Enhanced thermal-and impact-initiated reactions of PTFE/Al energetic materials through ultrasonic-assisted core-shell construction
2022-08-30ZhouyngWuJinxuLiuSongZhngXinqingLiuXioXuWeizheShukuiLiChunHe
Zhou-yng Wu ,Jin-xu Liu ,** ,Song Zhng ,Xin-qing Liu ,Xio Xu ,Wei-zhe M ,Shu-kui Li ,c,Chun He ,*
a School of Materials Science and Engineering,Beijing Institute of Technology,Beijing,100081,China
b Science and Technology on Transient Impact Laboratory,No.208 Research Institute of China Ordnance Industries,102202,China
c Department of Materials Science and Engineering,Shenzhen MSU-BIT University,Shenzhen,518172,China
Keywords:PTFE/Al Core-shell structure Energetic materials Ultrasonic-assisted mixing
ABSTRACT A facile and economical approach was developed for the large-scale production of powdered core-shell structured PTFE/Al (CS-PA) energetic materials through ultrasonic-assisted mixing.The low-cost micrometer-sized PTFE and Al particles were used as starting materials.Under high-power ultrasonic waves,the PTFE powder was dispersed into nano-to sub-micrometer-sized particles and then encapsulated the Al microparticles to form the core-shell structure.The heat of combustion,burning rate,and pressurization rate of the powdered CS-PA were measured.The thermal-initiated reaction behavior was further evaluated using thermogravimetry-differential scanning calorimetry.Subsequently,the bulk CSPA with a uniform microstructure was obtained via cold isostatic pressing of the powdered CS-PA followed by vacuum sintering.For the bulk CS-PA,the quasi-static compression behavior was characterized,and the impact-initiated reaction processes were conducted using the Split Hopkinson Pressure Bar(SHPB) and evaluated by a high-speed camera.Compared to physically mixed PTFE/Al materials,the powdered and bulk CS-PA demonstrated enhanced thermal-and impact-initiated reaction characteristics respectively,proving the effectiveness of our approach for constructing core-shell structures.
1.Introduction
PTFE/Al energetic materials (EMs),both in powder and bulk form,have drawn great attention due to their superior exothermicity and great potential in versatile applications[1].Compared to traditional explosives,such as TNT,the PTFE/Al EMs have a higher energy density,and the theoretical energy density can reach up to 21 kJ/cmat a stoichiometric ratio [2].In the past decade,the powdered PTFE/Al EMs have demonstrated their capability in applications,such as propellants [3],pyrotechnics [4-6],and the synthesis of nanomaterials [7],while the bulk PTFE/Al EMs,also categorized as reactive structural materials,are of particular interest due to their impact-initiated characteristics [8].However,despite high energy density,the reaction rate and efficiency of the PTFE/Al EMs are largely restricted by the agglomeration of PTFE and Al particles,which largely increases mass transportation distance and decreases the interfacial contact area between the oxidizing and reducing agents[9].Thus,finding an approach to eliminate the agglomeration of the Al particles is of significant importance to further improve the reaction efficiency of the PTFE/Al EMs.
Recently,the construction of core-shell nanostructures has been proved to be an effective approach for reducing the agglomeration of the reactants of the PTFE/Al EMs [10].In this regard,various synthesis methods have been proposed,such as the chemical vapor deposition (CVD) [10],wet-chemical method [11],and surface modification technique[12-14].By coating the Al particles with the PTFE,higher reaction rate and efficiency have been achieved for the core-shell structured PTFE/Al EMs.However,these synthesis methods normally involve relatively complex processes and raise challenges for industrial production.Besides,the effect of the coreshell structure on the microstructures and impact-initiated behaviors of bulk PTFE/Al materials has not been studied.
In this article,we prepared the powdered core-shell structured PTFE/Al EMs (CS-PA) by using high-power ultrasonic processing and bulk CS-PA through powder metallurgy technique.Under highpower ultrasonic waves,the micrometer-sized PTFE was dispersed into nano-to sub-micrometer-sized particles,which were then coated on the surface of the Al particles by magnetic stirring to form the core-shell structure.For the powdered CS-PA,the heat of combustion,pressurization rate,and burning rate were measured.The thermal reaction behavior of the powdered CS-PA was also analyzed using thermogravimetry (TG)-differential scanning calorimetry(DSC).For the bulk CS-PA,the microstructure was observed using the metallographic microscope and the quasi-static compression behavior was evaluated.Besides,the impactinitiated behaviors of the bulk CS-PA were characterized using the Split Hopkinson Pressure Bar (SHPB) under impact loading conditions,and the impact-initiated reaction processes were recorded and analyzed using a high-speed camera.
2.Experimental section
2.1.Preparation
The powdered CS-PA was prepared through high-power ultrasonic processing.The Al particles (Beijing Xingrongyuan Co.,Ltd.China) serve as the reducing agent and have an average size of~3 μm.For the oxidizing agent,two kinds of PTFE powders were utilized,one has an average size of~60 μm,named P-A (Shenyang Micro Powder Factory Co.,Ltd.China).The P-A consists of small PTFE particles with low molecular weight and was used to encapsulate the Al particles.The other one has an average particle size of~20 μm,named P-B (DuPont,USA),which was used as the binder for constructing the bulk materials.The mass ratio of the P-A,P-B,and Al was 14.7: 58.8: 26.5,where the Al and PTFE reached stoichiometric equilibrium.
For the preparation of the powdered CS-PA,the PTFE-coated Al particles were firstly fabricated.Commonly,the ball-milling process can not eliminate the agglomeration of the PTFE and Al particles,which results in poor microstructure uniformity of the bulk materials.Therefore,the P-A and Al powders were added into the anhydrous ethanol solution and then processed using a digital ultrasonic generator with a power of 2.4 kW and a frequency of 20 kHz.Under the high-power ultrasonic waves,the P-A powder was dispersed into nano-to sub-micrometer-sized particles,named as PTFE-NPs.Then,a magnetic stirring process at a speed of 30 r/s was employed for 4 h to further mix the PTFE-NPs and Al particles.Finally,the mixed liquid was filtered and dried at 60C for 24 h,hence the PTFE-coated Al particles were fabricated.The asprepared PTFE-coated Al particles were then evenly mixed with the P-B using a planetary ball milling at a speed of 300 r/min for 1 h,thus the powdered CS-PA was obtained.The mass ratio of the PA,P-B,and Al in the CS-PA was 14.7:58.8:26.5,in which the PTFE and Al reached stoichiometric equilibrium.For comparison,the physically mixed powdered PTFE/Al (PM-PA) was also prepared,where the P-A,P-B,and Al powders of the same mass ratio were physically mixed using planetary ball milling at a speed of 300 r/min for 1 h.
The bulk CS-PA and PM-PA were then prepared through powder metallurgy technique,including cold isostatic pressing and vacuum sintering processes.Firstly,the as-prepared powders were put into a cylindrical rubber hose and pressed at 300 MPa for 20 min.Next,the specimens were sintered in a vacuum sintering furnace.The oven temperature was ramped up to 360C and held for 2 h.Then the oven temperature was reduced to room temperature,and hence the bulk CS-PA and PM-PA materials were prepared.
2.2.Characterizations
The morphology of the powdered materials was observed by a scanning electron microscope (SEM) (GeminiSEM 300).The elemental composition and distribution of the PTFE-coated Al particles were characterized by an energy dispersive X-ray spectroscopy (EDS) system equipped on the SEM.The particle size distributions of the Al and PTFE-coated Al particles were both measured by the laser particle size distribution analyzer (MASTERSIZER 2000) to get the statistical particle size and thickness of the PTFE coating.
The heat of combustion of the powdered CS-PA and PM-PA in high-purity oxygen (99.999 %) and argon (99.999 %) atmospheres was measured by an oxygen bomb calorimeter (Parr 6200).The pressure of oxygen or argon in the bomb was 3 MPa.The burning rates of the powdered CS-PA and PM-PA were tested by a selfdesigned metal groove with a dimension of 70 × 5 × 5 mm under ambient atmosphere.The loose powder (0.5 g) was uniformly placed into the metal groove and then an electrical heating Ni-Cr wire was used to initiate the self-sustained exothermic reaction of the powder from one side.The combustion processes were recorded using a high-speed camera (Fastcam AX200),which was placed perpendicular to the propagation direction of the combustion wave,at a rate of 1000 frames per second.The pressurization rates of the powders were measured using a self-designed vessel of 250 mL internal volume with a pressure sensor (10 kHz,1 MPa)atop.A Ni-Cr filament (0.15 mm) was used to ignite the powders(0.5 g),and the pressure change in the vessel was recorded as a function of time [15,16].The thermal reaction behaviors of the powdered CS-PA and PM-PA were further characterized by TG-DSC(SDT Q600)tests in pure nitrogen(N)atmosphere at a heating rate of 20C/min.The mass of the sample was about 3 mg.The DSC baselines were obtained without the sample under the same condition.
For the bulk PTFE/Al materials,the microstructures of the bulk CS-PA and PM-PA were observed using the metallographic microscope.The quasi-static compression behaviors of the bulk CS-PA and PM-PA were both characterized at room temperature using a CMT4305 Universal Materials Machine with a 300 kN loading capacity.The cylinder-shaped specimens have a dimension of φ10 × 10 mm.The cross-head compression rate was 0.6 mm/min,corresponding to a strain rate of 10s.Besides,to characterize the impact-initiated reactions of the bulk CS-PA and PM-PA,the cylindrical specimens(φ5×3 mm)were prepared and tested using the Split Hopkinson Pressure Bar.Simultaneously,a high-speed camera placed perpendicular to the bar was used to characterize the impact-initiated reaction processes at a rate of 10,000 frames per second.
3.Results and discussion
3.1.Characterizations of powder morphology
The scanning electron microscope was used to characterize the morphology of the powders.Fig.1a-i and 1a-ii present the SEM images of the P-A and PTFE-NPs,respectively.Clearly,the P-A was severely aggregated at first,but after the ultrasonic processing it was dispersed into the PTFE-NPs under the ultrasonic cavitation effect [17].Fig.1b shows the images of the Al particles,and Fig.1c shows the SEM image and the EDS mapping of a typical PTFEcoated Al particle.Obviously,with the assistance of magnetic stirring,the PTFE-NPs encapsulated the Al particle to form the coreshell structure.Compared to the PM-PA,where the P-A,P-B,and Al particles were mixed through ball milling,few PTFE particles were adhered to the Al particles,as shown in Fig.1d.This demonstrates the effectiveness of the formation of the core-shell structure through ultrasonic processing.
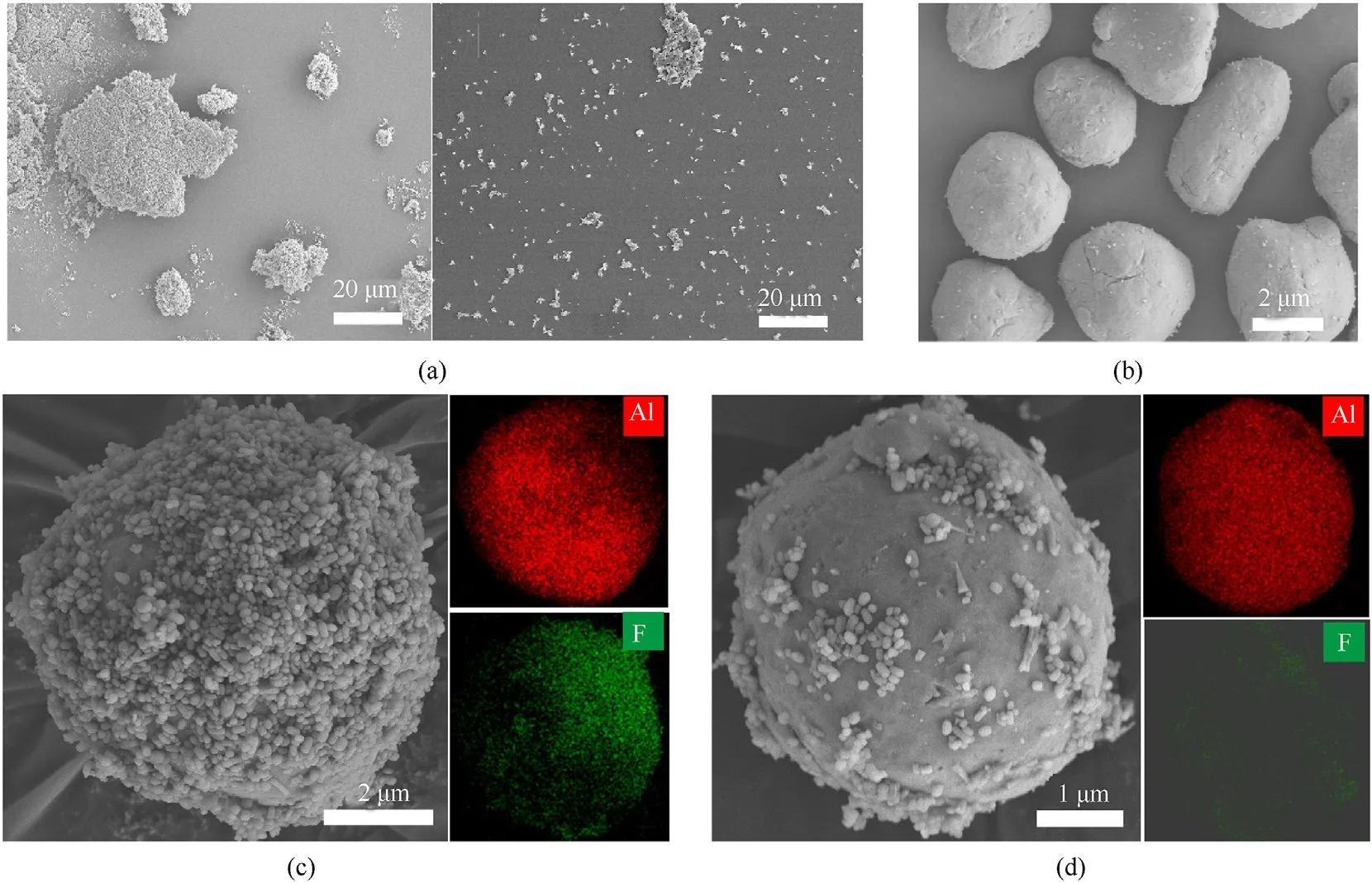
Fig.1.SEM images of(a-i)P-A and(a-ii)PTFE-NPs,(b)Al particles;SEM images and EDS mappings of the(c)the PTFE-coated Al particle of CS-PA and(d)the Al particle of PM-PA.
For the CS-PA,to evaluate the statistical thicknesses of the PTFE coating,the Al particles and the PTFE-coated Al particles were both characterized using the laser particle size distribution analyzer based on the laser diffraction method,and the particle size distributions are compared in Fig.2.For the Al powders,only a single peak positioned at 3.27 μm is seen,which corresponds to the diameter of the Al particles.As for the PTFE-coated Al particles,two peaks positioned at 0.53 μm and 5.83 μm are observed,which correspond to the PTFE-NPs and PTFE-coated Al particles,respectively.Clearly,an increment in the particle size of the PTFEcoated Al particles is observed compared to that of the Al particles.The difference in the particle sizes gives the statistical thickness of the PTFE shell,which is determined to be about 1.28 μm.This further confirms the effective coating of the PTFE-NPs on the Al particles.Then,the PTFE-coated particles were evenly mixed with the P-B,hence the powdered CS-PA was obtained.
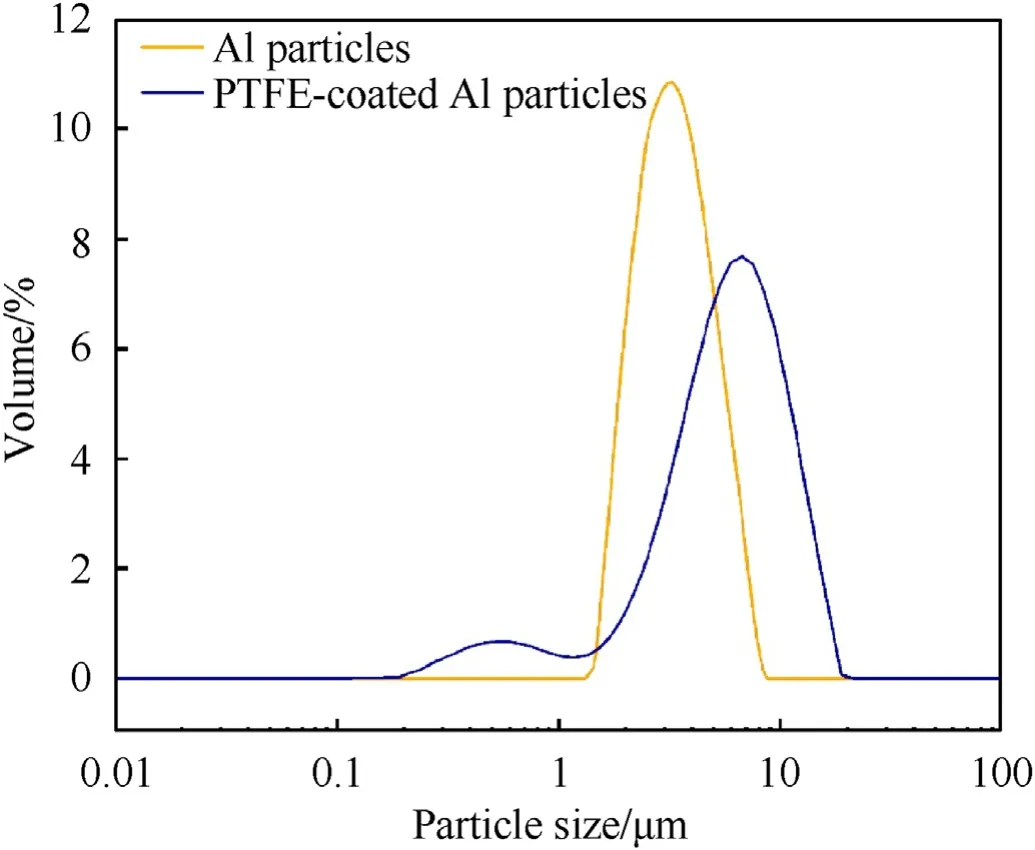
Fig.2.Particle size distributions of the Al and PTFE-coated Al particles.
3.2.Thermal-initiated behavior of powdered materials
The heat of combustion of the powdered PM-PA and CS-PA in high-purity oxygen (99.999 %) and argon (99.999 %) atmospheres was measured by the oxygen bomb calorimeter (Parr 6200).The heat of combustion of powdered CS-PA and PM-PA in an oxygen atmosphere is 13.94±0.05 kJ/g and 13.35±0.08 kJ/g,respectively,while the heat of combustion of the powdered CS-PA and PM-PA in argon atmosphere is 8.04 ± 0.02 kJ/g and 7.81 ± 0.04 kJ/g,respectively.The heat of combustion of CS-PA in pure oxygen atmosphere(13.94 ± 0.05 kJ/g) is higher than that (12.26 kJ/g) of a previously reported PTFE coated Al powder[12].Compared to the PM-PA,the powdered CS-PA has a higher heat of combustion in both highpurity oxygen and argon atmospheres.These results demonstrate that the core-shell structure is beneficial to improve the reaction efficiency of powdered PTFE/Al materials.

Fig.3.Photographic sequences of the combustion processes of (a) PM-PA and (b) CS-PA.
To investigate the thermal-initiated reaction characteristics,the combustion experiments under ambient pressure and temperature conditions were conducted.The loose powders of the CS-PA and PM-PA were evenly placed in a metal groove with a dimension of 70 × 5 × 5 mm and ignited using a Ni-Cr wire at one end of the groove.As the current passed the Ni-Cr wire,the heat generated induced the intense exothermic reaction between the PTFE and Al,which leads to a self-sustained combustion wave that propagates rapidly to the other end of the groove.Fig.3a and Fig.3b illustrate the photographic sequences of the combustion processes of the powdered PM-PA and CS-PA,respectively.As can be seen,after the ignition,the combustion waves were generated for both powdered materials,and traveled from the left-side to the right-side of the groove.The time required for the combustion wavefront traveling from the left-side to the right-side of the groove is 1792 ms for the PM-PA and 1207 ms for the CS-PA,respectively.In addition,the distance of the wavefront to the left-side of the groove is plotted as a function of the time,as illustrated in Fig.4.Through the linear fitting,the burning rates of the powdered CS-PA and PM-PA were determined to be 52.1±1.5 mm/s and 37.1±0.7 mm/s,respectively.These results demonstrate that the powdered CS-PA has a fast burning rate and reaction efficiency,which proves that the coreshell structure has a significant enhancement on the reaction performance of PTFE/Al powder.The reason can be attributed to the decreased Al agglomeration and increased interfacial contact between the PTFE and Al through the formation of the core-shell structure.
In addition to the burning rates,the pressurization rates of the powdered CS-PA and PM-PA were evaluated using a self-designed vessel of 250 mL internal volume with a pressure sensor atop.A Ni-Cr filament was used to ignite the powders,and the pressure change in the vessel was measured as a function of time [16].The transient pressure change can be attributed to the fact that the temperature at the reaction zone (3377C) exceeds the boiling point of the AlF(1272C)[18].Thus,shortly after the ignition,the AlFproduct existed in the gaseous state and a rapid pressure rise occurred at the beginning.The curves of the pressure change versus time are shown in Fig.5.For the powdered CS-PA,the pressure reached the peak at about 0.04 s,and the peak pressure is 0.34 MPa.Defined as the slope of the pressure rise,the pressurization rate of the powdered CS-PA is determined to be 8.5 MPa/s.Similar pattern of the pressure change is observed for the powdered PM-PA.The pressurization rate and the peak pressure are determined to be 5.5 MPa/s and 0.33 MPa,respectively.It can be seen that the pressurization rate of the CA-PA is higher than that of the PM-PA,while the peak pressures of the two materials are comparable.The reason for this phenomenon is that the powdered CS-PA has a higher burning rate and higher heat of combustion,which is favorable for the formation of gaseous products.
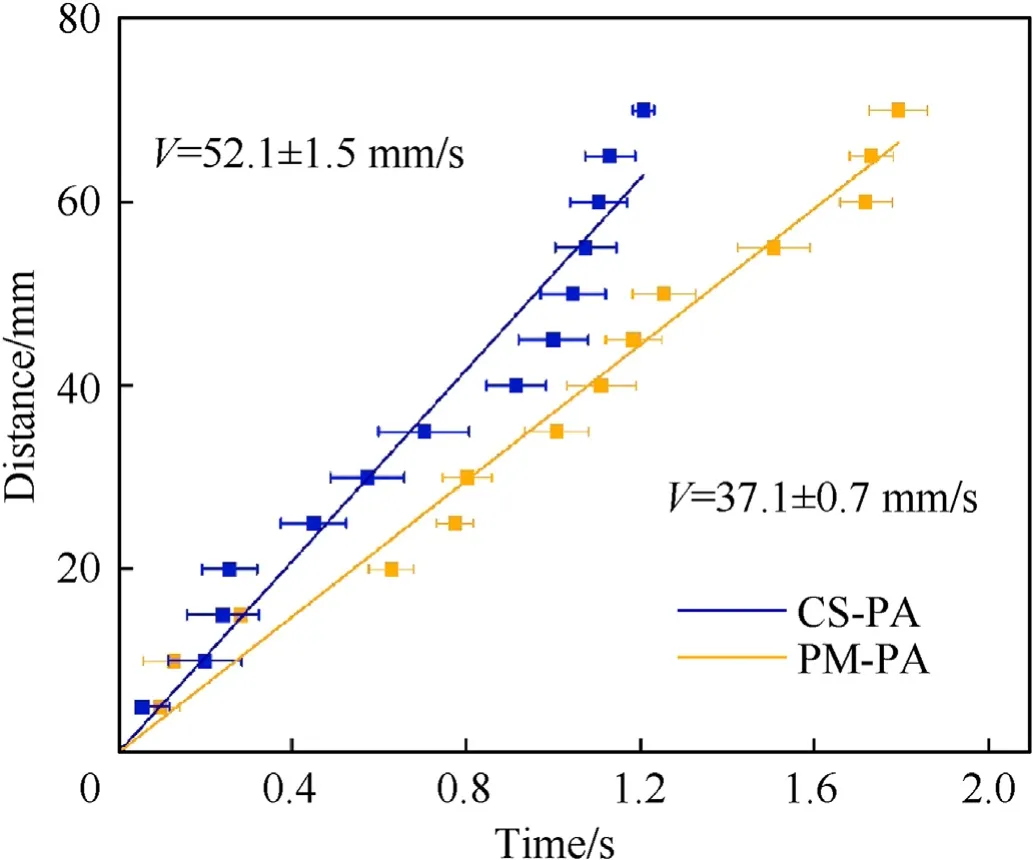
Fig.4.Burning rates of powdered CS-PA and PM-PA.
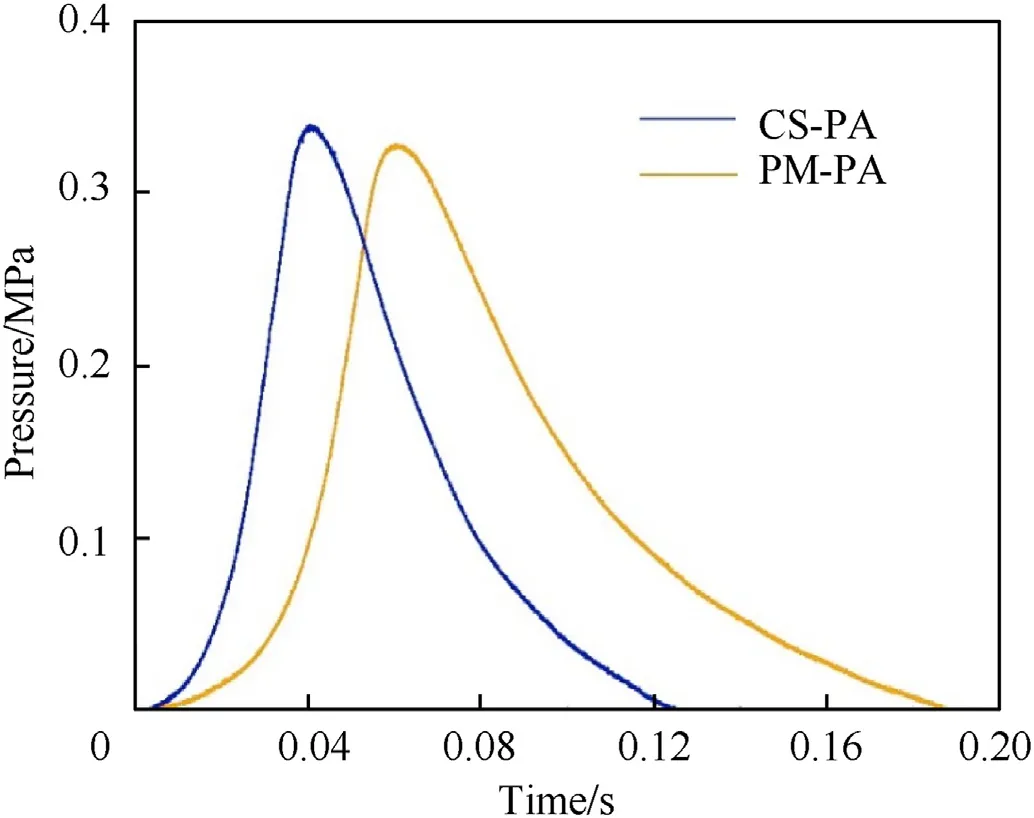
Fig.5.Curves of the pressure change versus time in the constant-volume vessel.
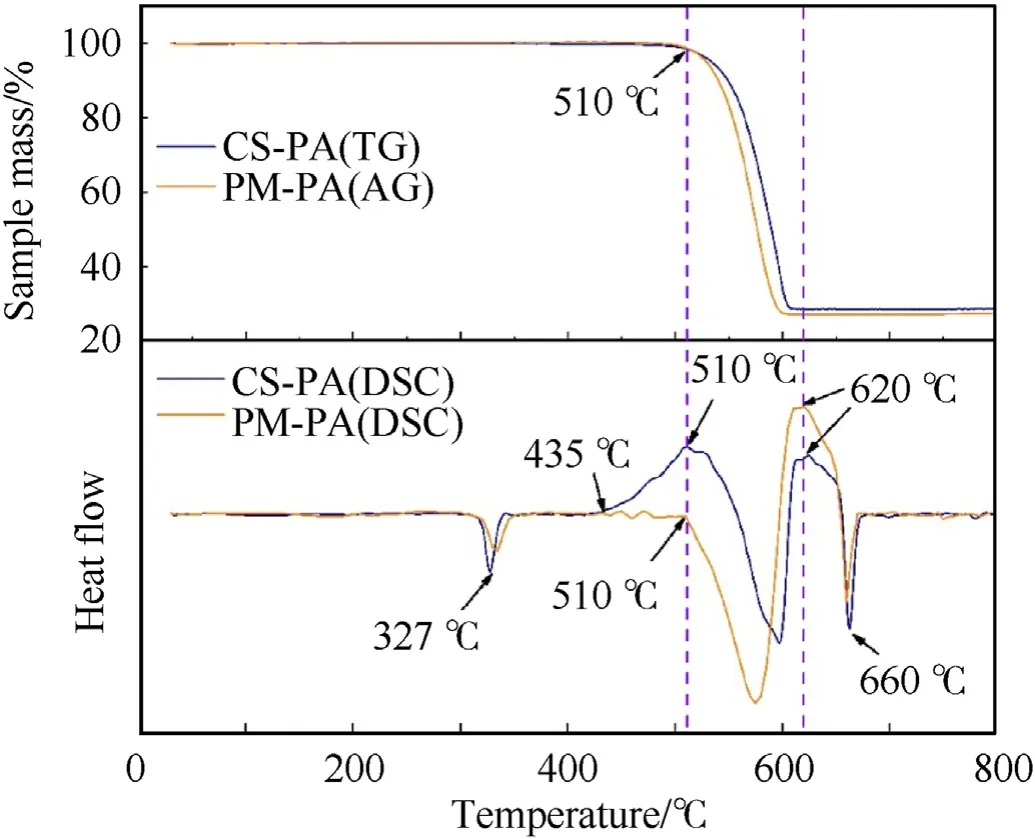
Fig.6.TG-DSC curves of CS-PA and PM-PA.
To further investigate the thermal-initiated behavior,the TGDSC measurements were carried out for the powdered CS-PA and PM-PA at a heating rate of 20C/min.The TG and DSC curves are illustrated and compared in the upper and lower panel of Fig.6,respectively.For both powdered materials,as the temperature increases,firstly,a minor endothermic peak at about 327C corresponding to the melting of the PTFE is seen on the DSC curves[19].Then it is observed on the TG and DSC curves that the curves begin to decline from 510C,which is ascribed to the anaerobic thermal decomposition of the PTFE with CFas the primary product[20,21].For the powdered CS-PA,a noticeable difference can be seen at 435C,where an exothermic peak is observed before the decomposition of the PTFE.Such a pre-ignition reaction phenomenon was not found in powdered PM-PA.Considering the PTFE and Al of the CS-PA have an intimate contact as a result of the core-shell construction,this exothermic peak might be attributed to the preignition reaction caused by the interaction of fluorine ions from the condense-phase PTFE with the native oxide layer (AlO) of the Al particles [22,23].Following the decomposition of the PTFE,an obvious exothermic peak corresponding to the reaction of the Al with the decomposition products of the PTFE is observed at 620C.As the temperature increases up to 660C,another endothermic peak appears as the Al melts[24].Besides,it is considered the heat of the pre-ignition reaction assists in the partial decomposition of PTFE,which is beneficial to improve the burning rate and pressurization rate of powdered CS-PA.
3.3.Impact-initiated behavior of bulk materials
The microstructures of the bulk CS-PA and PM-PA were observed using the metallographic microscope and the corresponding images are shown in Fig.7a and b,respectively.The brighter white parts in the images are the Al,while the darker gray parts are the PTFE.Obviously,the microstructure of the bulk CS-PA is more uniform compared to the bulk PM-PA.This is because the Al particles were pre-coated by PTFE-NPs to form PTFE-coated Al particles before mixing with P-B,thus the agglomeration was greatly reduced and the microstructure uniformity of the bulk material was improved.The densities of the bulk CS-PA and PM-PA were measured to be 2.265 g/cmand 2.229 g/cm,respectively,and the relative densities were 97.9 % and 96.4 %,respectively.
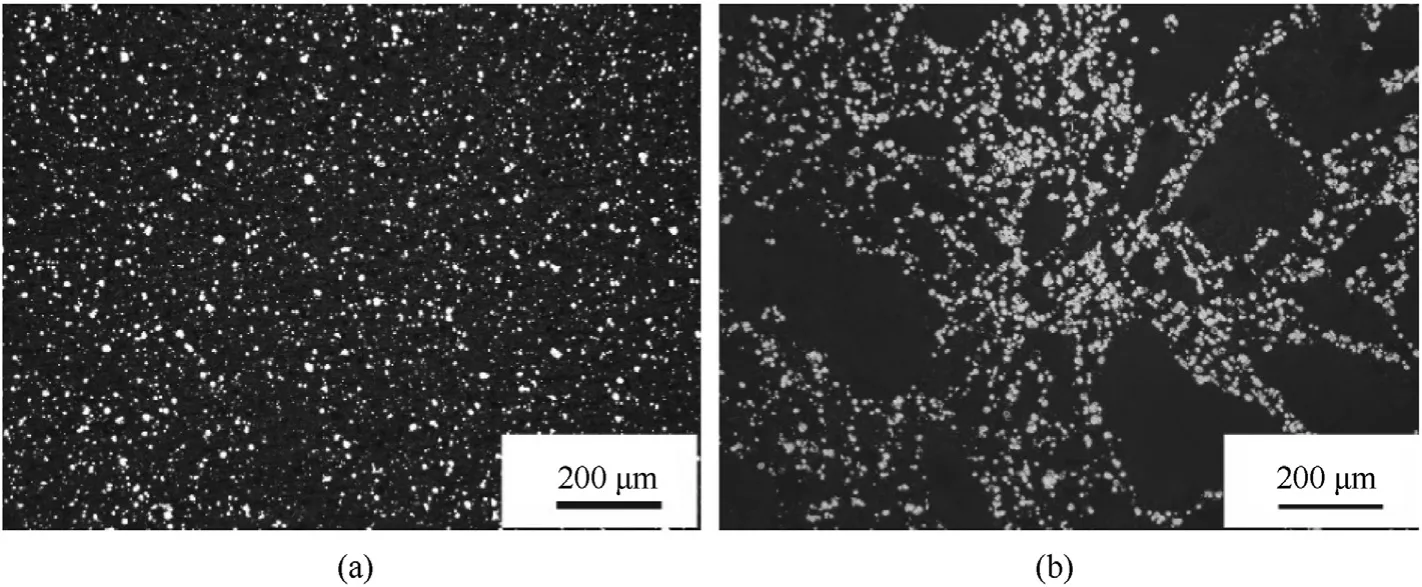
Fig.7.The microstructures of (a) CS-PA and (b) PM-PA.
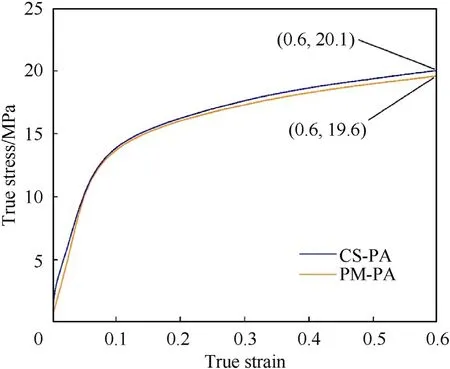
Fig.8.Quasi-static true stress-strain curves of CS-PA and PM-PA.
The quasi-static compressive mechanical properties of the bulk CS-PA and PM-PA materials were also tested.Fig.8 depicts the true stress-strain curves of the bulk CS-PA and PM-PA under quasi-static compression conditions.The two types of materials both exhibit good ductility,and a significant strain hardening effect is seen.The quasi-static yield strength of bulk CS-PA and PM-PA is 10.9 MPa and 10.5 MPa,respectively.When the strain reaches 0.6,the quasi-static compressive strength of the CS-PA and PM-PA is 20.1 MPa and 19.6 MPa,respectively.Therefore,the core-shell construction shows no obvious effect on the quasi-static compression properties of the bulk PTFE/Al materials.
The impact-initiated reactions of the bulk CS-PA and PM-PA(cylindrical specimen φ5 × 3 mm) were characterized using the SHPB under the same impact loading,as shown in Fig.9a.The strain rates are about 7500 sand the reaction processes were recorded by a high-speed camera at 10000 f/s.Fig.9b and c shows the highspeed photographic sequences of impact-initiated reaction processes of the bulk CS-PA and PM-PA,respectively.Under the highspeed impact conditions,both materials underwent severe deformation and broke into fine fragments.Simultaneously,the impact led to the localized heating,i.e.,the formation of “hot spot” in the materials [25].This initiated the exothermic reaction between the PTFE and Al and produced a bright flare.
The area of the bright flare generally gives an indication of the reaction efficiency of the bulk PTFE/Al.MATLAB software was used to process the high-speed photographic sequences of two types of bulk PTFE/Al and the flare area over time during the whole reaction process was shown in Fig.10.Upon ignition,the flare first increases and then decreases,and the duration of the flare for both materials is about 2.2 ms.The maximum areas of the flare of bulk CS-PA and PM-PA occurred at 0.3 ms and were determined to be about 22.6 and 17.2 cm,respectively.Besides,for the bulk PM-PA,many fine fragments were observed splashing out during the impact-initiated reaction,which indicates an incomplete reaction of the bulk PM-PA.On the contrary,few fragments are seen for the bulk CS-PA,demonstrating an enhanced reaction efficiency of the bulk CS-PA under impact loading.This might be attributed to the more uniform microstructure of bulk CS-PA which can increase the interfacial contact between the reactants and decrease the distance of mass transport.
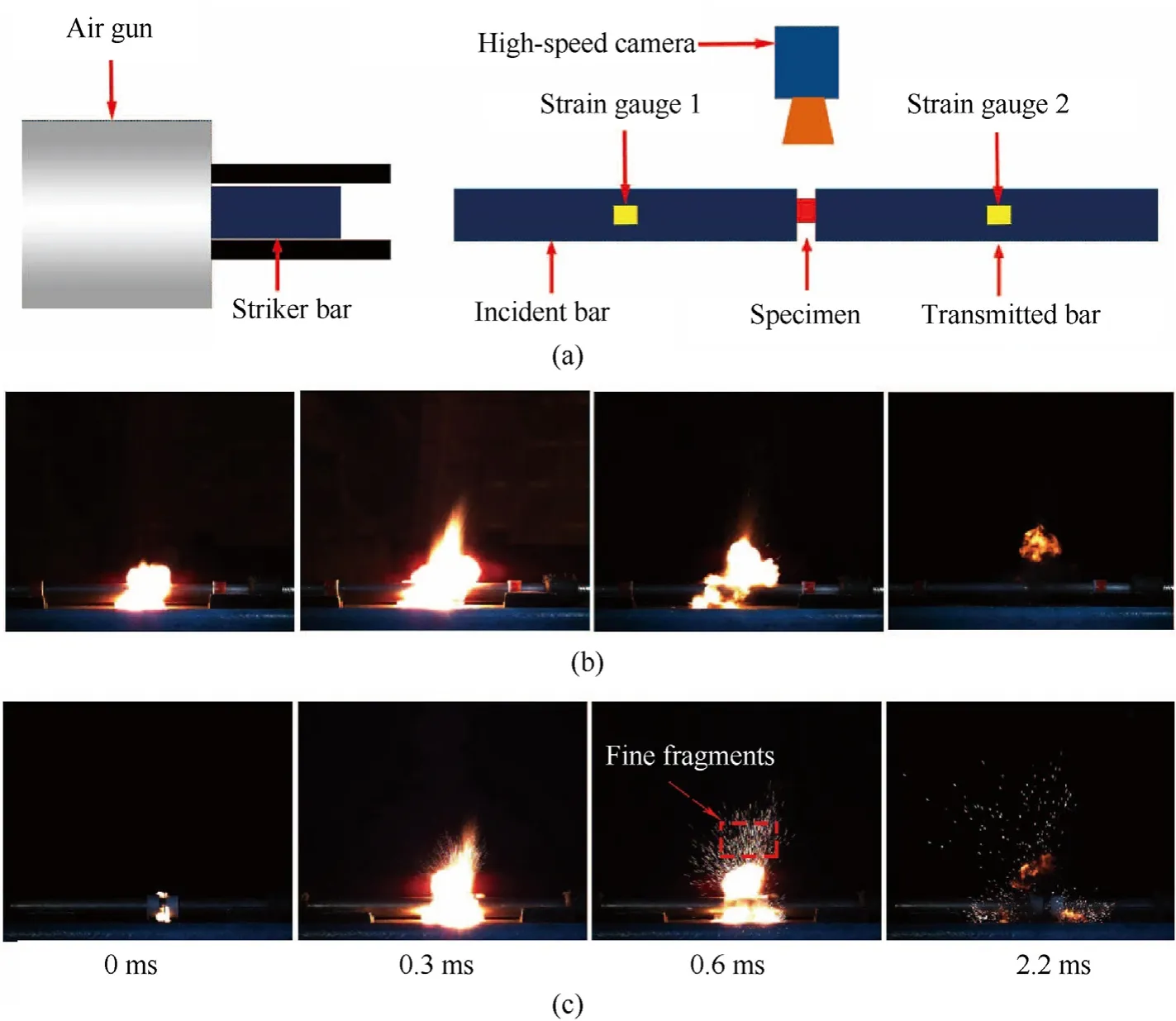
Fig.9.(a) Schematic diagram of SHPB device and the photographic sequences of reaction processes of (b) CS-PA and (c) PM-PA.
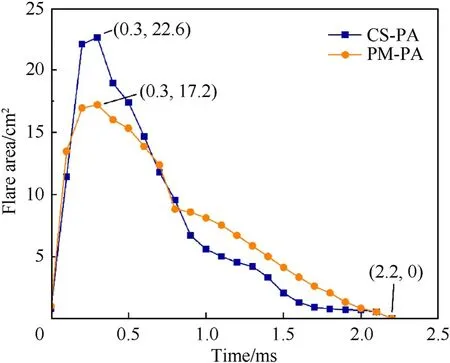
Fig.10.Flare area over time during the whole reaction processes of two types of materials.
4.Conclusions
In summary,we fabricated the powdered CS-PA through highpower ultrasonic processing and then the bulk CS-PA by powder metallurgy technique.Under high-power ultrasonic waves,the aggregated PTFE was dispersed into nano-to sub-micrometer-sized particles,which subsequently encapsulates the Al microparticles to form the core-shell structure.By employing the core-shell configuration,the thermal-and impact-initiated reactions of the CS-PA are enhanced compared to that of the physically mixed PTFE/Al powders.The conclusions are summarized as follows:
(1) Using PTFE and Al particles as starting materials,the PTFEcoated Al particles were obtained,and the average thickness of the PTFE shell was determined to be about 1.28 μm.
(2) Compared with the physically mixed PTFE/Al powders,the powdered CS-PA exhibited higher heat of combustion in oxygen and argon atmosphere,burning rate(52.1 mm/s),and pressurization rate (8.5 MPa/s).Furthermore,the TG-DSC analysis shows that the CS-PA has a pre-ignition reaction at about 435C,demonstrating a higher reactivity of the CS-PA.
(3) Utilizing the powdered CS-PA,the bulk CS-PA was obtained through cold isostatic pressing and vacuum sintering processes.The density of the bulk CS-PA was measured to be 2.265 g/cm,and the relative density was 97.9 %.Upon impact loading using SHPB,the observation using a highspeed camera illustrated that the CS-PA has an enhanced reaction efficiency.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by the National Natural Science Foundation of China(No.51571033,11804022)and the Science and Technology on Transient Impact Laboratory Foundation (No.6142606183208).
杂志排行
Defence Technology的其它文章
- Experimental study on propagation characteristics of rotating detonation wave with kerosene fuel-rich gas
- Adaptive robust control for triple avoidance -striking -arrival performance of uncertain tank mechanical systems
- Experimental study on WFeNiMo high-entropy alloy projectile penetrating semi-infinite steel target
- Numerical investigation of a muzzle multiphase flow field using two underwater launch methods
- Shock wave and bubble characteristics of underwater array explosion of charges
- A micro-chip exploding foil initiator based on printed circuit board technology
