Amebic liver abscess: Clinico-radiological findings and interventional management
2022-09-01RajeevNayanPriyadarshiRameshKumarUtpalAnand
INTRODUCTION
Amebic liver abscess (ALA) is an infection caused by the protozoan
(EH)
an intestinal parasite. The infection is acquired by ingestion of water or food contaminated by EH cysts (the infective stage of the parasite). The cysts resist gastric juice and reach the distal ileum, where they undergo excystation producing trophozoites (the feeding stage of the parasite). In > 90% patients, the trophozoites feed on intestinal tissue and bacteria without producing symptoms. In less than 1% of cases, however, the trophozoites penetrate the mucosa and, through the portal route, reach the liver causing liver abscess[1]. ALA is the most common and has the highest mortality of amebiasis manifestations. It continues to remain the most common cause of liver abscess in developing and underdeveloped countries[2-6].
ALA was known as a progressive and deadly disease a century ago; however, since the introduction of modern antibiotics, the mortality has drastically reduced to between 1% and 3%[7]. Metronidazole is the most effective agent, with cure rates of approximately 90%. Most patients become asymptomatic within 72 to 96 h of treatment, and drainage adds no benefit to uncomplicated cases[7,8]. This fact seems to be more relevant for a typical case where the patient presents the classic and the most common form of the disease,
, subacute mild disease. Reports from endemic areas have shown that a greater percentage of cases require drainage through either a needle or catheter. The reported prevalence of such cases varies from 44% to 80%[3-5,7,9-14]. A thorough literature search shows that two distinct clinical settings usually require drainage. In the first, the patients present acutely with severe and fulminant disease, and drainage is performed to control disease progression and prevent organ failure. Such abscesses, by different authors, have been denoted by different terms that indicate the aggressive nature of the disease, such as “acute aggressive ALA”, “severe ALA” or “fulminant ALA”[10,15-17]. In the second clinical setting of the disease, the patients present late with mild symptoms, usually tenderness; they usually have a large persistent abscess despite medical therapy. Various terms are used to describe such abscesses, such as “drug-resistant ALA”, “refractory ALA” or “chronic indolent ALA”[18-22]. Regardless of the presentations, most cases are usually associated with a few complications, such as rupture, secondary infection or biliary communication. Considering this fact, a few authors prefer referring to it as “complicated ALA”[13,14]. Therefore, ALA can be classified into three clinical subtypes: subacute mild, acute aggressive and chronic indolent. Not only do the ALAs have varied clinical presentations, but they are also associated with distinct imaging patterns[10].
This review describes the three major types of clinical presentations as well as three types of imaging patterns (correlating with clinical subtypes). Special emphasis has been placed on the two clinical types - acute aggressive and chronic indolent. This paper also discusses the complications of ALA and their percutaneous management.
OVERVIEW OF EPIDEMIOLOGY, RISK FACTORS AND PATHOGENESIS OF COMPLICATED ALA
Epidemiology
Although ALA occurs globally, most reports emerge from endemic countries, such as India, Sri Lanka, Bangladesh, Mexico, East and South Africa or parts of Central and South America[23]. A high endemicity in these countries is related to poor hygiene and sanitation since the parasite is transmitted
the fecal-oral route. Even in endemic countries, ALA occurs primarily in rural areas where defecation in the open air is a common practice[11,24-26]. The lack of adequate sewage disposal results in contamination of drinking water with EH cysts. Using polymerase chain reaction (PCR), a population-based study from India detected the prevalence of EH in 14% of stool samples[27]. In developed countries, ALA occurs mostly in travelers or immigrants from endemic areas[28]. Apart from endemicity, several other epidemiological factors also increase the risk of developing complicated disease.
Risk factors
The disease is found almost exclusively in men (male: female > 10:1)[11]. The reason for this is unknown but several investigators have speculated that it might be related to alcohol, particularly those prepared locally from the sap of palm trees (toddy)[11,24,25,29]. Not only is the toddy a risk factor for ALA, but in many studies it has been linked to severe disease[13,30]. The exact mechanism by which it contributes to the pathogenesis of ALA is unclear. It has been proposed that alcohol may alter the gut mucosa or convert the pathogen to a more virulent strain or render the liver more susceptible to the infection[23,24,29]. Most cases occur in middle age ranging from 20 to 50 years[30]. In older patients, the disease tends to be more severe possibly due to their poor immunity, whereas it is rare in children[31]. Another factor contributing to the pathogenesis of ALA is malnutrition[11,13,23]. For centuries, the disease has been a symbol of poverty. A typical patient with ALA, as we have observed, is a thin emaciated villager of low socioeconomic status. Their poor nutritional status is evidenced by low albumin, BMI and hemoglobin[11]. ALA has also been shown to be severe in diabetic patients[16,32].
Pathogenesis
The term “amebic liver abscess” is a misnomer as the cavity formation or liquefaction is not due to suppuration; rather, it is the result of a unique type of necrosis[33,34]. The necrotic area appears as if it has been dissolved by chemical or toxin. Considering this morphological pattern, it was believed that the parasite possesses a toxin that lyses the hepatocytes, and therefore the parasite was named “histolytica”[35]. It is now known that several proteolytic enzymes released by the inflammatory cells are responsible for tissue destruction[7,36,37].
Understanding the gross morphology is important because it is characteristic and, to a large extent, can be extrapolated to imaging findings[35,38,39]. The gross appearance varies depending on the severity and the duration of the disease. In the early stage, it is that of a necrotic area where the center has liquefied necrotic tissue (chocolate-colored sterile “pus”); however, the periphery has more solid tissue[10,35,38-40]. The peripheral solid and partially liquified tissue is responsible for the shaggy or ragged appearance on the abscess wall[10,40]. A mature wall is absent and the tissue surrounding the abscess is congested, compressed and edematous[41]. There may be pressure over the surrounding liver parenchyma or the hepatic capsule. Venous thrombosis and ischemic infarction are commonly observed in fatal cases[42]. As the abscess heals, a fibrous wall forms and the cavity becomes more sharply defined[38,43]. The edema and congestion regress and the abscess wall is surrounded only by a thin rim of edema. The peripheral solid tissue becomes more liquefied, the content is gradually resorbed, and the lesion heals completely without scar. However, a complicated or a very large abscess can persist in the form of a residual abscess with a thick fibrous wall. A mature wall, as opposed to the ragged wall, indicates chronicity or secondary infection[42].
ALA is usually solitary, located in the right lobe of the liver. The size varies from a few centimeters to 20 cm[35]. However, the risk of complications increases with the number and size. In autopsy series, unlike successfully treated series, 60% of cases show multiple abscesses varying in size from 10 to 15 cm[35]. Literature shows a higher incidence of large (> 5 to 10 cm) and multiple abscesses (occurring in about 50% of cases) among the Southeast Asian population compared to other studied populations[8-11,43-47].
CLINICAL PRESENTATION
The clinical presentation varies from mild to severe. Based on the duration and the severity, ALA can be classified into three main types: subacute mild, acute aggressive, and chronic indolent[10,15,23,28,48].
Subacute mild ALA
The decision to perform drainage is based largely on the clinical grounds. Any symptomatic patient with persistent symptoms after four days of treatment requires drainage, regardless of the imaging findings. In the most common scenario of percutaneous drainage, the patients continue to have symptoms, primarily pain or tenderness in the right upper quadrant, despite completed medical therapy. In another clinical setting, early drainage is performed for acute aggressive abscesses to control the disease severity[10]. The third clinical setting may be the patients in whom there is diagnostic uncertainty between ALA and pyogenic abscess. In such cases, most physicians prefer to drain the amebic abscesses considering them as a pyogenic abscess.
Acute aggressive ALA
Acute aggressive ALA is characterized by a more severe and rapidly progressive course. Considering the acuteness and severity of this form, Katzenstein
[15] named it “acute aggressive ALA”. The prevalence of this type of ALA may be high in endemic areas[10]. In a study of 317 patients with ALA, Balasegaram reported acute fulminating infection in 13% of cases[17]. The patients often present more acutely (< 10 d) with signs of severe disease including systemic toxicity, high fevers and chills, and an exquisitely tender hepatomegaly[15]. Signs related to rupture and other complications may be present. In fact, rupture is a common presenting manifestation of aggressive ALA, occurring in up to 57% of patients[10]. The patients with free intraperitoneal rupture often have features of generalized peritonitis. Sepsis-like features can occur in more severely affected patients. Up to 90% of patients require hospitalization and about 13% require intensive care unit management[10]. Signs of organ dysfunction, such as jaundice, may also be observed in most patients[9,12,32]. Renal dysfunction can occur in 5% to 12% of cases[6,10]. Hepatic failure and encephalopathy may also occur. Approximately, one-third to one-half of the patients will have gross fluid derangements including ascites, pleural effusion and edema[5,9,10,13,52]. Patients with aggressive ALA are often misdiagnosed as having acute cholecystitis, appendicitis or bowel perforation[30,53-55].
He put on socks and shoes, and a jacket, and went out. She watched Gaston trying to find out what to do next. Gaston wandered around the plate, but everything seemed wrong and he didn t know what to do or where to go.
Most patients with aggressive ALA will have markedly deranged laboratory parameters, such as severe leukocytosis, hyperbilirubinemia, hypoalbuminemia, elevated liver enzymes, and elevated alkaline phosphatase[10]. A high mortality has been recorded in this group of patients. Most deaths are usually related to intraperitoneal rupture, which is followed by sepsis and multiorgan failure. Many findings of aggressive disease have been identified as poor prognostic markers in different studies, such as multiple abscesses, large (> 500 cc) volume abscesses, presence of encephalopathy, hypoalbuminemia, and hyperbilirubinemia (> 3.5 mg/dL)[3,9,13,32]. Medical therapy alone is often suboptimal to control the disease and the laboratory tests do not return to near normal following treatment. Therefore, drainage with either a needle or catheter is usually required[10,15].
Chronic indolent ALA
Chronic presentation can occur in approximately 10 to 20% of cases[10,15,23,49,56,57]. This presentation has been designated in most studies as “chronic indolent ALA”. In this form, patients present late with mild symptoms for more than four weeks. Most patients complain of pain over the right lower chest or upper abdomen. Fever is usually absent or of low grade. However, a history of fever with chills at the onset of the disease may be obtained in most cases. Additionally, many patients will have a history of prior medical treatment or sometimes prior needle aspirations. On examination, right upper quadrant tenderness is usually present. Other low-grade symptoms include weight loss, anorexia, or malaise[10,15]. Laboratory tests are usually normal except elevated alkaline phosphatase level and low serum albumin. Leukocytosis in chronic abscesses suggests the presence of secondary infection, which is the most common complication in this form of the disease. In contrast to acute aggressive ALA, chronic ALA is rarely associated with intraperitoneal rupture.
LABORATORY EVALUATION
The diagnosis of ALA is based on recognition of the typical clinical features, imaging studies and serological tests. Serological tests are considered confirmatory (sensitivity > 94%; specificity > 95%)[7]. However, their usefulness in the diagnosis of acute ALA is limited in endemic areas because the tests remain positive for several months to years after resolution of infection. Moreover, the serological tests may be negative in the first seven to ten days of the infection, limiting their diagnostic use for acute ALA[7].
Joseph was only four years old, and still afraid of the dark, so Aunt Linda left the door open and the hall light on when she tucked4 us in to bed. Joe couldn’t sleep, so he just lay there staring at the ceiling. Just as I dozed5 off to sleep, he woke me up and asked, “Jennie, what are those ugly things near the light?”(I had always liked that he asked me questions because I was older and supposed to know the answers. I didn’t always know the answers, of course, but I could always pretend I did.) He was pointing to the moths fluttering6 around the hall light. “They’re just moths, go to sleep,” I told him.
Routine laboratory tests in ALA are nonspecific and do not differ from those in pyogenic abscess[58,59]. However, these tests are useful in assessing the severity and monitoring the treatment response. In most patients with acute benign ALA, mild to moderate leukocytosis is found with an average WBC count of 16000/μL. However, a high WBC count above 20000/μL should suggest either aggressive, or secondarily infected abscesses[9,56,60]. In our series, a mean of 24000/μL was found in patients with aggressive abscesses. Serum bilirubin and liver enzyme (AST/ALT) levels are normal or minimally elevated in mild cases. When elevated, the AST/ALT levels return to normal following medical therapy. However, the alkaline phosphatase level is elevated in 70 to 80% of cases, regardless of the severity of the disease and the duration of presentation[56,60]. A very high value of bilirubin (> 3.5 mg/dL) and liver enzymes indicates complications or aggressive disease. A low serum albumin (< 2 g/dL) is found in almost all patients; however, an exceedingly low value is a poor prognostic marker[34]. Inflammatory biomarkers, such as C-reactive protein and procalcitonin have been found to be nonspecifically raised in most patients with ALA[34,59,61].
IMAGING EVALUATION: IMAGING CLASSIFICATION AND CLINICORADIOLOGICAL CORRELATION
Chest radiographs, ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are the most employed modalities for diagnosis of ALA. Radiographs are insensitive, non-specific and are abnormal in only half of cases[23]. They can reveal elevated diaphragm, pleural effusion and basal consolidation or atelectasis. MRI seems to offer no advantage over CT[33,62]. Of all radiological tests, ultrasound and CT are the most employed tools; in fact, they are complementary to one another in many ways. For example, ultrasound can detect the degrees of liquefaction, differentiating solid necrotic tissue from more liquefied tissue; this information is not provided by CT. Ultrasound, however, can fail to detect an early abscess when the lesion is not liquid enough to be visible[63]. CT is more sensitive in this regard. Another concern with ultrasound may be that early aggressive abscesses might be mistaken for necrotic malignant masses because they often contain solid (non-liquefied) necrotic material[8,38,39,47]. Due to its ability to differentiate viable tissue from necrotic tissue, contrast-enhanced CT can distinguish between necrotic mass and aggressive abscesses. Additionally, CT is useful in the identification of various complications associated with ALA. Although both ultrasound and CT are highly sensitive (ultrasound, 85%-95%; CT, 100%)[64], their specificity is low for differentiating ALA from other infective abscesses or necrotic masses[45].
The imaging features of ALA on CT have been described as round oval hypodense lesions with a rim enhancing wall and on sonography as hypoechoic or anechoic lesions with internal echoes. This classic description of ALA, however, does not take into account the entire spectrum of the imaging findings, which are known to vary considerably. The varied morphology has largely been shown to reflect the underlying pathological changes, which occur as ALA evolves through the different phases of maturation. Acute abscesses consist mainly of solid necrotic tissue and their edges are irregular or ragged. As the abscesses heal, there is formation of a distinct wall, edges become smooth, and the contents become more liquefied[10,38,43]. This morphologic variation has prompted several investigators to classify the imaging features of ALA into distinct types[46,65,66]. Most investigators have classified ALA into three types based on sonographic appearance. In 1987, Léonetti
[65] divided the sonographic morphology into three stages: pre-suppurative stage (phase I), suppurative stage (phase II), and scarring stage (phase III). Subsequently, N'Gbesso
[66] proposed a similar sonographic classification: non-collected ALA (type I), collected ALA (type II), and healed ALA (type III).
I looked up in horror and disbelief. There was Justin on the roof of the house, filling his plastic bucket with the ripe juicy plums from his favorite tree.
On MRI, a variable degree of wall formation and edema surrounding ALA have been reported according to the status of abscess healing. Elizondo
[43], who examined 29 ALAs with MRI, reported that untreated ALAs are associated with an incomplete ring (corresponding to incomplete wall) and diffuse or wedge-shaped perilesional edema. Following successful treatment, the ring formation is complete and the edema regresses to form concentric rings around the abscess. Matching with the MRI findings, a double-target sign has been described on contrast-enhanced CT; the inner ring corresponds to the enhancing wall and the outer ring to the perilesional edema[10,67].
Bill was a retired, life long bachelor. He lived alone in the small terraced house() next door but two from us. On a number of occasions, I visited Bill s house, and it seemed that it hadn t really changed much from the 50s. There were hints that some articles had been undisturbed apart from the occasional silverfish or visiting woodlouse, since the 1930s.
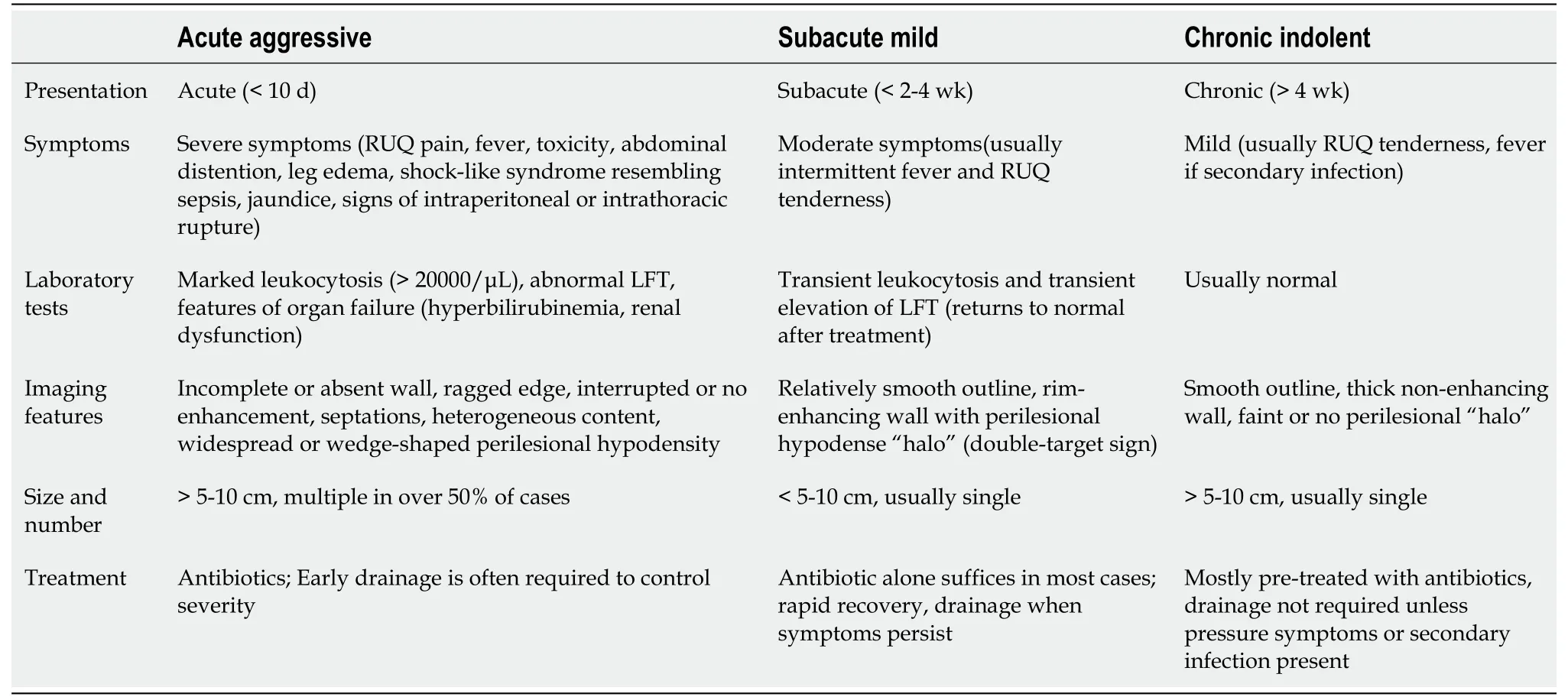
Type I: ALA with ragged edges
When Karen was in high school and I was married, living far away from home, we went through a second divorce. This time, however, I was careful to maintain ties. Gordon remained the father figure he d always been and even became Grandpa Gordon to my firstborn. Karen and Gordon grew apart some, but reestablished ties after graduation.
Type II: ALA with a complete rim enhancing wall
Although aspirations have been useful in the management of refractory abscesses for several decades, free rupture with peritonitis was typically considered an indication for surgery. The reported mortality rate in surgically treated patients was as high as 50%[90,91]. In the last three decades, a paradigm shift has been seen from surgical drainage to catheter drainage. All complications related to ALA are currently managed with percutaneous catheter drainage[11,19-21,92-94]. By using catheter drainage, we have achieved a success rate of 97%, without significant mortality[11]. Only the placement of multiple catheters, usually in multiple sessions, is required to drain intraperitoneal fluid collections. As the collections are sterile, the peritonitis is not as severe as that seen in cases of bowel perforation. Not only is catheter drainage curative for the intraperitoneal rupture, it also effectively treats pleuropulmonary ruptures[11]. The drainage of pleural fluid collections may require CT guidance as ultrasound has low sensitivity for pleuropulmonary pathology. Lung abscesses usually do not require drainage due to the presence of bronchial fistula, which provides natural drainage in most patients. Catheter drainage has also been proved to be excellent in the management of biliary communications. Agarwal
[22] evaluated 33 patients with refractory abscesses, nine of the patients were found to have an abscess with intrabiliary communication, and all patients were successfully treated with prolonged catheter drainage (12 to 50 d). None of the patients required endoscopic placement of stents. Endoscopic stenting or sphincterotomy, however, may be required to control bile leak prior to catheter removal when fistulous communication persists despite prolonged catheter drainage. Catheter drainage has also been shown to facilitate spontaneous healing of small arterial aneurysms resulting from ALA[83].
I...I thought you could use it for something. Susan s stammered3(,) explanation did nothing to help us understand why a twelve-by-eighteen-inch dark blue carpet remnant() was being presented as a birthday gift.
Type III: ALA with a nonenhancing wall
Type III pattern represents chronic indolent ALA. It is characterized by a thick fibrotic wall that is much smoother and does not enhance with contrast (Figure 1C). The absence of contrast enhancement excludes active inflammation. This pattern, in fact, represents persistence of amebic pus (usually more than four weeks), in which the liver fails to clear the necrotic tissue. The abscesses in this form are usually asymptomatic; however, when they are large enough to cause capsular stretching, they can cause right upper quadrant pain. Clinicians should be aware that healed ALAs in this pattern often resemble cysts and can persist for months or years following successful treatment[46,66,71,72].
COMPLICATIONS: CLINICO-RADIOLOGICAL FINDINGS
Rupture
Intraperitoneal rupture has been said to occur in only 7% of cases[7,56,57]. However, we found an incidence of intraperitoneal rupture of 33% in our series[10]. In fact, several series from endemic countries have reported similar findings[6,13,17]. Based on imaging findings, intraperitoneal ruptures can be divided into two types: contained rupture and free rupture[11,60]. The contained rupture is characterized by accumulation of the localized fluid collection around the liver, usually in the subphrenic or subhepatic space (Figure 3A)[11]. The localized fluid from the contained rupture may occasionally be palpable on abdominal examination. This type of rupture carries a good prognosis and fortunately, is more common than its counterpart - the free rupture. The free rupture is characterized by fluid collection that diffusely involves the entire peritoneal cavity; it can cause generalized peritonitis and carry a poor prognosis (Figure 3B). The differentiation between these two types is significant as more aggressive treatment for longer duration is required for free ruptures[11,21].
Intrathoracic rupture: Pleural empyema, lung abscess, hepatobronchial fistula
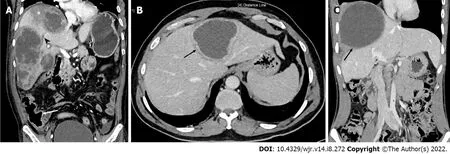
Pleuropulmonary rupture occurs in 7% to 20% of patients[7,56,57]. The abscesses located inferior to the diaphragm can perforate it to enter the pleural space causing amebic empyema, which is the most common intrathoracic complication. It is important that pleural empyema be differentiated from sterile pleural effusion, which occurs more frequently than empyema. The sterile effusion is reactionary and resolves spontaneously, and therefore, it requires no drainage[57]. The presence of loculations and septations on ultrasound indicate amebic empyema[11]. The next intrathoracic complication is the development of lung consolidation or lung abscess, which occurs when an abscess directly ruptures into lung parenchyma invading through both the diaphragm and pleura. The lung abscess may, in turn, communicate with the bronchi to cause hepatobronchial fistula or with pleura to cause bronchopleural fistula. Bronchial communication has been reported to occur in over one-third of thoracic complications[76]. The presence of air in the lung abscess or liver abscess or in the pleural collections indicates these fistulous complications (Figure 2)[11]. Clinically, the patients complain of productive cough, often expectoration of amebic pus-like material. The pleuropulmonary rupture is considered less severe than the intraperitoneal rupture because of spontaneous drainage of the abscesses following the hepatobronchial fistula.
When she came to the first jar the robber inside said softly: Is it time? Any other slave but Morgiana, on finding a man in the jar instead of the oil she wanted, would have screamed and made a noise; but she, knowing the danger her master was in, bethought herself of a plan, and answered quietly: Not yet, but presently
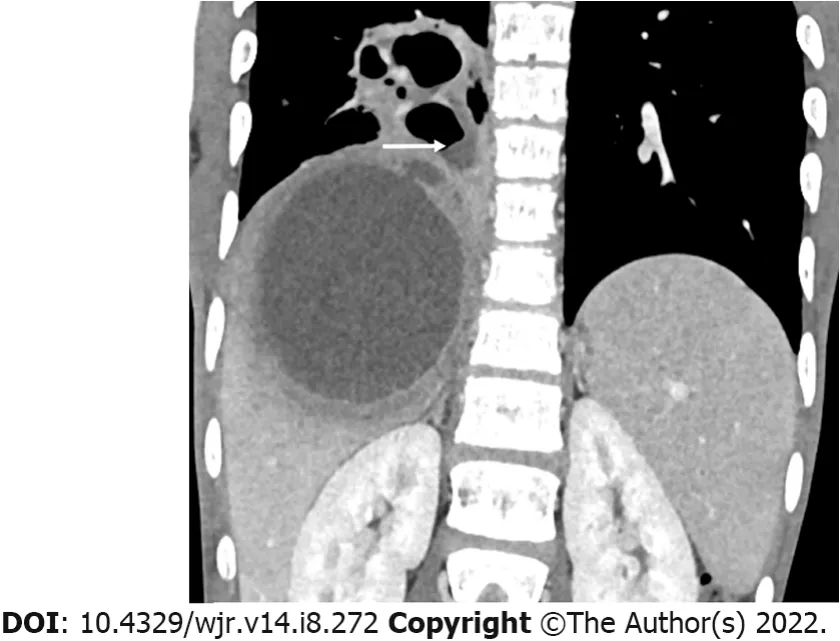
Intraperitoneal rupture: Contained rupture versus free rupture
The most feared complication of ALA is rupture. The overall incidence ranges from 6 to 40%[10,44,52,73]. ALA generally ruptures into the thoracic cavity or intraperitoneal space. Occasionally, the abscess can rupture into hollow viscera, such as the stomach, duodenum, or colon[20,60,74,75]. Of all ruptures, the gravest, but fortunately rare, is rupture into the pericardium[49]. In our experience, the risk of free intraperitoneal ruptures is high when the abscesses present acutely (type I pattern). However, intrathoracic ruptures, particularly the intrapulmonary ones, are noted more frequently in chronic cases (type II or III pattern). This may be due to development of adhesion between the diaphragm and pleura in older abscesses[10].
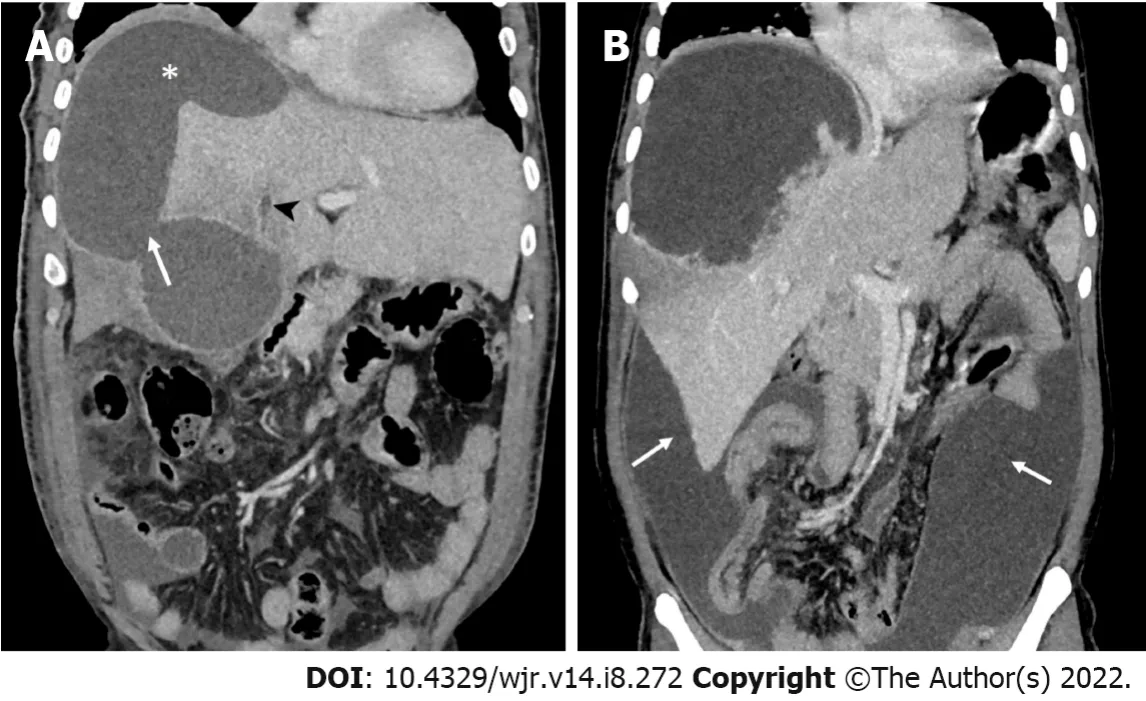
Biliary complication: Communication versus compression
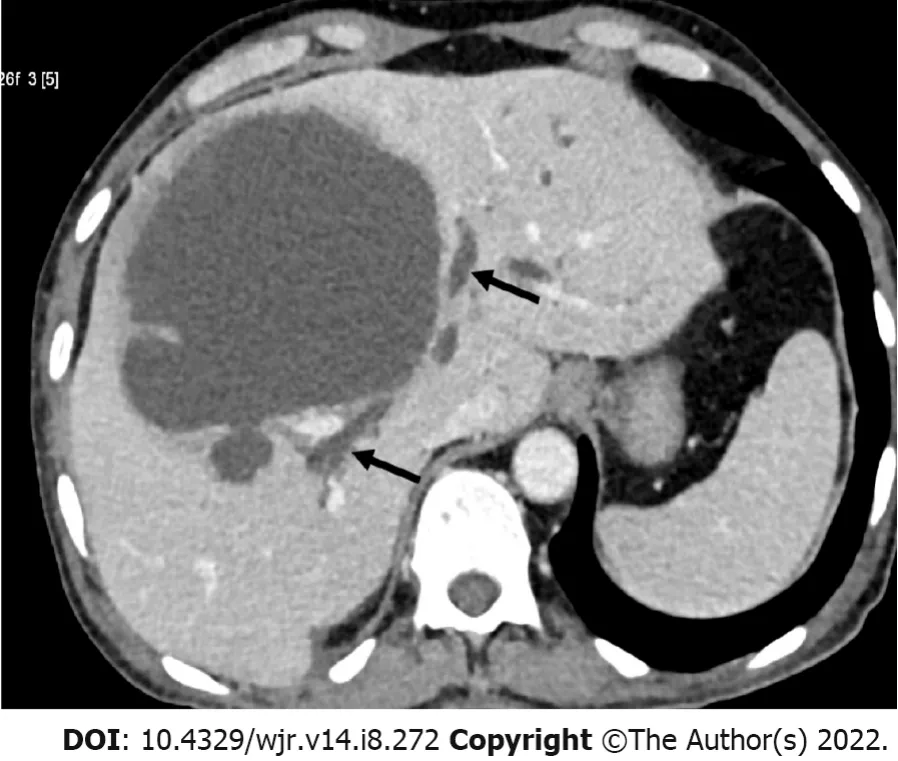
A common cause of drug failure is the presence of biliary complications, which has been reported to occur in up to 27% of refractory cases[12,22,77]. This occurs either from ductal communication with the abscess or from external compression by a large abscess[12,41]. When the liver parenchyma is destroyed by an aggressive abscess, the bile ducts are also damaged, producing ductal communications[12]. Usually, the communication is subtle, and therefore, ductal dilatation may not be evident on imaging. In several cases, the diagnosis is made only during percutaneous drainage when the initial aspirated fluid is bilious or when bile (usually persistent) appears thereafter[11,22,77]. Uncommonly, an abscess, particularly when large and aggressive, can rupture into the central bile ducts, causing duct dilation (Figure 4). In such cases, the diagnosis may be confirmed when endoscopic retrograde cholangiopancreatography (ERCP) or cavitogram demonstrates contrast extravasation into the abscess cavity[22,55]. Usually, the bile ducts are compressed by a large abscess, resulting in biliary duct dilation; these cases are evident on ultrasound or CT. The size and location of an abscess on imaging can provide anatomic clues to the presence of a biliary complication. The large (> 5 to 10 cm) and centrally located abscesses (near porta hepatis) are more likely to have biliary compilations than those smaller and with subcapsular locations[12]. Clinically, the presence of high jaundice may indicate biliary complications. Agarwal
[22] compared the abscesses with and without biliary communications and found that total bilirubin levels > 2 mg/dL were present only in the patients with biliary complications.
One evening she managed to get hold of it, and the young man watched carefully where she hid it away, in a secret place in the bedchamber of her mistress
Secondary bacterial infection
ALA is typically sterile. However, in 10% to 20% of cases, it can be complicated by secondary bacterial infections[58,78,79]. The incidence may be higher than generally recognized. Recently, in a PCR based study from liver aspirates, Singh
[2] found bacterial infection in 37% of cases, mostly anaerobes of intestinal microbiota. The authors suggested that intestinal bacteria reach the liver along with the trophozoites through the portal route, that is, concurrent or coinfection with bacteria. When secondary bacterial infection occurs as coinfection, the disease may take an aggressive course. This complication should be suspected in refractory cases, particularly those associated with persistent high fever and marked leukocytosis (> 20000/μL)[56]. Another mechanism of secondary infection is bacterial superinfection, which usually occurs in the stagnant fluid following unsuccessful needle aspiration or inadequate catheter drainage[18]. Since most of the abscesses are walled off at this point, symptoms are of chronic indolent disease. In contrast to sterile amebic aspirate, cultures of pus from secondarily infected ALA usually yield positive results. Blood cultures, however, may be negative because most patients are generally pretreated with antibiotics[80].
Vascular complication: Venous thrombosis, venous compression and arterial aneurysm
Venous thrombosis is a common phenomenon in this disease. Autopsy studies have shown that venous thrombosis occurs in up to 30% of cases; however, we have identified venous thrombus in 70% of cases with the use of the latest multidetector CT[42,68]. Venous thrombosis may involve the portal or hepatic vein, but usually both are involved. Thrombus typically occurs in the smaller segmental or subsegmental branches. The hepatic vein thrombosis can extend into the inferior vena cava (IVC) or even into the right atrium[68]. Rarely, it can cause a Budd-Chiari like syndrome[81]. Detection of thrombus in large veins may be indicative of severe ALA[68,82]. The diagnosis of thrombosis on CT can be suggested by the presence of a wedge-shaped hypoattenuating area surrounding the abscess, which might be due to thrombosis led hypoperfusion[68]. Another vascular complication is compression of the intrahepatic veins and the IVC. Venous compression may be a clue to the presence of a high intracavitary pressure in the abscess, which in turn indicates aggressive abscesses. IVC compression occurs when a large abscess located in the caudate lobe compresses the IVC, causing leg edema[48]. Additionally, portal vein compression near the porta hepatis has been reported to cause splenomegaly and portal hypertension[41]. Hepatic artery pseudoaneurysm is a rare, but serious complication of ALA that results from erosion of the arterial wall by an aggressive abscess[83].
Concurrent colitis and perforations
Although diarrhea is found in only 15% to 30% of patients with ALA, concurrent colonic ulcers are detected in approximately 50% of patients with ALA on colonoscopy[17,57,84,85]. The colonic lesions on colonoscopy appear as small discrete ulcers in the cecum or ascending colon. Approximately 70% of the ulcers are localized to cecum and contiguous involvement of the appendix (amebic typhlo-appendicitis) is common[10]. As the ulcers are usually small and localized, symptoms related to colitis are mild. In severe cases, however, other segments may also be involved or there may be cecal perforations. Furthermore, the severity of colitis seems to parallel the severity of abscesses. Recently, Premkumar
[85], in a study of 52 patients with ALA, reported bleeding and large ileocecal ulcers in the majority of their patients; most synchronous ALAs in this series had aggressive clinical and imaging features. In an autopsy study of 76 patients with fatal ALA, Aikat
[42] found that the incidence of colonic ulcers was 62%. With multidetector CT, we have observed concurrent colitis in 28% of patients, more frequently and possibly more severe in the patients with aggressive ALA than those with mild ALA. On CT, colitis generally manifests as nonspecific bowel wall thickening (Figure 1A)[10].
Secondly107, there is the Scorpion108 of Solomon (on whom be peace), which is a sword such as no king has; steel and stone are one to it; if you bring it down on a rock it will not be injured, and it will cleave109 whatever you strike
Our recent experience suggests that the latest generation CT can effectively evaluate several imaging characteristics, such as wall formation, degree of liquefaction, enhancement patterns, septa, or perilesional hypodensity[10]. These characteristics can provide considerable information on the patient’s clinical status. It appears that imaging findings of ALA can be classified into three distinct but overlapping patterns (type I, II and III) that correlate well with the clinical subtypes (Table 1)[10]. This classification may be useful for identifying those abscesses that would require more aggressive treatment.
MANAGEMENT: ROLE OF IMAGE-GUIDED PERCUTANEOUS DRAINAGE
ALA, in most patients, is mild and responds promptly to medical therapy. The drug of choice for the treatment of ALA is metronidazole, a nitroimidazole, which is given at a dose of 750 mg orally or intravenously three times daily for seven to ten days[31]. This regime results in resolution of fever, toxemia, and pain in 80% to 90% of patients with uncomplicated ALA within 72 to 96 h of treatment[7]. The disease resolves without complications or without the need for any invasive procedures. This treatment is followed by a luminal agent (paromomycin or diloxanide furoate) to clear the luminal parasites.
I ve run away from a little old woman,/ A little old man,/And I can run away from you, I can!: The Gingerbread Man is a popular type of tale called the cumulative13 tale
Type I pattern is observed in patients with acute aggressive ALA. It is characterized by incomplete or absent walls and ragged edges (Figure 1A). This pattern is observed in patients with acute aggressive ALA. Type I pattern indicates an early and progressive abscess, with no sign of healing. Surrounding the abscess, there is a diffuse or wedge-shaped hypodensity, which is usually due to the combined effect of hypoperfusion and edema[10,68]. Most cases show irregular and interrupted enhancement at the edges. Multiple irregular septa may be observed at the periphery, indicating the viable parenchyma that is yet to be necrotic[10]. On sonography, they appear heterogeneous due to the presence of both solid and liquefied necrotic tissue[38,47]. The heterogeneity accounts for the frequent misdiagnosis of aggressive ALA as malignant lesions[10,38,47,67]. Other imaging features often associated with type I morphology are large size, multiplicity, and irregular shape (due to coalescence of multiple lesions)[10].
Most patients (approximately 80%) have a subacute course characterized by mild symptoms that develop in less than 2 to 4 wk[23,28,30,49-51]. The disease typically begins with fever and chills, right upper quadrant pain and tender hepatomegaly. Other symptoms include anorexia, weakness, nausea and diarrhea. There may be right shoulder pain when an abscess located in the posterosuperior segments irritates the diaphragm. The typical finding on physical examination is point tenderness in the intercostal spaces[31]. The disease is associated with no or minimal organ dysfunction; the laboratory parameters are near normal except mild to moderate leukocytosis. Dramatic improvement is observed after medical therapy and no further complications occur. This pattern of presentation has also been referred to as “acute benign ALA” by a few authors; however, the term “subacute mild” may be preferable as it correctly defines the clinical course of the disease[15,48]. Additionally, the term also differentiates it from the two other forms of the disease,
, acute aggressive ALA and chronic indolent ALA.
In addition to clinical criteria, imaging-based criteria for the use of drainage was formulated by de la Rey Nel
[86]. They recommended that abscesses with the following risk factors should be drained: abscesses > 10 cm (because of their long healing time), abscesses located in the left lobe (because of the risk of rupture into the pericardium), and large superficial abscesses with a thin rim (because of the risk of rupture). In this context, it must be emphasized that lack of a mature wall is also an important risk factor that must be considered while assessing rupture risk. Most intraperitoneal ruptures in our series occurred when the abscesses lacked a mature wall[10].
Needle aspiration vs catheter drainage
Percutaneous drainage can be performed either by needle aspiration or catheter drainage under image guidance. Usually, sonographic guidance suffices for the placement of the catheter or needle into the abscess cavity[11]. CT guidance may be required in some cases, particularly in thoracic complications. Success of the procedure is dependent on its effectiveness in evacuation of the amebic pus. Needle aspiration is a simple, less invasive technique and requires less expertise. However, it is not as effective as catheter drainage, and presents several disadvantages. It fails to evacuate the solid necrotic tissue, which usually blocks the needle lumen during aspiration. Since tissue necrosis and its liquefaction is a dynamic process, not all tissue is completely liquid at the time of aspiration, and therefore, multiple sessions are generally needed to achieve complete drainage. This practice is perhaps related to the most serious drawback of needle aspiration,
, bacterial superinfections. The reported rate of superinfections following needle aspirations is 15%[18]. Nevertheless, needle aspirations may be useful in the appropriate settings, such as when the abscesses are small (< 5 cm) and the content is completely liquefied. Another common scenario includes multiple abscesses, where smaller and more liquefied abscesses are aspirated using an 18G spinal needle, whereas the larger and partially necrotic abscesses are drained using catheters[11]. Several randomized controlled studies have demonstrated that catheter drainage offers a higher success rate (up to 100%) compared to needle aspiration, particularly when abscesses are larger than 5 cm[78,87-89]. Due to its obvious advantage of having a large bore, it evacuates the necrotic tissue efficiently. It has an additional advantage of being indwelling, which makes it more effective in clearing those abscesses that liquefy over a period of time.
Percutaneous drainage in the management of complications
Type II pattern indicates subacute mild ALA. It is characterized by a well-defined enhancing wall (Figure 1B). The rim enhancement of the wall indicates active granulation tissue, a pathological sign of inflammation and beginning of healing[43]. A thin rim of edema surrounding the wall (in contrast to the more widespread edema of type I pattern) may be observed to form a perilesional “halo” on contrast CT. In many cases, a double-target sign (the inner ring of wall enhancement and outer ring of hypodense edema) is identified. The content is more liquefied and homogeneous compared to those presenting acutely. This pattern is nonspecific and resembles pyogenic abscesses[43,69,70].
Surgical management
The role of surgical drainage in the management of ALA has been reassessed due to the widespread use of radiologically guided drainage[95]. However, open drainage may be warranted in some cases where percutaneous drainage may fail to evacuate abscess content. Surgery may also be indicated in selected cases of intraperitoneal rupture with generalized peritonitis[96]. As an alternative to open surgical drainage, laparoscopic drainage can result in less morbidity and mortality[97].
CONCLUSION
Clinical and imaging features of ALA are variable and parallel to each other. Although the mild form of the disease is cured easily with antibiotics alone, the other two forms of the disease-acute aggressive and chronic indolent-often require percutaneous drainage. Most complications and mortality in ALA occur when it presents in its acute aggressive form. Imaging studies play a key role in identifying the different forms of the disease and assessing the complications. All complications, including free intraperitoneal ruptures, can be managed with percutaneous catheter drainage.
FOOTNOTES
Priyadarshi RN contributed to the concept and design of the manuscript, data collection and manuscript writing; Kumar R and Anand U contributed to the literature search, critical inputs and manuscript revision.
As far back as I can remember, the large pickle1 jar sat on the floor beside the dresser in my parents bedroom. When he got ready for bed, Dad would empty his pockets and toss his coins into the jar. As a small boy I was always fascinated at the sounds the coins made as they were dropped into the jar. They landed with a merry jingle2 when the jar was almost empty. Then the tones gradually muted to a dull thud as the jar was filled. I used to squat3 on the floor in front of the jar and admire the copper4 and silver circles that glinted like a pirate s treasure when the sun poured through the bedroom window.
All the authors declare that they have no conflict of interest related to this article.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Tyler wasn t willing to give up one single moment of his childhood to this deadly disease. It was not unusual to find him playing and racing3 around his backyard, wearing his medicine-laden(,) backpack and dragging his tank of oxygen behind him in his little wagon4(,) . All of us who knew Tyler marveled(,) at his pure joy in being alive and the energy it gave him. Tyler s mom often teased() him by telling him that he moved so fast she needed to dress him in red. That way, when she peered() through the window to check on him playing in the yard, she could quickly spot him.
India
Rajeev Nayan Priyadarshi 0000-0003-2890-8910; Ramesh Kumar 0000-0001-5136-4865; Utpal Anand 0000-0003-0653-4129.
Liu JH
Webster JR
Liu JH
1 Walsh JA. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality.
1986; 8: 228-238 [PMID: 2871619 DOI: 10.1093/clinids/8.2.228]
2 Singh A, Banerjee T, Kumar R, Shukla SK. Prevalence of cases of amebic liver abscess in a tertiary care centre in India: A study on risk factors, associated microflora and strain variation of Entamoeba histolytica.
2019; 14: e0214880 [PMID: 30943253 DOI: 10.1371/journal.pone.0214880]
3 Khan R, Hamid S, Abid S, Jafri W, Abbas Z, Islam M, Shah H, Beg S. Predictive factors for early aspiration in liver abscess.
2008; 14: 2089-2093 [PMID: 18395912 DOI: 10.3748/wjg.14.2089]
4 Ghosh S, Sharma S, Gadpayle AK, Gupta HK, Mahajan RK, Sahoo R, Kumar N. Clinical, laboratory, and management profile in patients of liver abscess from northern India.
2014; 2014: 142382 [PMID: 25002869 DOI: 10.1155/2014/142382]
5 Khanna S, Chaudhary D, Kumar A, Vij JC. Experience with aspiration in cases of amebic liver abscess in an endemic area.
2005; 24: 428-430 [PMID: 15928909 DOI: 10.1007/s10096-005-1338-2]
6 Jindal A, Pandey A, Sharma MK, Mukund A, Vijayaraghavan R, Arora V, Shasthry SM, Choudhary A, Sarin SK. Management Practices and Predictors of Outcome of Liver Abscess in Adults: A Series of 1630 Patients from a Liver Unit.
2021; 11: 312-320 [PMID: 33994714 DOI: 10.1016/j.jceh.2020.10.002]
7 Stanley SL Jr. Amoebiasis.
2003; 361: 1025-1034 [PMID: 12660071 DOI: 10.1016/S0140-6736(03)12830-9]
8 Ralls PW, Barnes PF, Johnson MB, De Cock KM, Radin DR, Halls J. Medical treatment of hepatic amebic abscess: rare need for percutaneous drainage.
1987; 165: 805-807 [PMID: 3317505 DOI: 10.1148/radiology.165.3.3317505]
9 Nigam P, Gupta AK, Kapoor KK, Sharan GR, Goyal BM, Joshi LD. Cholestasis in amoebic liver abscess.
1985; 26: 140-145 [PMID: 3967831 DOI: 10.1136/gut.26.2.140]
10 Priyadarshi RN, Sherin L, Kumar R, Anand U, Kumar P. CT of amebic liver abscess: different morphological types with different clinical features.
2021; 46: 4148-4158 [PMID: 33893854 DOI: 10.1007/s00261-021-03093-w]
11 Priyadarshi RN, Prakash V, Anand U, Kumar P, Jha AK, Kumar R. Ultrasound-guided percutaneous catheter drainage of various types of ruptured amebic liver abscess: a report of 117 cases from a highly endemic zone of India.
2019; 44: 877-885 [PMID: 30361869 DOI: 10.1007/s00261-018-1810-y]
12 Datta DV, Saha S, Singh SA, Aikat BK, Chhuttani PN. The clinical pattern and prognosis of patients with amebic liver abscess and jaundice.
1973; 18: 887-898 [PMID: 4355077 DOI: 10.1007/BF01073340]
13 Jha AK, Jha P, Chaudhary M, Purkayastha S, Jha SK, Ranjan R, Priyadarshi RN, Kumar R. Evaluation of factors associated with complications in amoebic liver abscess in a predominantly toddy-drinking population: A retrospective study of 198 cases.
2019; 3: 474-479 [PMID: 31832547 DOI: 10.1002/jgh3.12183]
14 Sharma N, Sharma A, Varma S, Lal A, Singh V. Amoebic liver abscess in the medical emergency of a North Indian hospital.
2010; 3: 21 [PMID: 20181006 DOI: 10.1186/1756-0500-3-21]
15 Katzenstein D, Rickerson V, Braude A. New concepts of amebic liver abscess derived from hepatic imaging, serodiagnosis, and hepatic enzymes in 67 consecutive cases in San Diego.
1982; 61: 237-246 [PMID: 6806561 DOI: 10.1097/00005792-198207000-00003]
16 Chuah SK, Chang-Chien CS, Sheen IS, Lin HH, Chiou SS, Chiu CT, Kuo CH, Chen JJ, Chiu KW. The prognostic factors of severe amebic liver abscess: a retrospective study of 125 cases.
1992; 46: 398-402 [PMID: 1575285 DOI: 10.4269/ajtmh.1992.46.398]
17 Balasegaram M. Management of hepatic abscess.
1981; 18: 282-340 [PMID: 6263552]
18 Singh JP, Kashyap A. A comparative evaluation of percutaneous catheter drainage for resistant amebic liver abscesses.
1989; 158: 58-62 [PMID: 2662790 DOI: 10.1016/0002-9610(89)90316-4]
19 Hanna RM, Dahniya MH, Badr SS, El-Betagy A. Percutaneous catheter drainage in drug-resistant amoebic liver abscess.
2000; 5: 578-581 [PMID: 10995100 DOI: 10.1046/j.1365-3156.2000.00586.x]
20 Ken JG, vanSonnenberg E, Casola G, Christensen R, Polansky AM. Perforated amebic liver abscesses: successful percutaneous treatment.
1989; 170: 195-197 [PMID: 2909097 DOI: 10.1148/radiology.170.1.2909097]
21 Baijal SS, Agarwal DK, Roy S, Choudhuri G. Complex ruptured amebic liver abscesses: the role of percutaneous catheter drainage.
1995; 20: 65-67 [PMID: 7556258 DOI: 10.1016/0720-048x(95)00613-u]
22 Agarwal DK, Baijal SS, Roy S, Mittal BR, Gupta R, Choudhuri G. Percutaneous catheter drainage of amebic liver abscesses with and without intrahepatic biliary communication: a comparative study.
1995; 20: 61-64 [PMID: 7556257 DOI: 10.1016/0720-048x(95)00603-n]
23 Hughes MA, Petri WA Jr. Amebic liver abscess.
2000; 14: 565-582, viii [PMID: 10987110 DOI: 10.1016/s0891-5520(05)70121-5]
24 Kumanan T, Sujanitha V, Sreeharan N. Amoebic liver abscess: a neglected tropical disease.
2020; 20: 160-162 [PMID: 32006496 DOI: 10.1016/S1473-3099(19)30696-6]
25 Kannathasan S, Murugananthan A, Kumanan T, de Silva NR, Rajeshkannan N, Haque R, Iddawela D. Epidemiology and factors associated with amoebic liver abscess in northern Sri Lanka.
2018; 18: 118 [PMID: 29316900 DOI: 10.1186/s12889-018-5036-2]
26 Ray G. Sociodemographic and Clinical Profile of Amoebic Liver Abscess observed at a Tertiary Referral Hospital over 10 Years.
2021; 42: 126-33
27 Nath J, Ghosh SK, Singha B, Paul J. Molecular Epidemiology of Amoebiasis: A Cross-Sectional Study among North East Indian Population.
2015; 9: e0004225 [PMID: 26633890 DOI: 10.1371/journal.pntd.0004225]
28 Wuerz T, Kane JB, Boggild AK, Krajden S, Keystone JS, Fuksa M, Kain KC, Warren R, Kempston J, Anderson J. A review of amoebic liver abscess for clinicians in a nonendemic setting.
2012; 26: 729-733 [PMID: 23061067 DOI: 10.1155/2012/852835]
29 Kumar R, Priyadarshi RN, Anand U. Toddy consumption and amoebic liver abscess in India: An unexplored link.
2019; 63: 89-90 [PMID: 30880745 DOI: 10.4103/ijph.IJPH_192_18]
30 Mukhopadhyay M, Saha AK, Sarkar A, Mukherjee S. Amoebic liver abscess: presentation and complications.
2010; 72: 37-41 [PMID: 23133202 DOI: 10.1007/s12262-010-0007-6]
31 Haque R, Huston CD, Hughes M, Houpt E, Petri WA Jr. Amebiasis.
2003; 348: 1565-1573 [PMID: 12700377 DOI: 10.1056/NEJMra022710]
32 Sharma MP, Dasarathy S, Verma N, Saksena S, Shukla DK. Prognostic markers in amebic liver abscess: a prospective study.
1996; 91: 2584-2588 [PMID: 8946991]
33 Singh R, Adhikari DR, Patil BP, Talathi NR, Hanamshetti SR, Joshi RM. Amoebic liver abscess: an appraisal.
2011; 96: 305-309 [PMID: 22808611 DOI: 10.9738/cc9.1]
34 Khim G, Em S, Mo S, Townell N. Liver abscess: diagnostic and management issues found in the low resource setting.
2019; 132: 45-52 [PMID: 31836890 DOI: 10.1093/bmb/ldz032]
35 Brandt H, Tamayo RP. Pathology of human amebiasis.
1970; 1: 351-385 [PMID: 4330002 DOI: 10.1016/s0046-8177(70)80072-7]
36 Martínez-Palomo A. The pathogenesis of amoebiasis.
1987; 3: 111-118 [PMID: 15462926 DOI: 10.1016/0169-4758(87)90048-2]
37 Tsutsumi V, Mena-Lopez R, Anaya-Velazquez F, Martinez-Palomo A. Cellular bases of experimental amebic liver abscess formation.
1984; 117: 81-91 [PMID: 6385728]
38 Simjee AE, Patel A, Gathiram V, Engelbrecht HE, Singh K, Rooknoodeen F. Serial ultrasound in amoebic liver abscess.
1985; 36: 61-68 [PMID: 3905191 DOI: 10.1016/s0009-9260(85)80027-1]
39 Missalek W. Ultrasonography in the diagnosis of amoebic liver abscess and its complications.
1992; 22: 59-64 [PMID: 1318595 DOI: 10.1177/004947559202200205]
40 Jimenez F. Pathology of amebiasis.
1981; 57: 217-223 [PMID: 6938282]
41 Knight R. Hepatic amebiasis.
1984; 4: 277-292 [PMID: 6098014 DOI: 10.1055/s-2008-1040657]
42 Aikat BK, Bhusnurmath SR, Pal AK, Chhuttani PN, Datta DV. The pathology and pathogenesis of fatal hepatic amoebiasis--A study based on 79 autopsy cases.
1979; 73: 188-192 [PMID: 473308 DOI: 10.1016/0035-9203(79)90209-8]
43 Elizondo G, Weissleder R, Stark DD, Todd LE, Compton C, Wittenberg J, Ferrucci JT Jr. Amebic liver abscess: diagnosis and treatment evaluation with MR imaging.
1987; 165: 795-800 [PMID: 2891154 DOI: 10.1148/radiology.165.3.2891154]
44 Basile JA, Klein SR, Worthen NJ, Wilson SE, Hiatt JR. Amebic liver abscess. The surgeon's role in management.
1983; 146: 67-71 [PMID: 6869681 DOI: 10.1016/0002-9610(83)90261-1]
45 Radin DR, Ralls PW, Colletti PM, Halls JM. CT of amebic liver abscess.
1988; 150: 1297-1301 [PMID: 3259367 DOI: 10.2214/ajr.150.6.1297]
46 Nari GA, Ceballos Espinosa R, Carrera Ladrón de Guevara S, Preciado Vargas J, Cruz Valenciano JL, Briones Rivas JL, Moreno Hernández F, Góngora Ortega J. [Amebic liver abscess. Three years experience].
2008; 100: 268-272 [PMID: 18662078 DOI: 10.4321/s1130-01082008000500004]
47 Boultbee JE, Simjee AE, Rooknoodeen F, Engelbrecht HE. Experiences with grey scale ultrasonography in hepatic amoebiasis.
1979; 30: 683-689 [PMID: 509870 DOI: 10.1016/s0009-9260(79)80020-3]
48 Sharma MP, Ahuja V. Amoebic liver abscess.
2003; 4: 107-11
49 Adams EB, MacLeod IN. Invasive amebiasis. II. Amebic liver abscess and its complications.
1977; 56: 325-334 [PMID: 875719 DOI: 10.1097/00005792-197707000-00004]
50 Wells CD, Arguedas M. Amebic liver abscess.
2004; 97: 673-682 [PMID: 15301125 DOI: 10.1097/00007611-200407000-00013]
51 Anesi JA, Gluckman S. Amebic liver abscess.
2015; 6: 41-43 [PMID: 31040985 DOI: 10.1002/cld.488]
52 Alam F, Salam MA, Hassan P, Mahmood I, Kabir M, Haque R. Amebic liver abscess in northern region of Bangladesh: sociodemographic determinants and clinical outcomes.
2014; 7: 625 [PMID: 25204395 DOI: 10.1186/1756-0500-7-625]
53 Wallace RJ Jr, Greenberg SB, Lau JM, Kalchoff WP, Mangold DE, Martin R. Amebic peritonitis following rupture of an amebic liver abscess. Successful treatment of two patients.
1978; 113: 322-325 [PMID: 205190 DOI: 10.1001/archsurg.1978.01370150094024]
54 Ajao OG, Adebo OA. Unruptured amoebic liver abscess presenting as acute abdomen.
1983; 13: 109-111 [PMID: 6879689 DOI: 10.1177/004947558301300305]
55 Ibrarullah M, Agarwal DK, Baijal SS, Mittal BR, Kapoor VK. Amebic liver abscess with intra-biliary rupture.
1994; 7: 305-10; discussion 310 [PMID: 8204550 DOI: 10.1155/1994/36160]
56 Peters RS, Gitlin N, Libke RD. Amebic liver abscess.
1981; 32: 161-174 [PMID: 7013659 DOI: 10.1146/annurev.me.32.020181.001113]
57 Reed SL. Amebiasis: an update.
1992; 14: 385-393 [PMID: 1554822 DOI: 10.1093/clinids/14.2.385]
58 Barnes PF, De Cock KM, Reynolds TN, Ralls PW. A comparison of amebic and pyogenic abscess of the liver.
1987; 66: 472-483 [PMID: 3316923 DOI: 10.1097/00005792-198711000-00005]
59 Neill L, Edwards F, Collin SM, Harrington D, Wakerley D, Rao GG, McGregor AC. Clinical characteristics and treatment outcomes in a cohort of patients with pyogenic and amoebic liver abscess.
2019; 19: 490 [PMID: 31159769 DOI: 10.1186/s12879-019-4127-8]
60 Greaney GC, Reynolds TB, Donovan AJ. Ruptured amebic liver abscess.
1985; 120: 555-561 [PMID: 3885916 DOI: 10.1001/archsurg.1985.01390290037006]
61 Recipon G, Piver É, Caille A, Le Pape P, Pihet M, Pagès JC, Chandenier J, Desoubeaux G. Is procalcitonin increased in cases of invasive amoebiasis?
2015; 83: 395-399 [PMID: 26388549 DOI: 10.1016/j.diagmicrobio.2015.08.014]
62 Ralls PW, Henley DS, Colletti PM, Benson R, Raval JK, Radin DR, Boswell WD Jr, Halls JM. Amebic liver abscess: MR imaging.
1987; 165: 801-804 [PMID: 3317504 DOI: 10.1148/radiology.165.3.3317504]
63 Elzi L, Laifer G, Sendi P, Ledermann HP, Fluckiger U, Bassetti S. Low sensitivity of ultrasonography for the early diagnosis of amebic liver abscess.
2004; 117: 519-522 [PMID: 15464710 DOI: 10.1016/j.amjmed.2004.01.031]
64 Seeto RK, Rockey DC. Amebic liver abscess: epidemiology, clinical features, and outcome.
1999; 170: 104-109 [PMID: 10063397]
65 Léonetti P, Moncany G, Soubeyrand J. [Amebic abscess of the liver. Contribution of ultrasonics to developmental diagnosis apropos of 983 cases].
1987; 68: 259-264 [PMID: 3295224]
66 N'Gbesso RD, Kéita AK. [Ultrasonography of amebic liver abscesses. Proposal of a new classification].
1997; 78: 569-576 [PMID: 9537173]
67 Terrier F, Becker CD, Triller JK. Morphologic aspects of hepatic abscesses at computed tomography and ultrasound.
1983; 24: 129-137 [PMID: 6624514]
68 Priyadarshi RN, Kumar P, Kumar R, Anand U, Shyama. Venous thrombosis and segmental hypoperfusion in amebic liver abscess: MDCT demonstration and its implications.
2020; 45: 652-660 [PMID: 31955219 DOI: 10.1007/s00261-020-02409-6]
69 Qian LJ, Zhu J, Zhuang ZG, Xia Q, Liu Q, Xu JR. Spectrum of multilocular cystic hepatic lesions: CT and MR imaging findings with pathologic correlation.
2013; 33: 1419-1433 [PMID: 24025933 DOI: 10.1148/rg.335125063]
70 Mathieu D, Vasile N, Fagniez PL, Segui S, Grably D, Lardé D. Dynamic CT features of hepatic abscesses.
1985; 154: 749-752 [PMID: 3969480 DOI: 10.1148/radiology.154.3.3969480]
71 Ralls PW, Quinn MF, Boswell WD Jr, Colletti PM, Radin DR, Halls J. Patterns of resolution in successfully treated hepatic amebic abscess: sonographic evaluation.
1983; 149: 541-543 [PMID: 6622702 DOI: 10.1148/radiology.149.2.6622702]
72 Berry M, Bazaz R, Bhargava S. Amebic liver abscess: sonographic diagnosis and management.
1986; 14: 239-242 [PMID: 3084579 DOI: 10.1002/jcu.1870140402]
73 Sarda AK, Bal S, Sharma AK, Kapur MM. Intraperitoneal rupture of amoebic liver abscess.
1989; 76: 202-203 [PMID: 2702459 DOI: 10.1002/bjs.1800760231]
74 Pawar SV, Zanwar VG, Gambhire PA, Mohite AR, Choksey AS, Rathi PM, Asgaonkar DS. Unusual complication of amebic liver abscess: Hepatogastric fistula.
2015; 7: 916-919 [PMID: 26240693 DOI: 10.4253/wjge.v7.i9.916]
75 Mowji PJ, Cohen AJ, Potkin B, Viltuznik J. Amebic liver abscess with hepatoduodenal fistula.
1987; 82: 558-559 [PMID: 3578237]
76 Ibarra-Pérez C. Thoracic complications of amebic abscess of the liver: report of 501 cases.
1981; 79: 672-677 [PMID: 7226956 DOI: 10.1378/chest.79.6.672]
77 Sandeep SM, Banait VS, Thakur SK, Bapat MR, Rathi PM, Abraham P. Endoscopic biliary drainage in patients with amebic liver abscess and biliary communication.
2006; 25: 125-127 [PMID: 16877823]
78 Singh S, Chaudhary P, Saxena N, Khandelwal S, Poddar DD, Biswal UC. Treatment of liver abscess: prospective randomized comparison of catheter drainage and needle aspiration.
2013; 26: 332-339 [PMID: 24714320]
79 Ochsner A, DeBakey M. Liver abscess part I.
1935; 29: 173-194 [DOI: 10.1016/s0002-9610(35)91120-5]
80 Chemaly RF, Hall GS, Keys TF, Procop GW. Microbiology of liver abscesses and the predictive value of abscess gram stain and associated blood cultures.
2003; 46: 245-248 [PMID: 12944014 DOI: 10.1016/s0732-8893(03)00088-9]
81 Méchaï F, Aoun O, Ficko C, Barruet R, Imbert P, Rapp C. Budd-Chiari syndrome as a vascular complication of amebic liver abscess.
2009; 81: 768-769 [PMID: 19861608 DOI: 10.4269/ajtmh.2009.09-0230]
82 Yadav T, Patel RK, Bansal A, Chatterjee N, Patidar Y, Mukund A. Caudate lobe amebic abscesses: percutaneous imageguided aspiration or drainage.
2022; 47: 1157-1166 [PMID: 34964910 DOI: 10.1007/s00261-021-03395-z]
83 Priyadarshi RN, Kumar R, Anand U. Case Report: Spontaneous Resolution of Intracavitary Hepatic Artery Pseudoaneurysm Caused by Amebic Liver Abscess following Percutaneous Drainage.
2019; 101: 157-159 [PMID: 31162010 DOI: 10.4269/ajtmh.19-0103]
84 Sachdev GK, Dhol P. Colonic involvement in patients with amebic liver abscess: endoscopic findings.
1997; 46: 37-39 [PMID: 9260703 DOI: 10.1016/s0016-5107(97)70207-4]
85 Premkumar M, Devurgowda D, Dudha S, Kulkarni A, Joshi YK. Clinical and Endoscopic Management of Synchronous Amoebic Liver Abscess and Bleeding Colonic Ulcers.
2019; 67: 14-18 [PMID: 31304698]
86 de la Rey Nel J, Simjee AE, Patel A. Indications for aspiration of amoebic liver abscess.
1989; 75: 373-376 [PMID: 2711266]
87 Rajak CL, Gupta S, Jain S, Chawla Y, Gulati M, Suri S. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage.
1998; 170: 1035-1039 [PMID: 9530055 DOI: 10.2214/ajr.170.4.9530055]
88 Cai YL, Xiong XZ, Lu J, Cheng Y, Yang C, Lin YX, Zhang J, Cheng NS. Percutaneous needle aspiration versus catheter drainage in the management of liver abscess: a systematic review and meta-analysis.
2015; 17: 195-201 [PMID: 25209740 DOI: 10.1111/hpb.12332]
89 Ramani A, Ramani R, Kumar MS, Lakhkar BN, Kundaje GN. Ultrasound-guided needle aspiration of amoebic liver abscess.
1993; 69: 381-383 [PMID: 8346134 DOI: 10.1136/pgmj.69.811.381]
90 Kumar R, Anand U, Priyadarshi RN, Mohan S, Parasar K. Management of amoebic peritonitis due to ruptured amoebic liver abscess: It's time for a paradigm shift.
2019; 3: 268-269 [PMID: 31276048 DOI: 10.1002/jgh3.12144]
91 Eggleston FC, Handa AK, Verghese M. Amebic peritonitis secondary to amebic liver abscess.
1982; 91: 46-48 [PMID: 7054906]
92 Saraswat VA, Agarwal DK, Baijal SS, Roy S, Choudhuri G, Dhiman RK, Bhandari L, Naik SR. Percutaneous catheter drainage of amoebic liver abscess.
1992; 45: 187-189 [PMID: 1555372 DOI: 10.1016/s0009-9260(05)80639-7]
93 vanSonnenberg E, Ferrucci JT Jr, Mueller PR, Wittenberg J, Simeone JF. Percutaneous drainage of abscesses and fluid collections: technique, results, and applications.
1982; 142: 1-10 [PMID: 7053517 DOI: 10.1148/radiology.142.1.7053517]
94 vanSonnenberg E, Mueller PR, Schiffman HR, Ferrucci JT Jr, Casola G, Simeone JF, Cabrera OA, Gosink BB. Intrahepatic amebic abscesses: indications for and results of percutaneous catheter drainage.
1985; 156: 631-635 [PMID: 4023220 DOI: 10.1148/radiology.156.3.4023220]
95 Gibney EJ. Amoebic liver abscess.
1990; 77: 843-844 [PMID: 2203504 DOI: 10.1002/bjs.1800770803]
96 Akgun Y, Tacyildiz IH, Celik Y. Amebic liver abscess: changing trends over 20 years.
1999; 23: 102-106 [PMID: 9841772 DOI: 10.1007/s002689900573]
97 Tay KH, Ravintharan T, Hoe MN, See AC, Chng HC. Laparoscopic drainage of liver abscesses.
1998; 85: 330-332 [PMID: 9529485 DOI: 10.1046/j.1365-2168.1998.00617.x]
杂志排行
World Journal of Radiology的其它文章
- Triple rule-out computed tomography angiography: Evaluation of acute chest pain in COVID-19 patients in the emergency department
- Imaging volumes during COVID-19: A Victorian health service experience
- Progress in interventional radiology treatment of pulmonary embolism: A brief review
- Advanced magnetic resonance imaging findings in salivary gland tumors
- Augmenting prostate magnetic resonance imaging reporting to incorporate diagnostic recommendations based upon clinical risk calculators
