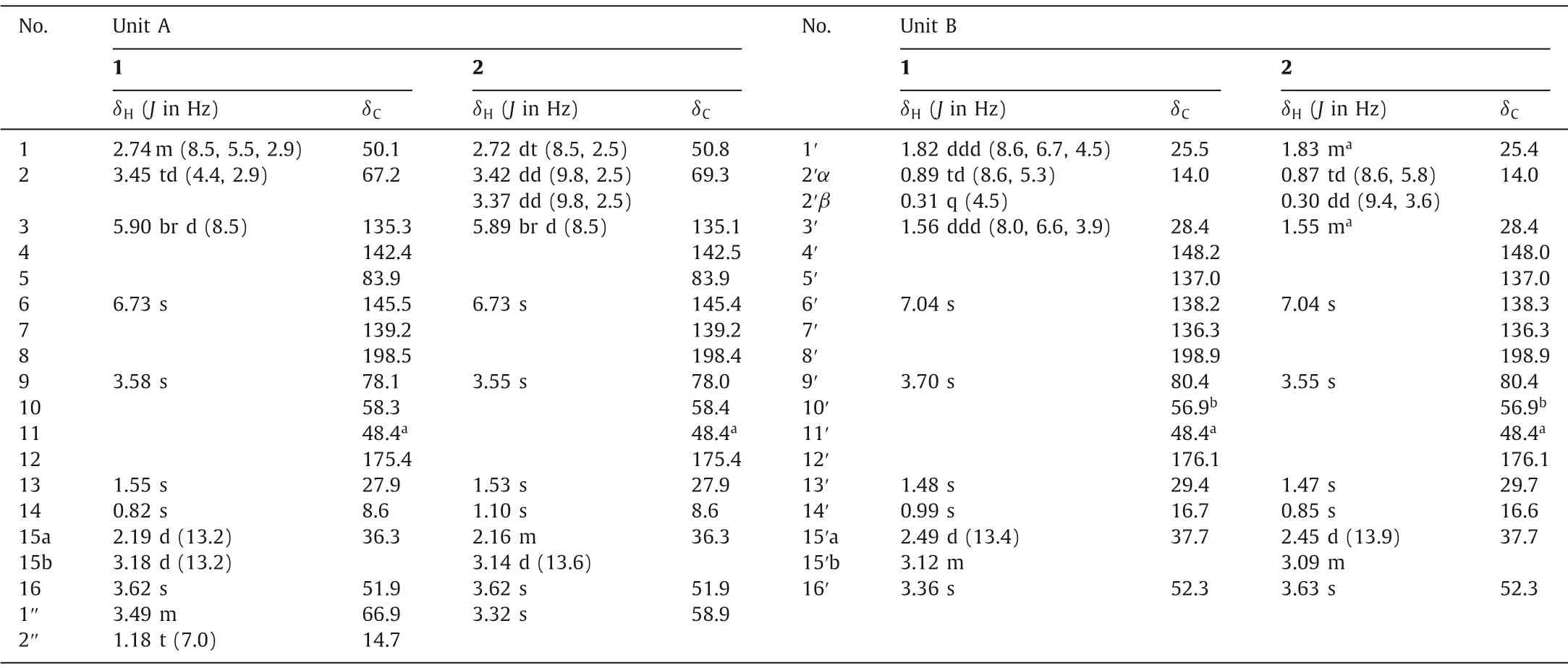
Table 1 1H (600 MHz) and 13C (150 MHz) NMR spectroscopic data for 1 and 2 in CDCl3.
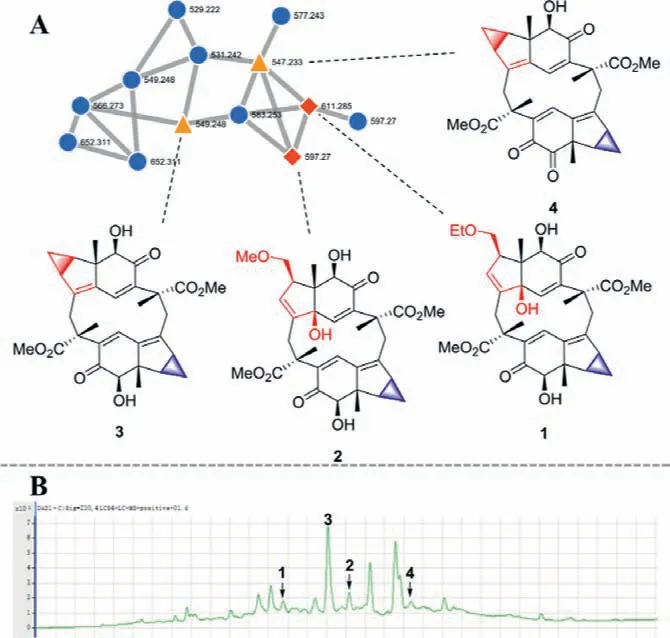
Fig.2.(A) A cluster for LSDs and structures of compounds 1–4.(B) ESI (+)-TIC full scan mass spectrum of fraction B.
The planar structure of compound 1 was established by interpretation of its 2D NMR spectroscopic data, which resolved the detailed assignments of two lindenane sesquiterpene units (A and B)in the aforementioned.The units A and B were linkedviaC-15/C-11′ and C-15′/C-11 and forming a 12-membered ring core like cycloshizukaol A was fixed by the key HMBC correlations from H2-15 to C-11′/C-12′, from H2-15′ to C-11/C-12, from H3-13 to C-15′, from H3-13′ to C-15.Thus, the basic lindenane sesquiterpenoid [6+6]Michael addition dimeric skeleton in a 12-membered ring was determined.Interpretation of its1H-1H COSY spectrums revealed three spin-coupling systems of H-1/H2-2/H-3, H-1′/H2-2′/H-3′,H2-1′′/H3-2′′, as drawn with bold bonds (Fig.3), including a pairs of specific upfield protons atδH0.31 (H-2′β, q,J=4.5 Hz)/0.89(H-2′α, td,J=8.6, 5.3 Hz) of unit B.The absence of characteristic cyclopropane proton signals for unit A indicated that the structural differences may occur at this moiety.Observed HMBC correlations (Fig.3) from olefinic H-3 (δH5.90) to C-1 (δC50.1), C-2 (δC67.2), C-4 (δC142.4), C-5 (δC83.9), indicated that the cyclopropane was cracked between C-2 and C-3, and forming ethylated hydroxymethyl branch by the HMBC correlations from H2-1′′ (δH3.49)to C-2 (δC67.2), andΔ3(4)double bond.At this stage, the cyclopropane cracked lindenane sesquiterpene Michael addition dimer skeleton of 1 was constructed.
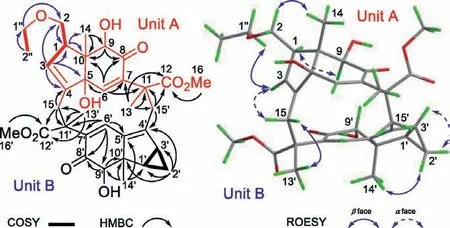
Fig.3.Key 2D NMR correlations of 1.
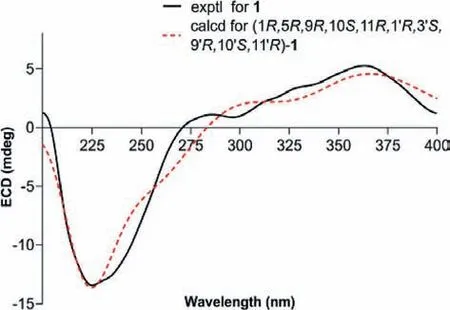
Fig.4.Experimental and computational ECD spectra of 1.
Similar ROESY correlations as those of cycloshizukaol A [18],such as H-1/H-9, H-1′/H-9′, H-2′α/H-3′, indicated that these protons were co-facial andα-oriented.In contrast, the ROESY crosspeaks of H3-14/H-2 and H3-14′/H-2′βrevealed that CH3-14 and CH3-14′ wereβ-configuration.In conclusion, newly ethylated hydroxymethyl branch (C-2) was adopted asβ-orientation.To conform the structure and establish the stereochemistry, the single crystals of 1 were obtained in methanol/water (9:1) system and subjected to an X-ray diffraction experiment with Mu Kαradiation [CCDC number: 2019872, Flack parameter: 0.0(2)].However,the quality of the crystals is insufficient to determine its absolute configurations, but the ethylated hydroxymethyl branch (C-2) from cracked cyclopropane moiety of unit A was very clear.(Fig.S1 in Supporting information).Subsequently, the absolute configuration of 1 was confirmed as 1R,5R,9R,10S,11R,1ʹR,3ʹS,9ʹR,10ʹS,11ʹRby consistent electronic circular dichroism (ECD) curves between experiment and theoretical calculation (Fig.4) [21].Hence, the novel structure of 1 was depicted as shown in Fig.1.
Chlospicene B (2), a white amorphous powder, was assigned to a molecular formula of C33H40O10by positive HRESIMSm/z619.2511 [M+Na]+(calcd.for 619.2514).The MS/MS molecular networks and 1D NMR data (Table 1) displayed that 2 also possesses a 12-membered ring core closely related to 1, together with a cyclopropane cracked.Comparison of the NMR data of 2 with those of 1, the noticeable differences in that the replacement of the ethoxy C-1ʹʹ(δC66.9) and C-2ʹʹ(δC14.7) in 1 with a methoxy C-1ʹʹ(δC58.9) of 2, which was verified by HMBC correlation(Fig.S2 in Supporting information) from H3–1ʹʹ(δH3.32) to C-2(δC69.3).The relative configurations of 2 were determined based on analysis of the ROESY spectrum, which is highly similar to 1(Figs.S1 and S2).The absolute configuration of 2 was also determined as 1R,5R,9R,10S,11R,1ʹR,3ʹS,9ʹR,10ʹS,11ʹRbased on the ECD theoretical calculations (Fig.S3 in Supporting information), and completely consistent experimental ECD curves of 1 and 2 (Fig.S4 in Supporting information).
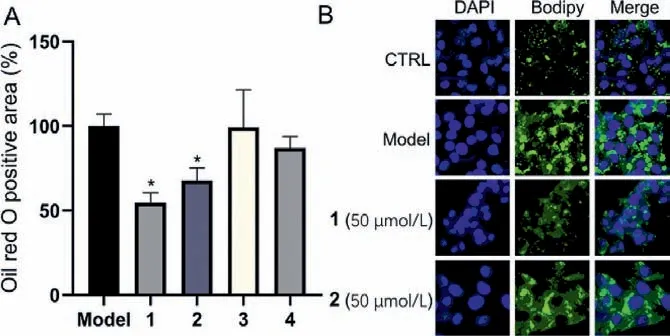
Fig.5.Anti-NASH screening of selected isolates in FFA-induced HepG2 cells.(A)Effects on reducing intracellular lipid accumulation of 1, 2, 3, and 4.The control group was treated with a blank medium and the model group was treated with 0.25 mmol/L FFA for 48 h.The other groups were treated with 0.25 mmol/L FFA and selected compounds at a concentration of 50 μmol/L for 48 h.The cells were carried out through oil red O staining, eluted with isopropanol, and measured the relative absorbance (n=3), compared with the model group (∗P < 0.05).(B) FFA-exposed HepG2 cells were treated with compounds 1 and 2 at a concentration of 50 μmol/L for 48 h.Bodipy staining (0.5 μmol/L, 30 min) was used to show the lipid droplets in the cells.
A plausible biogenetic pathway for the formation of cyclopropane cracked sesquiterpenoid dimers 1 and 2 was proposed as shown in Scheme 1.Given the abundant sesquiterpenoid monomer in this genus, chloranthalactone A was considered as the monomeric precursor.First, oxidation of chloranthalactone A initials the biogenetic pathway and affords the intermediates I.Subsequently, an intramolecular [6+6] cyclization reaction [18,19] of two molecules I produce the 12-membered ring of 3.Alternately,compound 4 might be also derived biogenetically from 3 through oxidation.After theO-cyclization ofΔ4,5in 3, the epoxide cleavage process occurs thus delivering the intermediate III, and III then undergoes a facile acid-catalyzed dehydration process leading to the unstable intermediate IV.Finally, a key cyclopropylcarbinyl rearrangement [22] of IV gives rise to the sesquiterpenoid dimers 1 and 2.
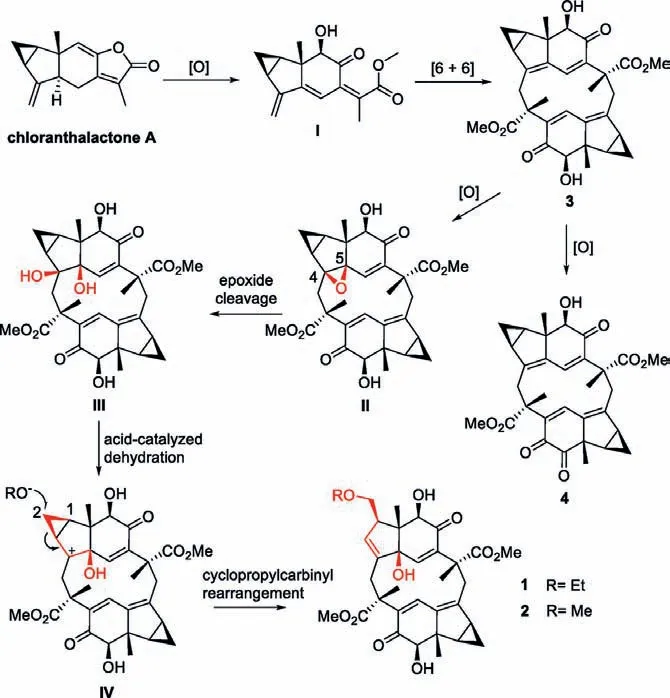
Scheme 1.Proposed biogenetic pathways of 1 and 2.
Numerous pieces of evidence have supported the beneficial role of diverse molecular frameworks in treating NASH [23].Given that these compounds had no cytotoxic activities (Fig.S6 in Supporting information), the anti-NASH (Nonalcoholic steatohepatitis) activities of these compounds were evaluated in free fatty acid (FFA)-induced HepG2 cells model [24].Oil red O staining was used to detect lipid content in the cells.Quantitative analysis of the staining showed that compounds 1 and 2 might have significant activities at a concentration of 50 μmol/L while compounds 3 and 4 had no effects (Fig.5A).Furthermore, Bodipy staining showed that lipid droplets deeply decreased in FFA-exposed HepG2 cells after treating with compounds 1 and 2 for 48 h (Fig.5B).The combined data revealed that chlospicenes A and B (1 and 2) are worthy of further study as potential protective agents against NASH.
In summary, guided by a molecular networking-based strategy,chlospicenes A and B (1 and 2), the first example of cyclopropane cracked sesquiterpenoid dimers fused by a 12-membered ring were rapidly discovered and isolated from the roots ofC.henryi.This successful discovery demonstrates the high efficiency of rapidly finding specific novel skeleton natural products through molecular networking-based strategy.The appealing anti-NASH (Nonalcoholic steatohepatitis) activities of chlospicenes A and B (1 and 2)indicated that these kinds of LSDs are worthy of further study as potential protective agents against NASH.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge supports from the National Natural Science Foundation of China (No.32070389), and the “Double First-Class” University Project (No.CPU2018GY08).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.084.
杂志排行
Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
