Effects of elevated atmospheric carbon dioxide concentration on nitrification of farmland soil
2022-09-07DUYilinLIANGJiabinGUOXinyuLUOJipengLIUYuankunMUQiliLITingqiangMinistryofEducationKeyLaboratoryofEnvironmentalRemediationandEcologicalHealthCollegeofEnvironmentalandResourceSciencesZhejiangUniversityHangzhou30058ChinaZhe
DU Yilin,LIANG Jiabin,GUO Xinyu,LUO Jipeng,LIU Yuankun,MU Qili,LI Tingqiang,2*(.Ministry of Education Key Laboratory of Environmental Remediation and Ecological Health, College of Environmental and Resource Sciences, Zhejiang University, Hangzhou 30058, China; 2. Zhejiang Provincial Key Laboratory of Agricultural Resources and Environment,Hangzhou 30058,China)
Abstract It has been widely reported that elevated carbon dioxide concentration(eCO2)affects soil nitrogen cycling dramatically, in which nitrification plays an important role in net primary productivity and greenhouse gas emission.However,the general patterns of soil nitrification and ammonia oxidizers in response to eCO2 remain uncertain.Here we performed a global meta-analysis to explore the effects of eCO2 on soil nitrification in agroecosystems and underlying microbial mechanisms. Our results showed that eCO2 significantly increased potential nitrification rate(PNR), nitrous oxide (N2O) emission and ammonia-oxidizing bacteria (AOB) abundance. Climates, experimental conditions, soil properties and agricultural practices were important factors in regulating the responses of soil nitrification to eCO2.The responses of nitrification potential were positively correlated with AOB abundance,while no evidence was found for ammonia-oxidizing archaea (AOA) abundance. The eCO2 response of AOB abundance was more closely related to the responses of soil nitrification than a wide range of environmental and experimental factors,as well as management practices. Furthermore, various effects of eCO2 on AOB abundance were determined by soil type and CO2 increase magnitude. Collectively, our results suggest that eCO2 strengthens soil nitrification by increasing AOB populations in agroecosystems.
Key words farmland soil;amoA gene;elevated CO2 concentration;meta-analysis;nitrification
Atmospheric carbon dioxide (CO2) concentration has gradually increased by 40% ever since preindustrial time due to build-up of fossil fuel combustion and land use change[1].It is predictable that CO2level will reach 650-1 200 μL/L by the end of 21st century, even with the mitigation policies of greenhouse gas[2]. The elevated atmospheric CO2has been shown to enhance plant photosynthesis and growth[3], and promote soil labile carbon input in terrestrial ecosystem[4]. Previous studies demonstrated that elevated carbon dioxide concentration (eCO2) can alter soil carbon and nitrogen biogeochemical cycles,thereby affecting the whole global ecosystem[5].
Nitrification is a critical process in soil nitrogen cycling, showing a profound impact on plant nitrogen availability and nitrogen loss through nitrate leaching and nitrous oxide (N2O) emission[6]. Hence, it is a key driver of terrestrial net primary production[7].Autotrophic nitrification is generally considered as a two-step process in which ammonia oxidation is the first and rate-limiting step, although comammox bacteria were recently identified in capable of performing complete nitrification[8-9]. Ammonia-oxidizing bacteria (AOB)and ammonia-oxidizing archaea(AOA)are key functional microbial guilds of ammonia oxidation[10]. In addition to autotrophic nitrification, heterotrophic nitrification,where soil organic nitrogen is sequentially oxidized by bacteria and fungi, was also important in acid soils(e.g., grassland and forest ecosystems)[11]. Ammonia oxidizers catalyze soil nitrification processes, and thus play a critical role in mediating nitrogen transformation among soil, plant and atmosphere. Therefore, recent researches were performed to understand how AOA and AOB respond to eCO2,and the results will help illuminate underlying mechanisms of soil nitrification patterns under global climate change.
Numerous field studies have investigated the effects of eCO2on soil nitrification in agricultural soils. However, the results showed that eCO2can have positive[12],neutral[13]or negative effects[14]on soil nitrification. These discrepancies may be ascribed to the diverse soil properties (i.e., soil type[15], soil texture[16], soil bulk density, and soil organic matter[17]).Besides, eCO2magnitude[18]and eCO2duration[19]can profoundly affect soil nitrification. Importantly, plant type[20], nitrogen fertilization rate[21]and agronomic additive[22]can profoundly change the impact of eCO2on soil nitrification. To date, only two meta-analysisbased studies reported the effects of eCO2on soil nitrification. One found that nitrifying enzyme activity was significantly reduced under eCO2, and only 11 observations from grassland and forest ecosystems were included in the synthesis[23]. Another review stated that neither gross mineralization nor gross nitrification was altered by eCO2[24]. Furthermore, the responses of functional genes involved in soil nitrification to eCO2have not been quantitatively synthesized.Therefore,it is important to compile the existing studies on the effects of eCO2on soil nitrification and ammonia oxidizers in agroecosystems.
To address these knowledge gaps in how eCO2affects soil nitrification in agroecosystems,we collected 147 observations from 34 articles and conducted a metaanalysis to identify the general pattern of soil nitrification and ammonia oxidizers under eCO2conditions. The objectives of this analysis are to interpret the following questions: 1) Does eCO2have an impact on soil nitrification rate in agroecosystems?2)How do climatic conditions,experimental conditions,soil properties and agricultural practices regulate the effects of eCO2on soil nitrification rate? 3) What are the microbial mechanisms underlying eCO2-induced changes in soil nitrification rate? 4) Is there any relationship between soil nitrification rate and N2O emission under eCO2?
1 Materials and Methods
1.1 Data collection
The database was constructed by compiling data from peer-reviewed articles across the globe using the key terms, 1) “nitrification” or “ammonia oxidizing”or “amoA” or “AOA” or “AOB”, and 2) “soil”, and 3) “elevated CO2” or “increased CO2” in the Web of Science and China National Knowledge Infrastructure(CNKI) up to 20 June 2020, resulting in 318 articles.Subsequently, we selected eligible articles according to the following criteria: 1) only field experiments in agroecosystems were included to better represent responses of nitrification rate and ammonia oxidizers under natural environment; 2) responses of soil nitrification rate or/and ammonia oxidizers were measured;3)eCO2and ambient CO2(aCO2)treatments were conducted under the same climate, experimental condition, soil property and agricultural practice to avoid confounding factors; 4) eCO2magnitude and eCO2duration were clearly described; 5) the means,standard deviations and sample sizes of the variables were directly reported or could be indirectly calculated.Finally, 34 articles which met the above criteria were included in our database (attached Table S1,http://www.zjujournals.com/agr/EN/10.3785/j.issn.1008-9209.2021.02.082).
For each study included in our database, we extracted the variables including potential nitrification rate, the abundance of archaealamoAand bacterialamoA, N2O emission and soil properties [water-filled pore space (WFPS), soil pH (pH), total carbon (TC),soil carbon to nitrogen ratio (C/N), dissolved organic carbon (DOC), dissolved organic nitrogen (DON),ammonium nitrogen (N-N) content, nitrate nitrogen(N-N) content, microbial biomass carbon (MBC),and microbial biomass nitrogen (MBN)]. In total, the constructed database included 147 observations from 34 articles (attached Table S1). We extracted the means, standard deviations and sample sizes of each variable from all studies. If the concerned data were presented as figures rather than texts or tables,we used GetData Graph Digitizer v2.0 (http://getdata-graphdigitizer.com/) to extract the numerical values from digitized graphs.
We also collected information on:1)experimental locations(longitude and latitude);2)climatic conditions[mean annual temperature(MAT),mean annual precipi‐tation (MAP)]; 3) experimental conditions, including experimental method, eCO2magnitude and eCO2dura‐tion;4)soil properties,including soil type,soil physical properties(texture and bulk density),and soil chemical properties [pH, organic carbon, and total nitrogen(TN)]; 5) agricultural practices, including plant type,cropping system, nitrogen fertilization rate and agro‐nomic additive.Individual variables were further classi‐fied into categories as follows.The experimental method was grouped into free-air carbon dioxide enrichment(FACE), greenhouse (GH) and open top chamber(OTC). The increase magnitude of CO2concentration was grouped into <150 μL/L, 150-250 μL/L, >250-350 μL/L,and >350 μL/L.eCO2duration was grouped into <1 a, >1-5 a, and >5 a. Soil type was grouped into Anthrosol,Arenosol, Calcisol, Cambisol, Gleysol,Luvisol, Phaeozem, Regosol, Vertisol according to the classification system of the Food and Agriculture Orga‐nization(FAO).Soil texture was grouped into clay,clay loam, loam, and sand. Soil bulk density was grouped into ≤1.2 g/cm3,and >1.2 g/cm3.Soil pH was grouped into <6.5, 6.5-7.5, and >7.5. Soil organic carbon(SOC) was grouped into <10 g/kg, 10-20 g/kg,and >20 g/kg. Soil TN was grouped into <1 g/kg,1-2 g/kg, and >2 g/kg. Plant type was grouped into grass, cotton, maize, soybean, wheat and rice. Cropping system was grouped into monoculture and rotation.Nitro‐gen fertilization rate was grouped into <100 kg/(hm2·a),100-200 kg/(hm2·a), and >200 kg/(hm2·a).Agronomic additive was grouped into no additive, biochar, straw and pesticide. In addition, according to the Köppen-Geiger climate classification system[25],climate zones in these studies were extracted and classified into hotsummer Mediterranean climate (Csa), warm-summer Mediterranean climate(Csb),humid subtropical climate(Cfa), temperate oceanic climate (Cfb), monsooninfluenced warm-summer humid continental climate(Dwb), hot-summer humid continental climate (Dfa),and warm-summer humid continental climate(Dfb).
1.2 Statistical analyses
In this meta-analysis, natural logarithm of the response ratio(lnRr)was used as effect size to assess the effects of eCO2on nitrification rate,amoAabundance and other ancillary variables,which was calculated as:
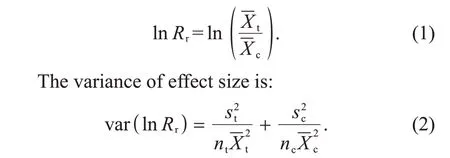

The weighted effect size and 95% confidence interval (CI) were calculated using the rma.mv function in the R package “metafor”[26]. Significance of effect size was assessed with the 95% CI. If the 95% CI of the effect size on a variable did not overlap zero, the effects of eCO2were considered to be significant; otherwise, the effects of eCO2were considered to be non-significant. For a better explanation, we also transformed lnRrto percentage change (eCO2effects, %) to present the effects of eCO2using the formula as follows:
eCO2effects/%=( )elnRr-1 ×100. (3)
Subgroup analysis was used to examine the effects of eCO2on concerned variables among different groups. Between-groupQstatistical test was applied to compare the heterogeneity of the weighted effect size of each categorical variable among different groups.A significantQbvalue indicated that the weighted effect sizes differed among groups. The relationships between effect size of potential nitrification rate(PNR)and predictor variables were examined using a linear regression model. Finally, the mixed-effects metaregression model analysis was performed using the R package“glmulti”[27].The relative importance of variables was calculated as the sum of the Akaike weights for all models that included this factor. A cutoff of 0.8 was set to identify the most important predictors[28].
2 Results
2.1 Effects of eCO2 on PNR, N2O emission and soil properties
As shown in the Fig. 1, the overall dataset indicated that eCO2had significantly positive effects on PNR (13%) and N2O emission (19%). However,abundance of AOA was not significantly changed,but AOB (15%) was significantly increased under eCO2.As for soil properties, eCO2significantly increased DOC,C/N,TC,and MBC by 19%,2%,3%and 24%,respectively. In contrast, eCO2significantly decreased pH,DON and NH4+-N by 2%,13%and 7%,respectively.In addition, eCO2had no effects on WFPS, NO-3-N and MBN.
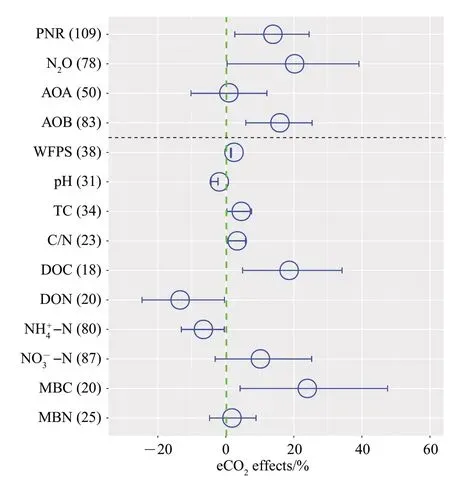
Fig.1 Effects of eCO2 on PNR, N2O emission,AOA,AOB and environmental variables
2.2 Factors driving the responses of PNR to eCO2
2.2.1 Latitude and climate
The effects of eCO2on PNR significantly decreased from low latitude to high latitude with a slope of-0.018 (P<0.001; Fig. 2A). The effects of eCO2on PNR significantly increased with the increase of MAT and MAP with a slope of 0.04 (P<0.001;Fig. 2B) and 0.001 (P=0.002; Fig. 2C), respectively.Besides, climate zone showed significant differences in PNR response to eCO2(P<0.001; Fig. 2D). eCO2significantly stimulated PNR by 105%, 15% and 17% in Csa, Cfa and Dfb, respectively. Conversely,eCO2significantly suppressed PNR by 28% in Dfa.Meanwhile, eCO2did not significantly affect PNR in Csb,Cfb and Dwb(Fig.2D).
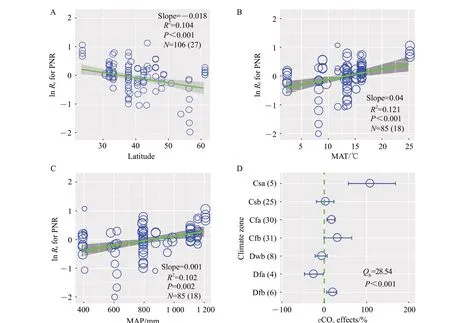
Fig.2 Relationships between responses of PNR(ln Rr)and latitude(A),MAT(B),MAP(C),and effects of climate zones on changes of PNR under eCO2(D)
2.2.2 Experimental conditions
The experimental methods significantly affected the effects of eCO2on PNR (P=0.002; Fig. 3). FACE significantly increased PNR by 16%, while GH and OTC had no effects on PNR.Additionally, the effects of eCO2on PNR depended on CO2increase magnitude(P<0.001;Fig.3).PNR increased by 33%and 105%when the CO2increase magnitude <150 μL/L and >350 μL/L,respectively.In contrast,eCO2showed no significant changes in PNR when the CO2increase magnitude was between 150 μL/L and 350 μL/L.Moreover,eCO2duration showed pronounced differences in PNR(P<0.001;Fig.3).PNR significantly increased by 48%and 19%when eCO2duration <1 a and 1-5 a,respectively. However, no significant effect on PNR was observed when eCO2duration more than 5 a.
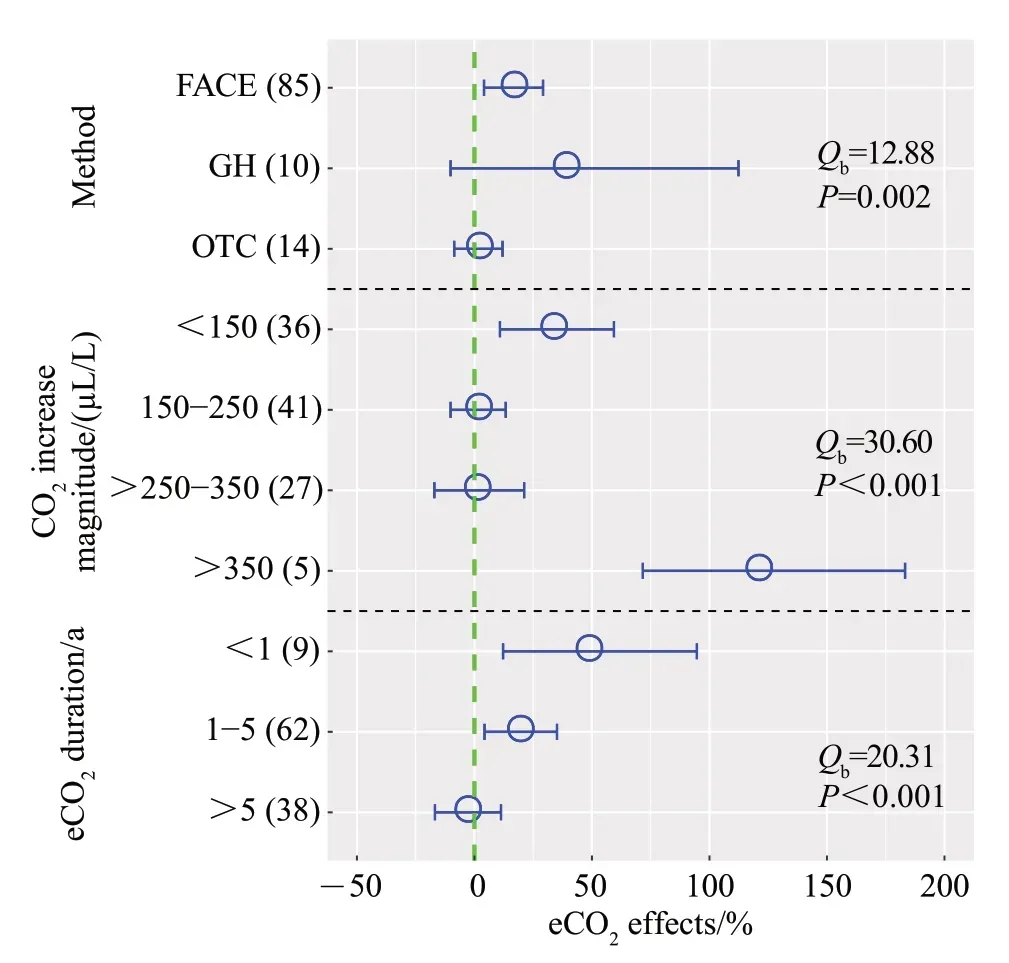
Fig.3 Effects of experimental conditions on changes in PNR under eCO2
2.2.3 Soil properties
Soil type significantly affected the effects of eCO2on PNR (P<0.001; Fig. 4A). Specifically,eCO2consistently stimulated PNR in Anthrosol(19%),Arenosol (97%), Cambisol (47%) and Vertisol(27%), respectively. On the contrary, eCO2significantly restrained PNR in Phaeozem (-28%).Besides, eCO2did not change PNR in Calcisol,Luvisol and Regosol. Soil texture also affected the response of PNR to eCO2(P=0.002; Fig. 4B). eCO2enhanced PNR by 23% in clay soil, while did not change PNR in clay loam, loam and sand soil.Additionally, SOC showed significant differences in PNR, and eCO2significantly increased PNR by 60%in soils with low SOC content (P<0.001; Fig. 4C).Moreover, soil bulk density, pH and TN had no significant effects on the response of PNR to eCO2(P>0.05;Fig.4B-C).

Fig.4 Effects of soil types(A),soil physical properties(B)and soil chemical properties(C)on changes in PNR under eCO2
2.2.4 Agricultural practices
The effects of eCO2on PNR significantly differed among plant type, nitrogen rate and agronomic additive (P<0.05; Fig. 5). More specifically, PNR increased in cotton (3%), wheat (27%) and rice (29%)fields,while did not significantly changed in grassland(Fig. 5A). Besides, eCO2induced significant increase in PNR in rotation system(22%;Fig.5B).Interestingly,PNR negatively (-20%) responded to low nitrogen rate[<100 kg/(hm2·a)],whereas PNR positively(5%)responded to high nitrogen rate [>200 kg/(hm2·a)]. In addition, both biochar (134%) and pesticide (24%)application had positive effects on PNR.
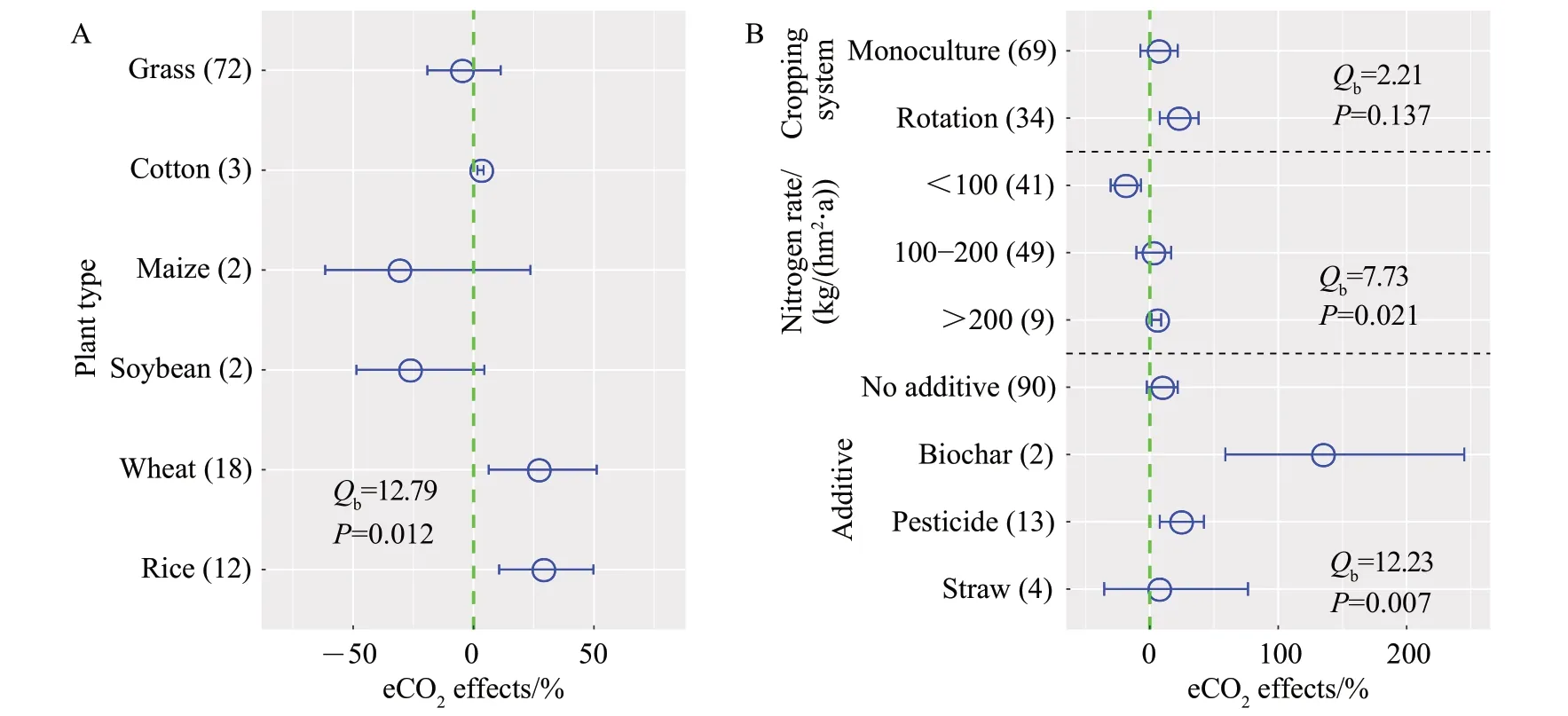
Fig.5 Effects of plant types(A)and agricultural practices(B)on changes in PNR under eCO2
2.3 Relationships between the eCO2 effects on PNR and ammonia oxidizers
The relationship between the effect size of AOA abundance and the effect size of PNR was not significant (P=0.525; Fig. 6A). However, the effect size of AOB abundance was positively correlated with the effect size of PNR (slope=0.388,P=0.001;Fig. 6B). Furthermore, the model selection analysis showed that the effects of eCO2on PNR was best predicted by the responses of AOB abundance over a wide range of climatic conditions, soil characteristics,agricultural practices and eCO2protocols(Fig.6C).
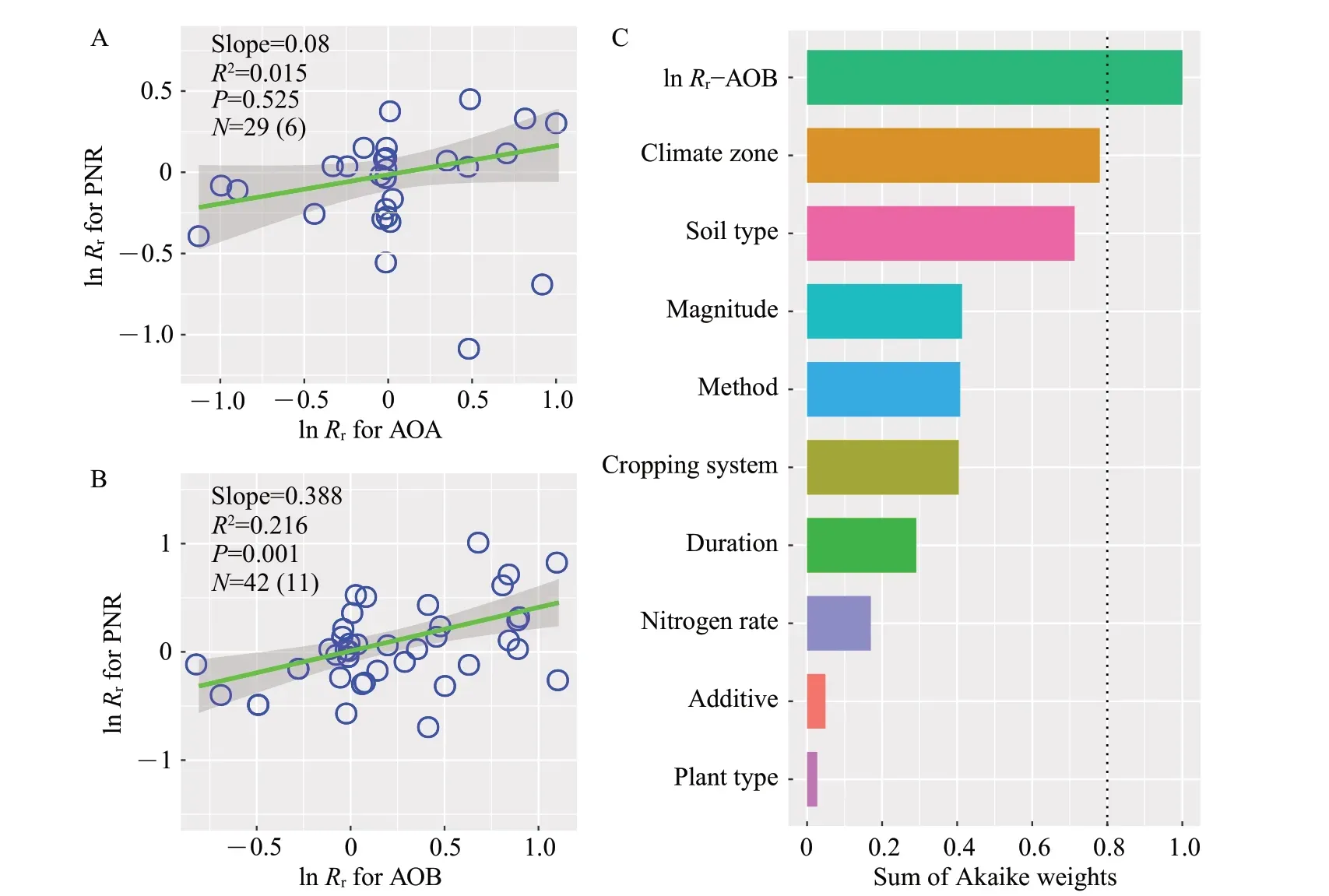
Fig.6 Relationships between the eCO2 effects on PNR and ammonia oxidizers
2.4 Key drivers of eCO2 effects on changes in AOB
The model selection analysis showed that the most important moderators of the effects of eCO2on AOB abundance were soil type and CO2increase magnitude (Fig. 7A). Subgroup analysis confirmed that the effects of eCO2on AOB abundance were significantly dependent on soil type and CO2increase magnitude (P=0.002 andP<0.001, respectively;Fig. 7B). AOB abundance significantly increased in Anthrosol (37%) and Cambisol (28%), while significantly decreased in Luvisol (-51%) and Phaeozem (-11%). AOB abundance significantly increased when the CO2increase magnitude <150 μL/L(39%), and tended to decrease with the increase of CO2increase magnitude, except the experiments with the CO2increase magnitude >350 μL/L.
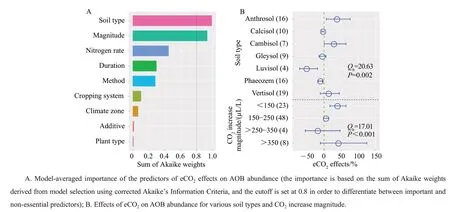
Fig.7 Key drivers of eCO2 effects on changes in AOB
2.5 Relationships between the eCO2 effects on PNR and soil N-N contents,N2O emissions
The responses of soil NO-3-N content significantly increased with the responses of PNR with a slope of 0.781 (R2=0.322,P<0.001; Fig. 8A).In contrast, there was no significant relationship between the responses of N2O emission and PNR(P=0.1;Fig.8B).
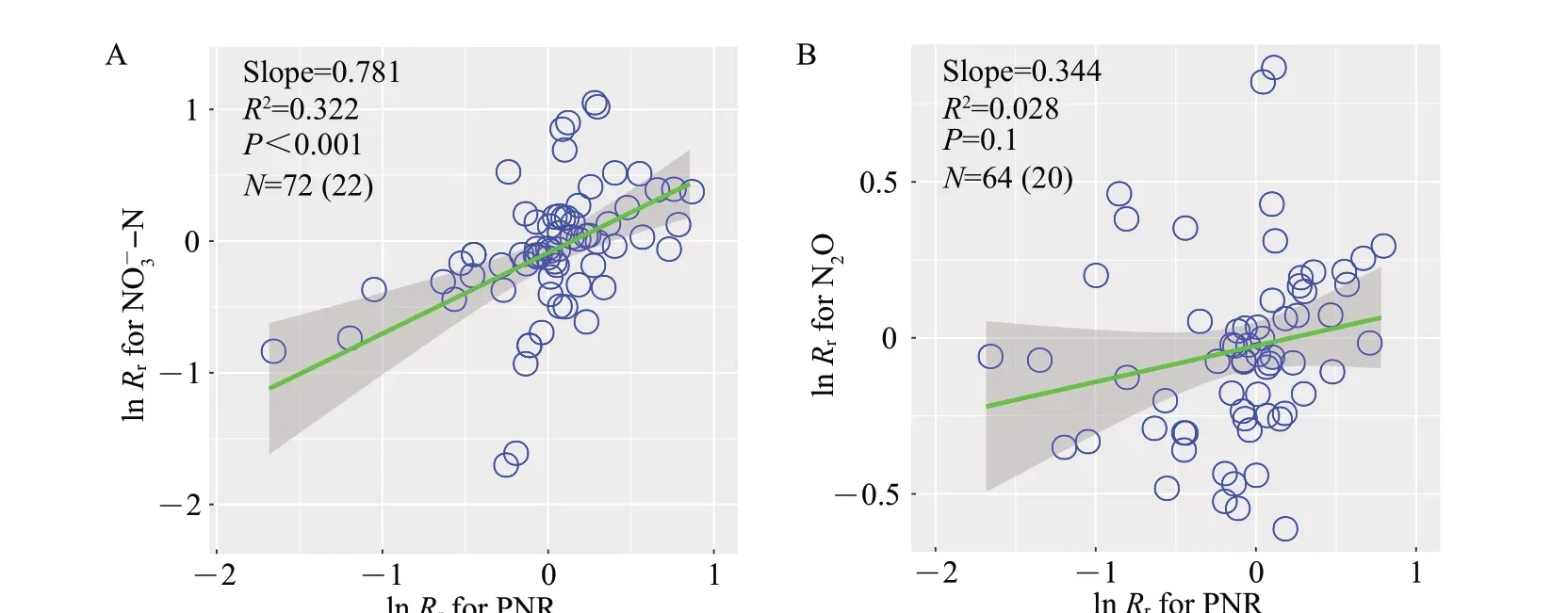
Fig.8 Response relationships between soil NO-3-N content(A),N2O emission(B)and soil nitrification rate
3 Discussion
3.1 Factors affecting the responses of PNR to eCO2
Our results indicated that the effects of eCO2on PNR significantly increased with MAT. Similarly, a meta-analysis showed that warming significantly increased net nitrification rates by 32% in terrestrial ecosystems[29]. Higher temperature can potentially resuscitate some AOB phylotypes and alter the AOB abundance and community, and thus amplifies the effects of nitrogen fertilization on soil nitrification[30].The alternative explanation is that elevated temperature drives the microbes to participate in nitrogen mineralization rather than nitrogen immobilization,thus results in the accumulation of large proportion of inorganic nitrogen[31]. This change provides more substrates (e.g., NH4+-N) for soil nitrification and speeds it up. In addition, MAP is another important climatic factor affecting the effects of eCO2on PNR.Altered precipitation regulates nitrogen cycling by changing soil water content,which plays a vital role in the abundance and community structure of nitrifying communities[32]. Increased precipitation can enhance microbial growth and metabolic activity in soil, which leads to an acceleration in soil nitrification process and plant growth[33]. For example, increased precipitation was associated with a higher abundance of AOB in meadow-steppe grassland[34]. Moreover, increased precipitation can mitigate the negative effect of intensive nitrogen fertilization on soil microbial activity of nitrification[32]. The increasingly fertilization may decrease ammonia oxidizer abundance and dampen soil nitrification by decreasing soil pH in agroecosystems, which could be balanced by water-mediated increase in soil pH[35].
In regarding to the experimental method, our results show that eCO2has a positive effect on PNR in FACE, but has no obvious effect on GH and OTC.This effect may be attributed to the fact that greenhouse (GH) and open-top chamber (OTC)provide more suitable microclimate for plant growth and soil nitrogen cycle, and thus hide the effects of eCO2on nitrification process. By contrast, FACE is always conducted in natural environment, so that there is no interaction of eCO2and other essential factors such as temperature and soil moisture. Consequently,we could conclude that eCO2accelerate nitrification process in agricultural soils. The response of PNR to eCO2was also significantly affected by CO2increase magnitude and eCO2duration. We found a negative relationship between eCO2duration and the response of PNR to eCO2, supporting the theory that the improvement of plant growth by eCO2leads to more nitrogen sequestrated in plant and litter pools, such that available nitrogen declines in soil[36].This hypothesis of nitrogen restraint to eCO2is known as progressive nitrogen limitation (PNL), which may constrain the eCO2fertilization effect on nitrifying microorganisms on the long timescale[37]. Similarly, response of PNR showed a negative relationship with the CO2increase magnitude, except that eCO2significantly increased PNR when the CO2increase magnitude >350 μL/L.The possible mechanism is that higher eCO2induces a greater increase in nitrogen uptake by plants,resulting in less nitrogen substrate for microbial nitrogen cycling[18].
Soil texture represents soil porosity, which could shape soil pore size and distribution, regulating soil water content and O2availability[38]. Previous metaanalyses suggested that coarse-textured soils would favor nitrification,because they have large fraction of soil macropores, resulting in high air-filled porosity[39-40].However, our results showed that eCO2could increase WFPS, reduce gas diffusion rate in soil, and thus suppress nitrification rate. For instance, well-aerated soils (e.g., Phaeozem) showed negative responses in PNR to eCO2. Differing from coarse-textured soils,carbon substrates become a dominant factor affecting soil nutrient cycling in fine-textured soils. Increasing labile carbon such as DOC and MBC induced by eCO2may also stimulate the activity of nitrifiers. Our results revealed that eCO2significantly increased PNR in heavy clay soils (e.g., Vertisol). Moreover,eCO2increased PNR in soils poor in organic carbon.This is probably due to the eCO2effects that increase labile carbon input into soil and a subsequent accelerated nitrogen cycling[14,41-42]. Surprisingly, soil pH and TN had no significant effects on PNR,inconsistent with a recent global meta-analysis[43]. This discrepancy may be attributed to the long-term eCO2effects on soil, as continuous eCO2can greatly facilitate soil acidification and deplete soil nitrogen content. As a result, the distinction of nitrifying microbial activity between initially different pH/TN soils may not be as conspicuous as expected.
Optimization of cropping system and nitrogen fertilization management could alleviate the negative effects on soil nitrification induced by eCO2. Our meta-analysis showed that eCO2increased PNR under crop rotation rather than monoculture. This is supported by previous studies reporting that rotation may change soil structure and increase soil carbon and nitrogen sequestration, thereby increasing the abundance of AOB gene[44-45].Furthermore,our results suggested that increasing nitrogen fertilization rate can offset the negative eCO2effects on soil nitrification.Specifically, PNL caused by eCO2can be eliminated by nitrogen fertilization in agroecosystems. For example,eCO2tended to decrease PNR in grassland with little or no fertilization,while increased PNR in arable land(e.g.,wheat and rice fields)with intensive fertilization.
Agronomic additives might also regulate the impact of eCO2on soil nitrification and thus PNR.As for biochar, the porosity and large surface area provide aerobic condition, and high cation exchange capacity (CEC) increases availability of NH4+-N[46].Both agricultural benefits from biochar properties favor the growth of ammonia oxidizers,resulting in the strongly positive response of PNR to eCO2.Besides,we also found that PNR still showed positive response to eCO2in rice-wheat rotation system applied with pesticide, although it has been demonstrated that the toxicity of various pesticides on soil microbial community, particularly ammonia-oxidizing micro‑organisms[47-48]. Accordingly, we concluded that the negative influence of pesticide on nitrification can be subsidized by eCO2.
3.2 Microbial mechanisms underlying changes in PNR
In general, soil nitrification largely depends on ammonia oxidizers,and thus we hypothesized that the response of ammonia oxidizers would have a significant relationship with the response of PNR under eCO2condition. Our results indicated that the response of PNR was positively correlated with the response of AOB abundance, but not AOA abundance, partially supporting this hypothesis.Furthermore,AOB abundance also constituted the most important predictor of the eCO2effect on soil nitrification potential. These findings are in line with a previous meta-analysis,which reported that soil nitrification potential with nitrogen addition increased concomitantly with only AOB[49]. Similar pattern of ammonia oxidizers was also found in other empirical studies[50]. AOA and AOB have different cell sizes and ammonia oxidation pathways[51], leading to the suggestion that AOA have higher substrate affinity than AOB[52]. Especially in agricultural soils, AOB have 20 times higherVmax(apparent maximum nitrification rate) and 40 times higherKm(apparent half-saturation constant) than AOA[53]. This physiological heterogeneity between AOA and AOB may result in the dominant role of AOB in soil nitrification. However, significant variation in the relationship of AOB and PNR still remained(R2=0.216), which could be attributed to soil type and CO2increase magnitude.
3.3 Linkage between PNR and N2O emission
It has been generally considered that eCO2increases N2O emission from soils,coincident with our synthesized results in agroecosystems.N2O is produced by continuous microbial nitrogen cycling processes,where nitrification and denitrification contribute almost 70%of the total N2O production[54].Increasingly studies have indicated that nitrification is an important pathway to generate N2O[55]. Nevertheless, we did not find a significant relationship between nitrification potential and N2O emission, which means that nitrification may be less responsive to the stimulation of N2O emission induced by eCO2.The possible explanation is that longterm eCO2will promote soil moisture and reduce soil O2concentration, thereby favoring denitrifiers rather than nitrifiers[23]. Further, there is evidence suggesting that eCO2stimulates N2O emission through benefiting N2O-producingnirK-type denitrifiers[42,56].Hence,more studies are needed to seek for the main pathway of N2O production stimulated by eCO2in agricultural soils.
4 Conclusions
The effects of eCO2on soil nitrification in agroecosystems were investigated using a metaanalysis. The result showed that eCO2tended to decrease PNR in grasslands, whereas significantly increased PNR in wheat and rice fields.Additionally,the responses of PNR to eCO2significantly increased with MAT and MAP,and soil properties and agricultural practices were key factors in regulating the responses of PNR to eCO2. In contrast to AOA,AOB showed a significant positive relationship with PNR in response to eCO2and became the foremost predictor of the eCO2effects on PNR. Besides, soil type and CO2increase magnitude largely accounted for the variation in responses of AOB to eCO2. Taken together, the effects of climates, soil properties and agricultural practices on changes in nitrification potential under eCO2condition, along with the niche differentiation of ammonia-oxidizing archaea and bacteria will help develop more accurate biogeochemical models to predict soil nitrogen cycling under future CO2scenarios.
