Study on the relationship between relieving energy crisis in myofascial trigger points with An-Pressing manipulation and AMPK/PGC-1α pathway activation
2022-08-16KUANGXiaoxia匡小霞LIWu李武JIANGQuanrui蒋全睿WEIWei危威LITielang李铁浪LIJiangshan李江山
KUANG Xiaoxia (匡小霞), LI Wu (李武), JIANG Quanrui (蒋全睿), WEI Wei (危威), LI Tielang (李铁浪),LI Jiangshan (李江山)
1 Hunan University of Chinese Medicine, Changsha 410208, China
2 The Second Hospital, University of South China, Hengyang 421000, China
Abstract
Keywords: Tuina; Massage; An-Pressing Manipulation; Myofascial Trigger Point; Energy Metabolism; AMP-Activated Protein Kinases; Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-α; Signal Transduction
Myofascial pain syndrome (MPS) is a chronic skeletal muscle injury characterized by the presence of hyperirritable points within the taut bands of the skeletal muscles, which are the myofascial trigger points(MTrPs)[1]. MTrps can cause severe muscle pain,dysfunction, paresthesia, autonomic dysfunction, and even muscle atrophy and disability after activation[2-5].Studies have shown that MTrPs may be a structure of chronic muscle lesion caused by local energy supply disorder secondary to acute injuries[6], with a vicious cycle of local energy crisis[7]. Mitochondrial dysfunction is a significant cause of local energy crisis[8]. Adenosine 5’-monophosphate (AMP)-activated protein kinase(AMPK) is an energy sensor of skeletal muscle,participating in skeletal muscle glucose and lipid metabolism, regulating peroxisome proliferatoractivated receptor gamma coactivator 1-alpha (PGC-1α),increasing mitochondrial biosynthesis and enzymatic activity[9], and promoting the repair of skeletal muscle damages. Recent research has shown that mitochondrial dysfunction in rat MTrPs skeletal muscle model is due to the down-regulated AMPK/PGC-1α function[10].
MTrPs An-Pressing manipulation, also known as ischemic compression, MTrPs massage manipulation or trigger point pressure release, is a manipulation that directly presses MTrPs with bare hands or instruments to release the contractural nodules and improve local blood circulation by mechanical action. This manipulation is also one of the widely used and highly targeted treatment methods in clinical practice[11]. Our previous study identified that An-Pressing manipulation relaxed the sarcomere of MTrPs contracture and promoted the repair of muscle fiber damage[12], but its mechanism is unclear. Therefore, this work focused on the energy crisis of MTrPs to explore the relationship between MTrPs deactivation and energy crisis after An-Pressing manipulation and whether the mechanism is related to the improved mitochondrial function after the activation of AMPK/PGC-1α pathway.
1 Materials and Methods
1.1 Experimental animals and groups
Forty-eight specific-pathogen-free male adult Sprague-Dawley rats, weighing 250-280 g, were provided by the Animal Experiment Center of Hunan University of Chinese Medicine [License No. SYXK (Xiang)2019-0009]. Rats were kept in separated cages at the Animal Center of Hunan University of Chinese Medicine(3 rats/cage) under the condition of 24-26 ℃, 50%-70%humidity, 12 h/12 h alternated light and dark cycles,and free access to food and drinking water. After one week of routine feeding, the rats were divided into a blank group, a model group, a lidocaine group, and an An-Pressing manipulation group by the random number table method, with 12 rats in each group. During the experiment, two rats in the lidocaine group and one rat in the An-Pressing manipulation group died unexpectedly. Finally, data from 12 rats in the blank group, 12 rats in the model group, 10 rats in the lidocaine group, and 11 rats in the An-Pressing manipulation group were collected. The handling of experimental rats complied with the relevant provisions of theGuiding Opinions on the Treatment of Experimental Animals[13].
1.2 Laboratory apparatus and reagents
1.2.1 Laboratory apparatus
Mechanical An-Pressing stimulation instrument(Patent No. 201720875963.5); C100 treadmill (Yusheng Sports Company, Jinhua City, Zhejiang Province, China);Reward R500 Universal Respiratory Anesthesia Machine(Shenzhen Reward Life Technology Co., Ltd., China);MP150 electromyograph (BIOPAC, USA); EMUC7 ultramicrotome (Leica, Germany); HT7700 transmission electron microscope (HITACHI, Japan); MiniProtean 3 cell electrophoresis apparatus (BIO-RAD, USA); MK3 microplate reader (Rebo Company, Finland); Icen-24R high-speed refrigerated centrifuge (Hangzhou Aosheng Company, China); Tanon-5200 automatic chemiluminescence analyzer (Shanghai Tianneng Company,China); Electronic Von Frey 2390 dolorimeter (IITC Corporation, USA).
1.2.2 Experimental reagents
Isoflurane (Cat. No. R510-22-16, Shenzhen Reward Life Technology Co., Ltd., China); lidocaine [5 mL (0.1 g),Shandong Hualu Pharmaceutical Co., Ltd., China];adenosine triphosphate (ATP) assay kit (Cat. No.A095-1-1, Nanjing Jiancheng Bioengineering Institute,China); protein marker (Cat. No. P12103, Helix, USA);polyvinylidene fluoride (PVDF) transfer membrane (Cat.No. ipvh00010) and chemiluminescence reagent (Cat.No. WBKLS0500), (Millipore, USA); high-efficiency RIPA lysis buffer [RIPA buffer (high), Cat. No. R0010] and BCA protein concentration assay kit (Cat. No. PC0020),(Solarbio, USA).
1.3 Modeling method and model evaluation
Method of blunt striking and eccentric exercise was applied to prepare the model[3], including an 8-week modeling period and a 4-week recovery period. To reduce the stress response, rats participating in the modeling were fed adaptively for 7 d before modeling and underwent adaptive treadmill training on the small animal treadmill, once every other day, 15 min each time, a total of 3 times. The treadmill was set with a slope of 0° and a speed of 16 m/min.
Blunt striking was performed on the 1st day of each week during the treatment period. After isoflurane inhalation anesthesia, rats were immobilized to the bottom of the beater, and the left hindlimb vastus medialis muscle bellies of the rats were palpated and marked. Then a blunt wooden stick was dropped vertically from a height of 20 cm to strike the marked position once. The eccentric exercise was performed on the second day: the small animal treadmill was set to a downhill running mode of -16° at 16 m/min, and the rats were driven by mechanical or sound stimulation during the eccentric exercise to ensure a complete 90-minute continuous eccentric exercise. Rats were routinely fed without intervention in the other 5 d. The above-mentioned process was repeated for 8 weeks before the 4-week recovery period with routine feeding and activities without any intervention.
Twitching reactions with the electrode needle penetrating into the palpable contracture nodules in the modeling sites and high-frequency spontaneous electrical activity appearing on the electromyogram,indicated that the modeling was successful[14].
1.4 MTrPs positioning and An-Pressing manipulation
MTrPs positioning: The position of the taut band was first determined according to the blunt strike target,then the contracture nodules on the taut band were determined and pressed with fingernails to induce a local twitch response.
An-Pressing manipulation: Inhalation anesthesia and maintenance with isoflurane (induction concentration of 4%, maintenance concentration of 2%) were performed. The experimental rats were immobilized in a supine position, and the MTrPs taut band was pressed vertically with a self-made An-Pressing stimulation device. The An-Pressing manipulation parameters were the same as those in our previous experiments with a force of 0.7 kg, each manipulation lasting for 6 s and a total time of 7.5 min[15-18].
1.5 Intervention method
Rats in the blank group and the model group were fed routinely for 13 d without intervention. Rats in the An-Pressing manipulation group received MTrPs An-Pressing manipulation, once every other day for a total of 7 times at the time points of the 1st, 3rd, 5th,7th, 9th, 11th, and 13th days after modeling. The lidocaine group was injected with lidocaine. A 1 mL syringe was used to inject 0.5 mL 1% lidocaine into the MTrPs, once every 6 d for a total of 3 times at the time points of the 1st, 7th, and 13th days after modeling.
1.6 Sampling and detection indicators
After intervention, the rats were fasted for 12 h and sacrificed after blood sampling from the abdominal aorta under anesthesia. The vastus medialis muscle of the left hindlimb was isolated in an ice bath (4 ℃ ice tray), trimmed, placed in liquid nitrogen, and stored in a-80 ℃ ultra-low temperature refrigerator until testing.
1.6.1 General observation indicators
The rat’s gait, the left hindlimb posture, the muscle tonus, and the licking and biting behavior were observed every 2 d during the intervention.
1.6.2 Measurement of pain threshold for mechanical stimulation
Rats were put on the metal mesh of the rat vitreous body for 20-30 min to perform the tactile sensation test.The sole of the rat’s left hindlimb was stimulated with the Electronic Von Frey stimulation needle when the rat was quiet. The pain measurement was read when the rat showed foot withdrawal reflex. According to the instrument-displayed strength, the stimulation was repeated 5 times for each foot at a 5-minute interval between the two stimulations. Rats will respond with foot lift, foot retraction, rapid foot flick, and foot licking,which were measured during 13:00-18:00 before modeling and on day 0, the 4th, 8th, 12th, and 14th days after modeling for each group.
1.6.3 Determination of ATP content by highperformance liquid chromatography
The rat muscle tissue of the contracture tubercle of the vastus medialis muscle (30 mg) was put into PC tubes after freeze-drying. The tissue was homogenized and centrifuged at a low temperature to prepare muscle extracts, which were stored at -80 ℃ for future testing. A 0.2 μm water-based membrane was used to filter the NaH2PO4aqueous solution with a mobile phase of 0.1 mol/L, followed by ultrasonic degassing at a flow rate of 0.2 mL/min with a column temperature of 40 ℃. Diode array detector detection wavelength was 254 nm. A 10 μL sample was injected each time with an autosampler for chromatographic analysis.
1.6.4 Detecting the expression of skeletal musclerelated proteins
The frozen left medial vastus muscle tissue of the rats was minced. The skeletal muscle tissue proteins were extracted with a total protein extraction kit. Proteins were quantified with a BCA protein quantitative assay kit. For each sample, 20 μg protein was loaded into the lane, electrophoresed, and electro
transferred to PVDF membrane, and then blocked with 5% nonfat milk at 4 ℃ overnight. The primary antibody was diluted to the desired concentration by adding into the blocking solution according to the instructions, and incubated with the membrane for 1 h at room temperature. The membrane was then washed 3 times with phosphate buffered solution (PBST),3 min/time. The secondary antibody labeled with horseradish peroxidase (HRP) was added and incubated for 1 h at room temperature. Washed it 3 times with PBST for 3 min/time. Enhanced chemiluminescence detection kit was used for exposure. TANON GIS software was used to read the gray value. The relative expression levels of AMPK, phosphorylated AMPK(phospho-AMPK), PGC-1α, and glucose transporter 4(GluT4) were compared and analyzed with β-actin as the internal reference. The ratio of the protein bands to the internal reference was the relative expression level.
1.6.5 Observation of mitochondrial ultrastructure
MTrPs tissue blocks of the left vastus medialis muscle(about 1 mm × 1 mm × 3 mm) were fixed in 2.5%glutaraldehyde phosphate buffer over 2 h. The samples were fixed, dehydrated, immersed, embedded, and solidified following the routine electron microscope sample preparation process. The ultrathin sections were double-stained with 3% uranyl acetate and lead nitrate,observed and filmed under the transmission electron microscope.
1.7 Statistical methods
The SPSS version 19.0 statistical software was used.Chi-square test was used for enumeration data.Measurement data were expressed as mean ± standard deviation (±s), and one-way analysis of variance was used for data meeting normality and homogeneity of variance, and the least significant difference method was used for pairwise comparison between groups; the Kruskal-WallisH-test was used for data with heterogeneity of variance. Nonparametric test was used if neither normality nor homogeneity of variances was met.P<0.05 indicated statistically significant.
2 Results
2.1 Comparison of the general conditions of rats
Rats in the blank group showed a strong physique,smooth and moist fur, and sensitive reactions. Rats in the model group showed low spirits; decreased activities, diet, and drinking; lusterless and yellowish hair; limped left hindlimb, often hanging in the air, and afraid to touch the ground; licking the paws, irritability,and fighting. After treatment, the left hindlimb muscles of the model group rats were atrophied, and the muscle tone was high, and the other conditions were basically the same as before. There was no obvious muscle atrophy of the left hindlimb in the An-Pressing manipulation group or the lidocaine group, and the muscle tone was slightly higher than that of the healthy side with normal walking and activities, without obvious limp, paw lifting, or licking the paws.
2.2 Comparison of the mechanical pain thresholds of rats
After modeling and before intervention (day 0),compared with the blank group, the mechanical pain thresholds of the model group, lidocaine group, and the An-Pressing manipulation group were statistically significantly lower (P<0.05). After 4 times of intervention (the 8th day), compared with the model group, the mechanical pain threshold of the An-Pressing manipulation group was statistically significantly increased (P<0.05); there was no statistical difference in the mechanical pain threshold between the An-Pressing manipulation group and the lidocaine group (P>0.05).After the intervention (the 14th day), the mechanical pain threshold of the An-Pressing manipulation group was recovered to the normal level and had no statistical difference with the blank group or the lidocaine group(P>0.05); compared with the model group, the mechanical pain threshold in the An-Pressing manipulation group was statistically significantly increased (P<0.05). It is shown in Table 1.
Table 1. Comparison of the pain threshold among groups ( ±s g)

Table 1. Comparison of the pain threshold among groups ( ±s g)
Note: Compared with the blank group, 1) P<0.05; compared with the model group, 2) P<0.05
Group n Day 0 4th day 8th day 12th day 14th day Blank 12 23.06±1.07 22.98±0.99 23.93±1.29 24.08±1.23 22.92±1.43 Model 12 12.18±0.691) 13.08±0.891) 13.59±1.211) 13.78±0.981) 13.02±0.491)Lidocaine 10 12.63±1.291) 17.03±1.962) 19.65±0.972) 22.08±1.632) 22.98±1.742)An-Pressing manipulation 11 11.45±0.541) 15.54±1.39 18.08±1.632) 21.80±0.892) 23.08±1.642)
2.3 Comparing the ATP content of local tissue
Compared with the blank group, the ATP contents in the local muscle tissue of MTrPs were decreased after modeling (P<0.05). After treatment, compared with the model group, the ATP contents in the An-Pressing manipulation group and the lidocaine group were increased (P<0.05). There was no significant difference between the An-Pressing manipulation group and the lidocaine group (P>0.05). It suggests that An-Pressing manipulation treatment can increase the ATP content in the local muscle tissue of MTrPs (Table 2).
Table 2. Comparing the ATP content of MTrPs in local tissue among groups ( ±s μmol/g prot)

Table 2. Comparing the ATP content of MTrPs in local tissue among groups ( ±s μmol/g prot)
Note: Ten rats were randomly selected from each group for this test; ATP=Adenosine triphosphate; MTrPs=Myofascial trigger points; compared with the blank group, 1) P<0.05; compared with the model group, 2) P<0.05
Group n ATP content Blank 10 417.01±43.20 Model 10 223.42±15.041)Lidocaine 10 307.45±8.472)An-Pressing manipulation 10 339.89±8.922)
2.4 Comparison of the mitochondrial ultrastructure
Under the electron microscope, the mitochondrial structure of muscle fibers was oval or elongated with a normal shape and size; the double-layer membrane structure was visible; the cytoplasm had no edema, and the sarcoplasmic reticulum was clear in the blank group.Mitochondria in the model group were deformed,decreased in number, and smaller in size. The structure of the double-layer membrane was blurred; the mitochondrial cristae were broken and deformed, and the sarcoplasmic reticulum was blurred. Mitochondria in the An-Pressing manipulation group were oval or elongated and increased in number. The shape and size were basically normal. The double-layer membrane structure was satisfactory. There was no obvious edema in the cytoplasm, and the sarcoplasmic reticulum was clearly visible. Mitochondria in the lidocaine group showed an increased number and more regular shape with a few swollen ones, without obvious vacuole. The double-layer membrane structure was acceptable;there was no obvious edema in the cytoplasm, and the arrangement of the sarcoplasmic reticulum was disordered. The mitochondria numbers in the An-Pressing manipulation group and the lidocaine group were similar, but the mitochondria in the An-Pressing manipulation group were more regular in shape without swelling, and the sarcoplasmic reticulum was clear (Figure 1).
2.5 Comparison of the protein expression levels of AMPK, phospho-AMPK, PGC-1α, and GluT4 in skeletal muscles
Compared with the blank group, the protein expression levels of phospho-AMPK, PGC-1α, and GluT4,as well as phospho-AMPK/AMPK in the model group decreased significantly (P<0.05). Compared with the model group, the expression levels of phospho-AMPK,PGC-1α, and GluT4, as well as phospho-AMPK/AMPK in the lidocaine group and the An-Pressing manipulation group increased significantly (P<0.05). Compared with the lidocaine group, the expression levels of phospho-AMPK, PGC-1α, and GluT4, as well as phospho-AMPK/AMPK in the An-Pressing manipulation group increased significantly (P<0.05), (Figure 2 and Table 3).

Figure 1. Local electron microscope images of rat MTrPs in each group
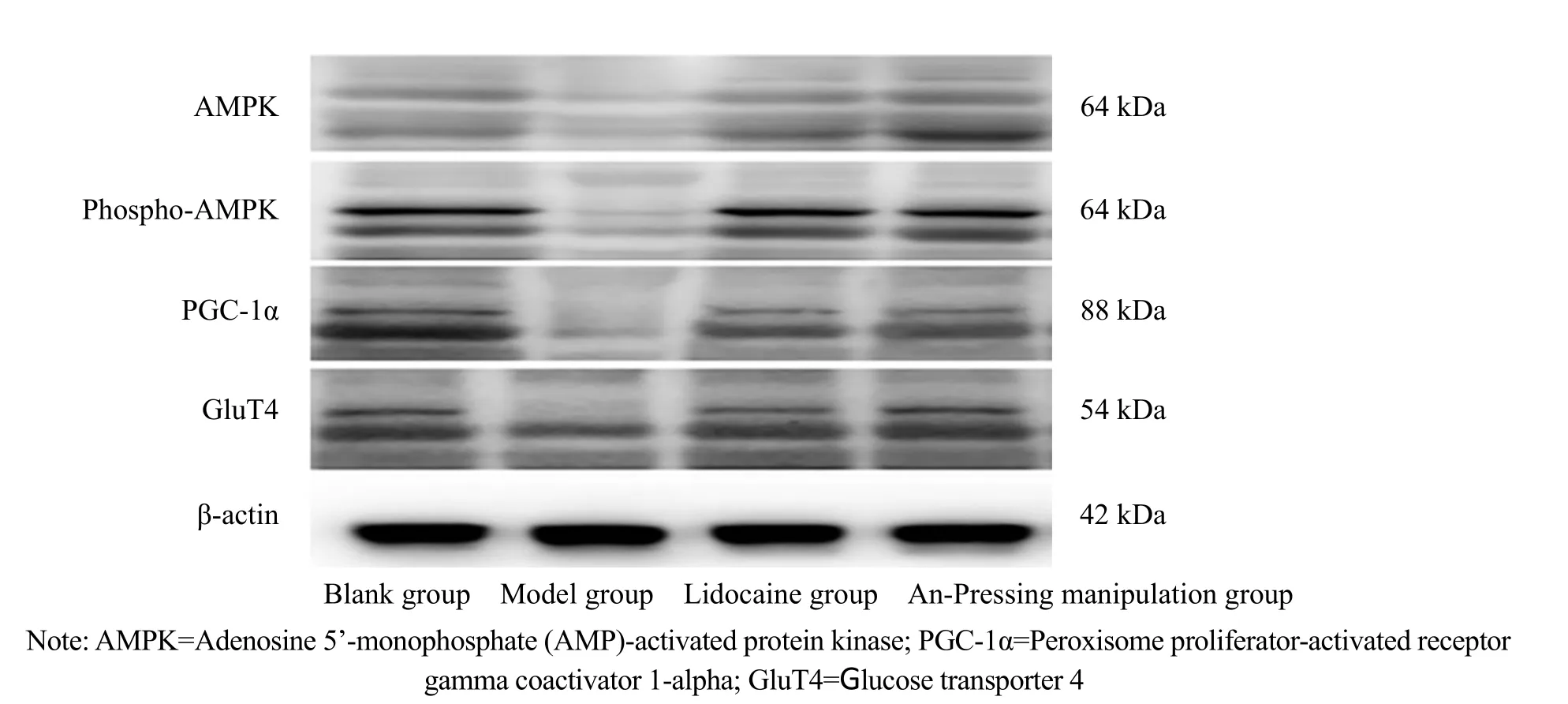
Figure 2. AMPK, phospho-AMPK, PGC-1α, and GluT4 protein expression bands in each group
Table 3. Comparison of the protein expression levels of AMPK, phospho-AMPK, PGC-1α, and GluT4 among groups ( ±s)
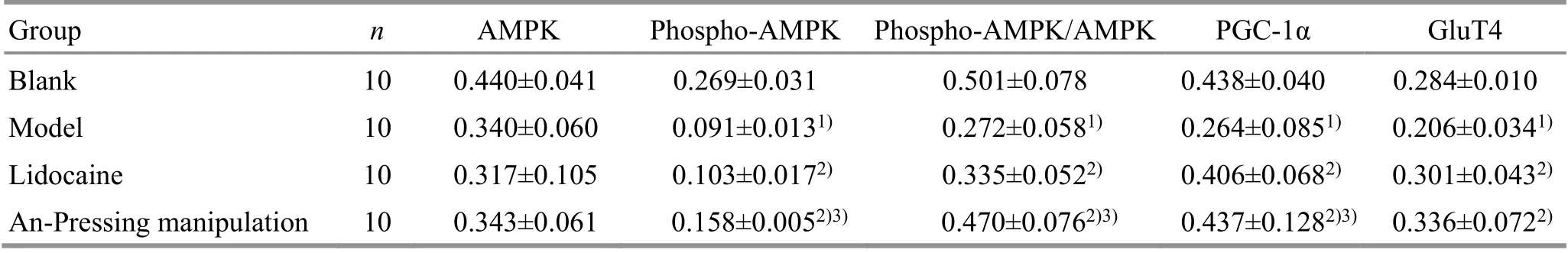
Table 3. Comparison of the protein expression levels of AMPK, phospho-AMPK, PGC-1α, and GluT4 among groups ( ±s)
Note: Ten rats were randomly selected from each group for these tests; AMPK=Adenosine 5’-monophosphate (AMP)-activated protein kinase; PGC-1α=Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; GluT4=Glucose transporter 4;compared with the blank group, 1) P<0.05; compared with the model group, 2) P<0.05; compared with the lidocaine group, 3) P<0.05
Group n AMPK Phospho-AMPK Phospho-AMPK/AMPK PGC-1α GluT4 Blank 10 0.440±0.041 0.269±0.031 0.501±0.078 0.438±0.040 0.284±0.010 Model 10 0.340±0.060 0.091±0.0131) 0.272±0.0581) 0.264±0.0851) 0.206±0.0341)Lidocaine 10 0.317±0.105 0.103±0.0172) 0.335±0.0522) 0.406±0.0682) 0.301±0.0432)An-Pressing manipulation 10 0.343±0.061 0.158±0.0052)3) 0.470±0.0762)3) 0.437±0.1282)3) 0.336±0.0722)
3 Discussion
The MTrPs are the presence of pain points and contracture nodules in the muscles, belonging to the clustered nodules in traditional Chinese medicine. The disease is located in the tendons, mostly due to a lack of Qi and blood, and further invasion by the wind, cold,and dampness pathogens. The acute and chronic injuries result in local muscle spasms and pain.An-Pressing manipulation has the functions of warming meridians, dispelling cold, promoting Qi, promoting blood circulation, and tonifying the deficiency, and can treat various pains caused by blocked meridians[19-20].
The depletion of intracellular energy substances and the accumulation of metabolites are important causes of muscle damage. Tuina (Chinese therapeutic massage)manipulation regulates muscle energy metabolism.Initially, SIMONS D G,et al[21]reported that manipulation regulated MTrPs energy due to the reduced overlap between actin and myosin by stretching,which greatly reduced the energy consumption, thus breaking the vicious circle of energy crisis.
With the deepening of research, it has been found that the manipulation may regulate skeletal muscle energy metabolism through the following related mechanisms. First, to improve skeletal muscle microcirculation and promote blood and oxygen supply.Manual stimulation dilates the local blood vessels,reduces peripheral resistance, increases the blood flow and the blood flow velocity, and brings abundant glucose and oxygen to muscle tissue, thus meeting the metabolic energy needs. The stimulation of Tuina manipulation affects the endothelial cells and the smooth muscle cells of blood vessels, making them produce more substances to regulate endogenous vasodilation, such as nitric oxide[22]. The rhythm extrusion of manipulation promotes the material exchange between blood and tissue fluid, thus improving the microcirculation of the body[23]. Studies have demonstrated that both the local glucose concentration and the blood flow are increased after ischemic An-Pressing at the MTrPs[24-25]. Second, the stimulation of Tuina manipulation promotes mitochondrial biosynthesis in muscle tissue. PGC-1α is a key regulator of mitochondrial biosynthesis and energy metabolism[26]. The stimulation of Tuina manipulation activates the skeletal muscle mechano- sensitive receptors, promotes the phosphorylation of PGC-1α,and then promotes the repair of skeletal muscles[27].Third, the stimulation of Tuina manipulation enhances the activity of ATPase and promotes tissue repair.Muscle tissue damage can lead to abnormal potassium-sodium ATPase on the sarcolemma, which affects energy conversion during oxidative phosphorylation. Tuina can increase ATPase mRNA expression and promote the synthesis of K+-Na+-ATPase,a key enzyme in the repair of skeletal muscle damage,and improve its activity[28-29]. The nature of An-Pressing manipulation is a form of mechanical stimulation, and the An-Pressing manipulation technique involves magnitude, duration, and frequency. To study the mechanisms of An-Pressing manipulation, our research group previously designed an An-Pressing stimulator based on analyzing the kinematic and dynamic characteristics of the An-Pressing manipulation, which can simulate the mechanical stimulation of An-Pressing manipulation and ensure the standard of each stimulation. In this study, the mechanical An-Pressing of the stimulator was used to interfere with the MTrPs to explore the mechanism of An-Pressing manipulation.The results showed that mechanical An-Pressing significantly increased the ATP content of MTrPs tissue,suggesting that An-Pressing manipulation can alleviate the energy crisis of MTrPs by elevating energy synthesis.
AMPK is the most critical regulatory molecule to maintain intracellular energy homeostasis. AMPK can mediate PGC-1α and downstream nuclear transcription factors, promote mitochondrial biogenesis, enhance mitochondrial enzyme activity, and promote the repair of skeletal muscle damage[30]. AMPK can promote GluT4 expression in the skeletal muscle cells and increase the uptake of glucose by muscle cells[31]. AMPK can also phosphorylate downstream target proteins, enhance the body’s catabolism to generate ATP, inhibit anabolism, reduce energy consumption, and promote the body’s energy reserves[32-33]. Mitochondrial dysfunction caused by the down-regulated AMPK/PGC-1α pathway is an important cause of tissue energy crisis in MTrPs[10]. Studies have found that Tuina manipulation participates in glucose metabolism regulation in type 2 diabetic rats by regulating the AMPK/GluT4 signal chain in skeletal muscles[34]. Tuina manipulation activates mechanosensitive channels in the muscle tissue, promotes the accumulation of PGC-1α in the nucleus, boosts mitochondria biogenesis,and accelerates the healing of injured muscles[27]. After An-Pressing manipulation, the protein expression of phospho-AMPK, PGC-1α, and GluT4, as well as the value of phospho-AMPK/AMPK were increased significantly. The mitochondria number was increased,and the shape, size, and structure of mitochondria basically returned to normal. These findings indicate that the An-Pressing manipulation may activate the energy metabolism AMPK/PGC-1α/GluT4 pathway,promote the generation and function improvement of mitochondria in muscle cells, as well as the uptake of glucose, which further increases the synthesis of ATP,thereby alleviating the energy crisis of MTrPs.
In conclusion, the production of MTrPs is related to ATP energy crisis, AMPK/PGC-1α pathway inhibition,and mitochondrial dysfunction. An-Pressing manipulation can improve the energy crisis of MTrPs,which may strengthen the mitochondrial function by activating the AMPK/PGC-1α pathway and promoting the mitochondrial function, thus promoting glycogen uptake and damaged tissue repair. The results of this study provide a scientific basis for the treatment of MTrPs by An-Pressing manipulation and provide a new idea for explaining the mechanism of An-Pressing manipulation based on the energy metabolism AMPK/PGC-1α pathway.
Conflict of Interest
There is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Project of National Natural Science Foundation of China (国家自然科学基金项目, No. 81973975); Youth Project of Hunan Provincial Natural Science Foundation of China (湖南省自然科学青年基金项目, No. 2021JJ40482); Excellent Youth Project of Hunan Education Department (湖南省教育厅优青项目, No. 20B439); Science and Technology Guidance Project of Hengyang (衡阳市科技指导性项目,No. 2019JH011041); Hunan Academician Expert Workstation (SHI Xuemin) Open Fund of the First Hospital of Hunan University of Chinese Medicine [湖南中医药大学附属第一医院湖南省院士专家工作站(石学敏)开放基金, No. 201901].
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.Received: 11 March 2021/Accepted: 18 August 2021
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Study on the mechanism of moxibustion for rheumatoid arthritis based on liquid chromatography-mass spectrometry
- Regulatory effect of acupuncture on electrical activity level of optic cortex in amblyopia model rats
- Influence of Tuina plus oxiracetam on serum inflammatory factors and oxidative stress in mild vascular dementia patients
- Effects of acupuncture on nutritional status in patients in a persistent vegetative state:a prospective randomized controlled study
- Clinical observation of acupuncture plus acupoint sticking therapy for insomnia and its influence on subjective and objective sleep indicators
- Clinical observation of warm needling moxibustion plus lumbar traction for lumbar disc herniation
