Contrast-enhanced ultrasound of a traumatic neuroma of the extrahepatic bile duct: A case report and review of literature
2022-08-13ZhiQiangYuanHuaLinYanJiaWuLiYanLuo
Zhi-Qiang Yuan, Hua-Lin Yan,Jia-Wu Li, Yan Luo
Abstract
Key Words: Traumatic neuroma; Bile duct; Contrast-enhanced ultrasound; Enhancement;Cholangiocarcinoma; Case report
lNTRODUCTlON
A traumatic neuroma is a chronic reparative proliferative response of the nerve after trauma or surgery.It is composed of disorganized nerve fiber bundles with fibrous stroma, Schwann cells, perineural cells,axons, and endoneural fibroblasts[1]. The common sites of traumatic neuromas are the necks and extremities[2,3]. Although some studies have described traumatic neuromas in the bile duct, cases of sonographic features of contrast-enhanced ultrasound (CEUS) have not been published before. The clinical manifestation and imaging examination of a traumatic neuroma of the bile duct are not specific,which makes it challenging be accurately diagnosed preoperatively. Herein, we report a traumatic neuroma of the extrahepatic bile duct with detailed ultrasonographic imaging features. We also reviewed the literature on the imaging findings for traumatic neuromas.
CASE PRESENTATlON
Chief complaints
A 55-year-old man was admitted to our hospital with unexplained abdominal distension and anorexia 3 mo ago.
History of present illness
The patient suffered from unexplained abdominal distension and anorexia for 3 mo. The patient developed darkened urine 2 mo ago. He experienced a weight loss of 5 kg over the course of the disease.He underwent contrast-enhanced computed tomography (CECT) examination at a local hospital, and a lesion was found in the extrahepatic bile duct, which was believed to be a tumor.
History of past illness
The patient underwent cholecystectomy for gallbladder stones with an uneventful postoperative recovery 4 years ago. He had a 10-year history of hypertension.
Personal and family history
There was no other personal or family history of acute or chronic disease.
Physical examination
The patient showed no tenderness, rebound tenderness or muscle tension on abdominal palpation.
Laboratory examinations
The liver function tests demonstrated increased levels of alanine aminotransferase (185 IU/L, normal range: < 50 IU/L), aspartate aminotransferase (148 IU/L, normal range: < 40 IU/L) and total bilirubin(37.0 μmol/L, normal range: 5 µmol/L to 28 µmol/L). Tumor markers included carbohydrate antigen 19-9 (98.6 U/mL, normal range: < 22 U/mL), carcinoembryonic antigen (0.97 ng/mL, normal range: < 5 ng/mL), and alpha-fetoprotein (4.67 ng/mL, normal range: < 7 ng/mL).
Imaging examinations
The patient underwent an abdominal ultrasound (US) examination by a Resona7 US system (Mindray Medical International, Shenzhen, Guangdong Province, China) equipped with an SC6-1U (1-6 MHz)transducer. The US revealed mild to moderate dilatation of the intrahepatic bile duct, and the diameter of the upper extrahepatic bile duct was 1.2 cm (Figure 1A). A hyperechoic nodule sized 0.8 cm × 0.6 cm was found in the upper extrahepatic bile duct with an almost regular shape and slightly clear margins(Figure 1B). The patient underwent CEUS with the patient’s consent for further diagnosis. A 2.4-mL US contrast agent SonoVue (Bracco, Milan, Italy) suspension was injected through the left cubital vein followed by a flush with 5 mL saline. In the arterial phase, the nodule showed slight heterogeneous hyperenhancement without rim-like enhancement (Figure 1C). The nodule appeared heterogeneous isoenhancement in the venous phase (Figure 1D). Additional CECT in our hospital showed a hypoenhancement nodule approximately 1.3 cm × 1.0 cm in size in the upper extrahepatic bile duct (Figure 2).
FlNAL DlAGNOSlS
Based on the incidence of bile duct diseases, imaging findings and laboratory tests, the patient's clinical diagnosis was hilar cholangiocarcinoma. However, postoperative pathology of the common bile duct lesion showed a neoplastic proliferation of submucosal nerve tissue and fibrous tissue (Figure 3A), and an immunohistochemistry marker was positive for S-100 (Figure 3B). The above pathological findings indicated that the lesion in the bile duct was a traumatic neuroma.
TREATMENT
During the surgery, intraoperative frozen pathology showed no tumor cells within the bile duct lesion.Therefore, hilar bile duct resection and end-to-end bile ductal anastomosis (EE) were performed. The patient recovered uneventfully after surgery.
OUTCOME AND FOLLOW-UP
There was no obvious abnormality on CECT for half a year after the operation.
DlSCUSSlON
Extrahepatic bile duct masses are commonly malignant tumors, while benign tumors account for only 6%[4-7]. Consequently, the possibility that extrahepatic bile duct lesions are traumatic neuromas is easily overlooked. It has been reported that most traumatic neuromas of the biliary tract arise in the cystic duct stump after cholecystectomy[8]. If a nerve is transected and its continuity cannot be reestablished, a traumatic neuroma may develop[9].
We reviewed the literature from 2000 to 2021 and found 18 publications regarding the imaging features of traumatic neuromas in the bile ducts[2,10-26]. The clinical findings and imaging features of these 18 reported cases are summarized in Table 1. Finally, 22 patients were included in the literature review for further analysis. The age of patients ranged from 17 to 81 years of age, and there was a significant male predominance, with 15 males (68.2%), 2 females, and 5 patients of unreported sex. Most cases were secondary to cholecystectomy, but a few were secondary to liver transplantation,hepatectomy and hilar cholangiocarcinoma. The major symptoms found in these patients were jaundice,abdominal pain, and weight loss, while some patients had no apparent symptoms.
Unfortunately, no specific imaging features for traumatic neuromas of the bile duct have been found at present. Although some imaging modalities, such as US, computed tomography (CT), and nuclear magnetic resonance imaging (MRI), are valuable to some extent, it remains a challenge to diagnose traumatic bile duct neuromas preoperatively[17]. Imaging findings in these 22 patients varied from nodules or masses to localized bile duct stenosis with dilatation of the upper bile duct. It has been reported in the literature that the US imaging findings of extraabdominal nerve tumors and traumatic neuromas are generally hypoechoic masses, larger than the nerve trunk and continuous with the nerve[27]. However, the nerve injury related to cholecystectomy may be too small, so we could not find that the nerve is connected to traumatic neuroma of the bile duct. US was performed in 5 of the 22 patients, 2 of whom showed hypoechoic nodules, and the remaining 3 patients showed stenosis and dilatation of the bile ducts. However, our patient's US sonogram showed a hyperechoic nodule, indicating that the echogenicity of the nodule of traumatic neuroma was variable.

Figure 1 Ultrasound images of the patient. A and B: The ultrasound (US) showed mild to moderate intrahepatic bile duct dilatation (orange arrow) and a hyperechoic nodule sized 0.8 cm × 0.6 cm (orange arrow) in the extrahepatic bile duct; C and D: In the arterial phase, contrast-enhanced US (CEUS) showed slight hyperenhancement (orange arrow); in the venous phase, CEUS showed isoenhancement (orange arrow).
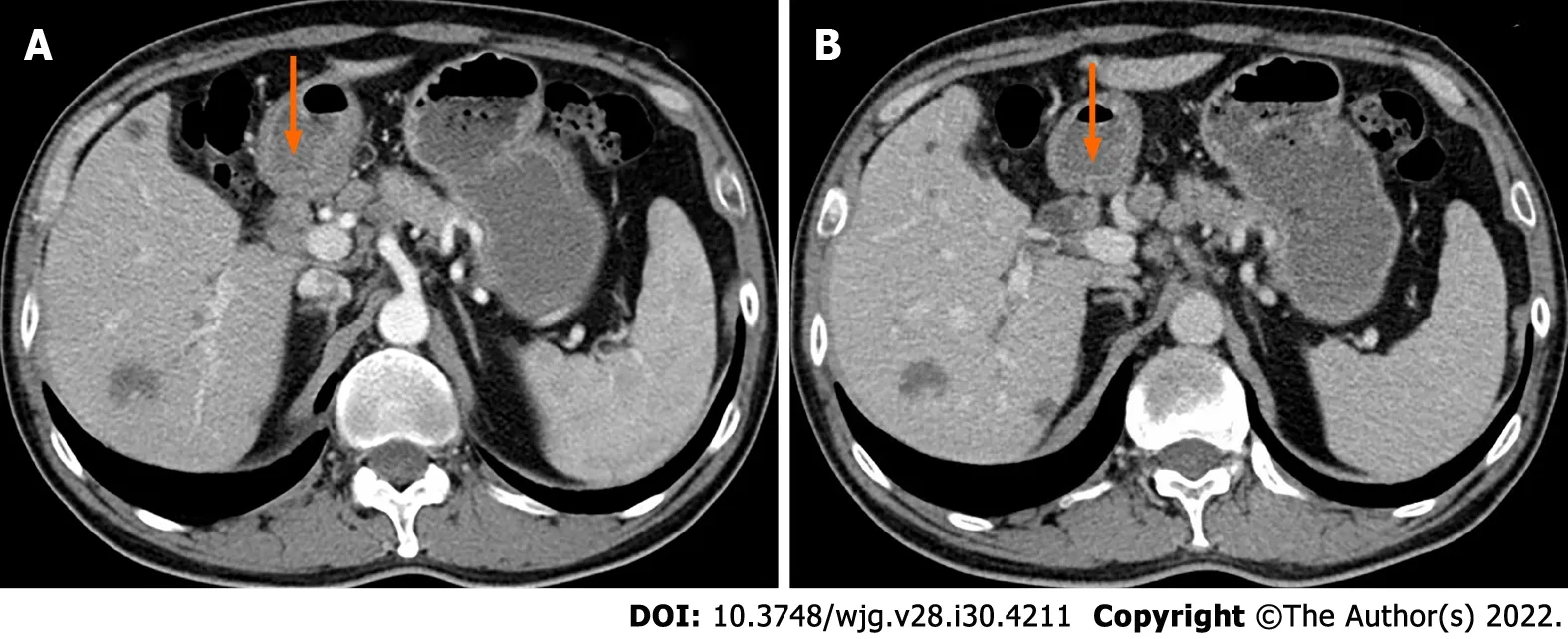
Figure 2 Contrast-enhanced computed tomography images of the patient. Contrast-enhanced computed tomography showed a hypoenhancement nodule in the upper extrahepatic bile duct (orange arrow).
CECT was performed in 2 of the 18 cases, and an enhancing nodule was seen, which was consistent with the CECT findings of our patient. Traumatic neuromas also show enhancement on MRI when a contrast agent is used[28], which may be related to a damaged peripheral nerve blood barrier that occurred during a prior insult to the nerve[29-32]. One of these 18 cases described the enhancement pattern of traumatic neuroma on MRI in detail, which showed a marked homogeneously enhanced nodule that was iso-intense to the aorta in the atrial phase and a homogeneously enhanced nodule that was iso-intense to the aorta in the portal phase. There have been a few reports of other imaging techniques for diagnosing traumatic neuromas, such as magnetic resonance cholangiopancreatography,endoscopic US, contrast-enhanced harmonic endoscopic ultrasonography, intraductal ultrasonography and percutaneous transhepatic cholangiography. None of these imaging methods revealed specific features for traumatic neuromas.
It is challenging to distinguish bile duct traumatic neuroma from other lesions before surgery, so it is often misdiagnosed. The diagnosis of bile duct traumatic neuroma was correctly diagnosed in 1 of the 18 cases examined and confirmed by biopsy. The remaining cases were not correctly diagnosed, and it was difficult to distinguish between benign and malignant lesions in most cases. Therefore, surgery would be performed on a large proportion of patients. Once the patient underwent surgery, an intraoperative frozen section examination helped to confirm that the lesion was benign and extensive surgicalresection of the traumatic neuroma was avoided[2,3]. The primary treatment reported in the literature consists of bile duct excision and hepaticojejunostomy (HJ). Although HJ is frequently recommended for reconstruction, the indications, surgical options and suture selection are also controversial. Some investigators also recommend EE because it is more physiological and can maintain physiological balance[33]. It is possible to achieve excellent long-term results and high quality of life using both HJ and EE when it is feasible for the proximal and distal ductal ends to permit EE[34]. Therefore, the choice of the optimum method is strictly correlated with the morphological nature of the lesion, which is different from one stage to the other, depending upon the moment of detection, and therefore have different surgical implications[35]. The surgeon found that the anastomosed edges blood supply was good and that there was no tension of the anastomosed edges in this patient. Therefore, according to the actual conditions of patients, as well as to maintain physiological balance, our hospital professor implemented EE for this patient.
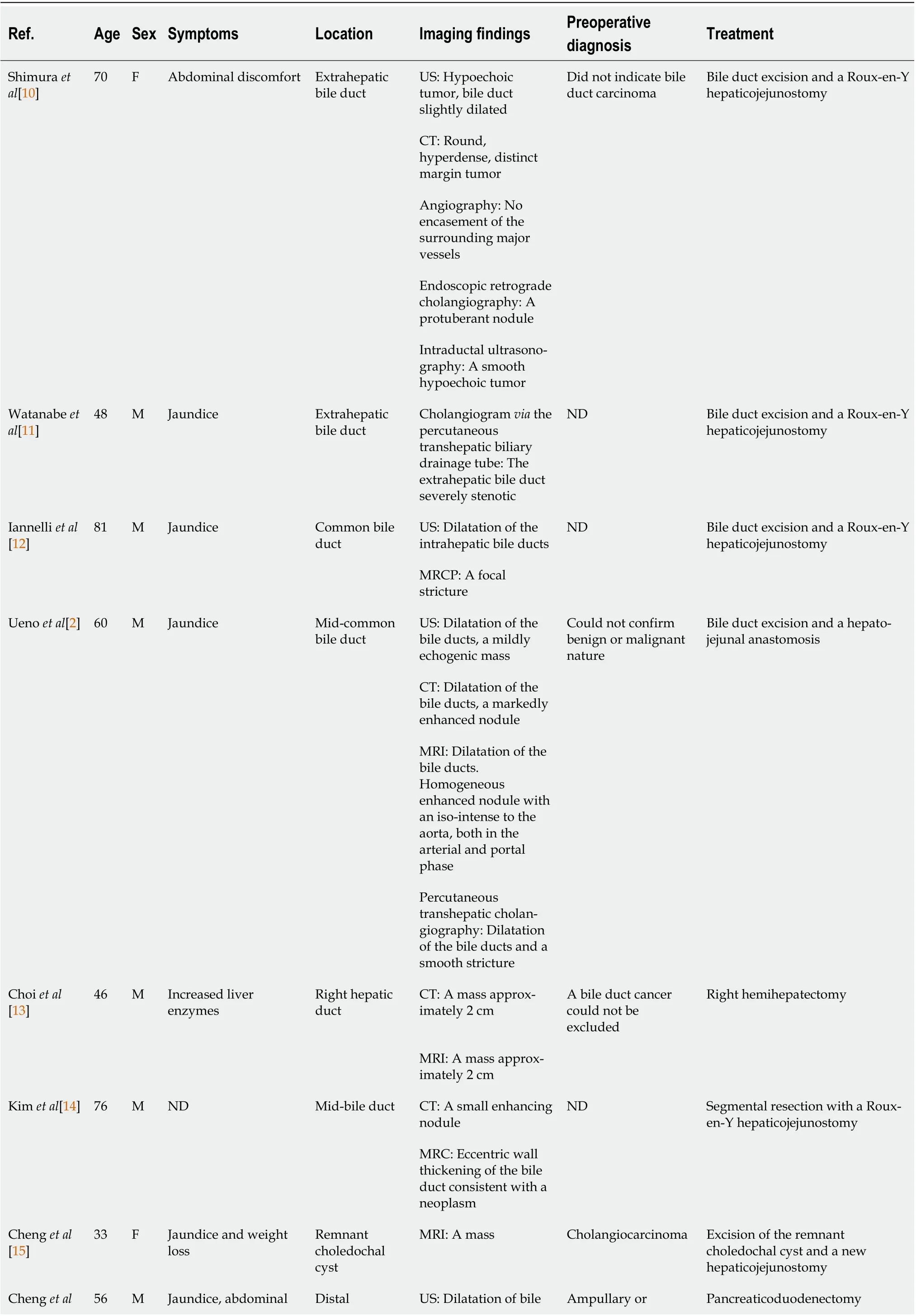
Table 1 Traumatic neuroma of the bile duct reported in the literature between 2000 and 2020

[16]pain and weight loss extrahepatic bile duct duct periampullary carcinoma MRI: Dilatation of bile duct, a filling-defect in the distal bile duct and a thickened biliary wall around the ampulla of Vater Cheng et al[17]68 M Progressive jaundice and abdominal pain Bifurcation of the left and right hepatic duct MRI: A mass with enhancement, a stricture of the hilar bile duct, dilatation of bile ducts Cholangiocarcinoma Excision of the mass and a new Roux-en-Y hepaticojejunostomy Navez et al[18]ND ND Jaundice (3 patients) or liver function test alteration (1 patient), a retro-obstructive choleperitoneum on the downstream biliary stenosis (1 patient)Anastomotic biliary stricture CT: Anastomotic biliary stricture (4 patients)ND Traumatic biliary neuromas resection combined with hepaticojejunostomy (1 patient);traumatic biliary neuromas resection and duct-to-duct biliary reconstruction protected by a Ttube (4 patients)MRI: A markedly homogeneous high intensity nodule enhanced on portalphase (1 patient),anastomotic biliary stricture (4 patients)Terzi et al[19]17 F Persistent elevated transaminase and bilirubin levels Anastomotic biliary Percutaneous transhepatic cholangiography: A biliary stricture at the anastomosis ND Resection of the bile duct stricture and a Roux-en-Y hepaticojejunostomy Toyonaga et al[20]76 F A bile duct nodule Proximal common bile duct CT: An 8 mm, smooth,and uniformly enhanced nodule Submucosal tumor Biopsy, observation for 1 year, no changes to the nodule Contrast enhanced endoscopic ultrasonography: A clear boundary and a low echoic nodule,uniformly enhanced at early Cholangioscopy: A smooth elevated lesion, covered with normal mucosa Yang et al[21]65 M Jaundice Right bile duct MRI: A 1.0 cm × 1.5 cm mass Cholangiocarcinoma Resection of the mass and Rouxen-Y hepaticojejunostomy.Hirohata et al[22]60 F No chief complaint Junction of the cystic duct US: A 6 mm round tumor, surrounding lymph nodes were not swollen Cholangiocarcinoma Surgery MRI: A slightly high signal on T2 and the periphery remnant cystic duct of the tumor presented as a high-intensity lesion on T2 EUS: A residual cystic duct tumor with enhancement ERCP: Not invade the common bile duct Yasuda et al[23]76 M ND Stump of the dilatated cystic duct EUS: A hypoechoic oval mass with a hyperechoic rim on the surface, 14 mm in Amputation neuroma Biopsy, observation
diameter, hypervascularity Cholangiogram: A hemispherical defect Cholangioscopy: A hemispherical mass covered with thin normal cystic duct epithelium Lalchandani et al[24]41 M Epigastric pain, weight loss, tea-colored urine Common hepatic duct US: Dilation of the bile ducts Acute cholangitis First: Biliary stent Finally: Bile duct resection and hepaticojejunostomy ERCP: A 3-4 cm stricture Kim et al[25]72 M A duodenal subepithelial tumor during a medical checkup Near the duodenal wall and the cystic duct stump CT: A 1.4 cm mass Duodenal subepithelial tumor Resection of the mass and duodenal wall, en-block resection of the mass and cystic duct origin EUS: An 18 mm hypoechoic mass Nechi et al[26]76 M Jaundice The transition zone between the common hepatic duct and the main bile duct US: Dilation of the bile ducts, a 5 mm hypoechoic nodule Could not confirm benign or malignant nature Resection of the main bile duct with a choledocho-duodenal anastomosis MRI: Dilation of the common hepatic duct ND: Not described; US: Grayscale ultrasound; CT: Computed tomography; CECT: Contrast-enhanced computed tomography; MRI: Magnetic resonance imaging; MRC: Magnetic resonance cholangiogram; MRCP: Magnetic resonance cholangiopancreatography; EUS: Endoscopic ultrasonography; ERCP:Endoscopic retrograde cholangiopancreatography.
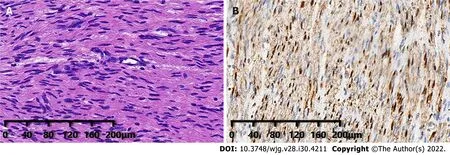
Figure 3 Postoperative histopathological images of the patient. A: Hematoxylin and eosin staining showed proliferation of submucosal nerve tissue(magnification, × 100); B: Immunohistochemical staining displayed S100(+) (magnification, × 100).
In this patient, the symptoms of anorexia, weight loss and jaundice mimicked those often caused by malignant tumors of extrahepatic bile ducts. CEUS and CECT showed enhancement of the nodule.Based on the incidence of bile duct diseases and imaging findings, the surgeons misdiagnosed it as cholangiocarcinoma. Periductal infiltrative cholangiocarcinomas account for the majority of extrahepatic cholangiocarcinomas[36]. Extrahepatic cholangiocarcinomas may show hyperenhancement,isoenhancement, or hypoenhancement in the early phase of CEUS, and most of them show hypoenhancement in the late phase[37]. If we find a nodule in the bile duct, we should rule out the diagnosis of cholangiocarcinoma when the nodule does not show hypoenhancement in the late phase of CEUS.However, when traumatic neuroma presents as localized bile duct stenosis, it is relatively difficult to distinguish it from malignant lesions. When a patient has a history of biliary system surgery and the tumor markers are not significantly elevated, suspicion of traumatic neuroma increases. If conditions permit, patients can be protected from unnecessary surgeries by confirming the diagnosis with a biopsy.CEUS is beneficial for differentiating cholangiocarcinoma from traumatic neuromas, but more cases are needed to summarize the sonographic features of this disease. Recognizing of traumatic neuromas may aid in preoperative work up, planning, and patient counseling[24].
CONCLUSlON
It is difficult to correctly diagnose traumatic neuroma of the bile duct before surgery. We should rule out malignant differential diagnoses, such as cholangiocarcinoma preoperatively, to avoid unnecessary surgery. The enhancement mode of CEUS may provide information to distinguish traumatic neuromas from malignant lesions. We need to combine the history of biliary tract surgery, clinical findings,imaging findings and laboratory tests to diagnose this disease.
FOOTNOTES
Author contributions:Yuan ZQ performed the literature review and wrote the manuscript; Yan HL and Li JW supported the data collection and manuscript revision; Luo Y supervised the writing and revision of the manuscript;all authors read and approved the final manuscript.
Supported byNational Natural Science Foundation of China, No. 82071940.
lnformed consent statement:Written informed consent for publication was obtained from the patient.
Conflict-of-interest statement:The authors declare that they have no conflicts of interest to report.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Zhi-Qiang Yuan 0000-0002-3037-7576; Hua-Lin Yan 0000-0003-1338-1124; Jia-Wu Li 0000-0003-0844-5883; Yan Luo 0000-0003-2985-1768.
Corresponding Author's Membership in Professional Societies:Society of Ultrasound, Abdomen Ultrasound Subcommittee, Chinese Medical Doctor Association, No. 199174.
S-Editor:Yan JP
L-Editor:A
P-Editor:Yan JP
杂志排行
World Journal of Gastroenterology的其它文章
- Role of one-step nucleic acid amplification in colorectal cancer lymph node metastases detection
- Current perspectives on the role of liver transplantation for Langerhans cell histiocytosis: A narrative review
- Gut microbiota, inflammatory bowel disease and colorectal cancer
- Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures
- P2X7 receptor blockade decreases inflammation, apoptosis, and enteric neuron loss during Clostridioides difficile toxin A-induced ileitis in mice
- Serological profiling of Crohn’s disease and ulcerative colitis patients reveals anti-microbial antibody signatures
