Surface Modification of NiCo2O4 Nanowires using Organic Ligands for Overall Water Splitting
2022-08-10孙轲,赵永青,殷杰等
Abstract: Bifunctional electrocatalysts in alkaline media play an important role in the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), owing to the considerable influence of water splitting in the green energy sector. Herein, we present surface-modified NiCo2O4 nanowires (NWs)with rich defects as a highly efficient overall water splitting electrocatalyst in alkaline media, where the surface modification is accomplished using organic ligands.X-ray photoelectron spectroscopy reveals that the increase in the Co2+/Co3+ ratio is responsible for the excellent bifunctional electrocatalytic performance of the surface-modified NiCo2O4 NWs. As expected, benefiting from the organic ligand-dominated surface modification, the optimized NiCo2O4 NWs can display an overpotential of only 83 mV for the HER and 280 mV for the OER, with a current density of 10 mA·cm-2 in 1.0 mol·L-1 KOH solution. More importantly, the NiCo2O4 NWs surface-modified using organic ligands exhibit outstanding performance for overall water splitting, with a voltage of 2.1 V and current density of 100 mA·cm-2, and also maintain their activity for at least 15 h.The present work highlights the importance of increasing the content of Co2+ in the spinel structure of NiCo2O4 NWs for enhancing their performance in overall water splitting.
Key Words: Bifunctional electrocatalyst; Organic ligand; Interface; Surface modified nanowire; Water splitting
1 Introduction
The rapid growth of energy consumption and deterioration of ecological environment, a kind of environmentally friendly energy, forcing people to look for1,2. Hydrogen (H2) is one of the most promising fuels to meet the renewable and clean energy demand of human society3. Electrolyzing water has been regarded as the most efficient way for the industrial production of hydrogen, however, water electrolysis is often limited by the sluggish anodic oxygen evolution reaction (OER) due to its multi-step pro-ton-coupled electron transfer process4. To date,IrO2and RuO2have been identified as the most active electrocatalysis for OER, while Pt and Pt-based catalysts for HER, how-ever the high cost severely restrain their large scale application4,5. Therefore, electrocatalyst is the critical part of the electrode, a great deal of efforts has been devoted toward meeting the target of electrochemical water splitting efficient and economically feasible.
Recently, transition metal oxides (TMO) materials have been attracting great attention as low-cost bifunctional electrocatalysts in alkaline electrolyte for both the HER and OER6,7. In order to improve catalytic efficiency of TM-based catalysts, the nanostructure and composition of catalysts should be optimized to overcome the inherent deficiencies of single material and expose more catalytically active sites for water splitting. Among the TMO, Ni and Co oxides display superior electrocatalytic capability toward water splitting because of their high corrosion-resistant properties in alkaline solution and richly variable valence states. Recent studies revealed that the water splitting electrocatalytic performance of (Ni, Co)-based catalysts can be improved via tuning interface8,9, valence states10, and vacancies11. However, the performance of the most of the catalysts are still much lower than that of the noble catalysts for water splitting. NiCo2O4nanowires (NWs) as a typical of spinel oxide have been widely investigated owing to their unique physicochemical properties and potential applications in water splitting12-15. To the best of our knowledge, Co occupied octahedral site in NiCo2O4and mainly exists at positive +3 valence state16, however, catalytic activity of Co2+is better than Co3+for water splitting. Thus, precisely tuning the Co2+/Co3+ratio is vital important for boosting the electrocatalysis activity.Furthermore, Metal-Organic Framework (MOF) with abundant pores and porous structure shows promising application in widely fields17. During the reaction generation process of MOF,we can build porous structure and change the ratio of Co valence state on the surface structure of NiCo2O4and improve the catalytic activity.
Herein, we successfully develop a novel bifunctional catalysts NiCo2O4NWs-MOFs. As shown in Scheme 1, the NiCo2O4NWs-MOFs were synthesized through a two-step procedure(See the details in the experimental section (Supporting Information online)). Firstly, the sea urchin NF/NiCo2O4NWs were synthesized by a facile hydrothermal method at 120 °C for about 16 h, and then dried at 80 °C18. Then the MOFs were grown on the NiCo2O4NWs by solvothermal reaction without the additional metal salts. The materials were in a ten fold relationship between the two samples, which we named them as NiCo2O4NWs-MOFA, NiCo2O4NWs-MOFB and NiCo2O4NWs-MOFC. During the process of MOF-74 grown on the NiCo2O4NWs, a new series of material were born. i) Ni-based and Co-based MOF derived materials with large surface area and rich pore structures can provide more accessible active sites and be favorable for enhanced electro-catalytic activity18-20of NiCo2O4. ii) The surface lattice of NiCo2O4can be further altered through the decorated Ni-MOF-74 and Co-MOF-74 which is the key factor for tuning the ratio of Co2+/Co3+21modifying their interface and increasing the content of vacancies13. iii) The surficial Ni-MOF-74 and Co-MOF-74(MOFs) can be formed by drawing both cobalt and nickel ions from the parent Ni-Co2O4NWs onto their surface15. Thus, the original Ni-Co2O4NWs can be effectively activated because of the modified of valence state of surface Co ion via organic ligand of MOF-74.
2 Results and discussion
In this paper, innovatively modified the ratio of valence superficial Co ion of the NiCo2O4NWs via organic ligand of MOF-74. Furthermore, many defects can be introduced to the NiCo2O4NWs and will produce more active sites.
Scheme 1 Illustration of the synthesis of NiCo2O4 NWs -MOFs.
In this method, both the catalytic properties and the durability can be improved, which confirmed by electrochemical test. The XPS indicates that the binding energy of the outer Co valence has changed after organic ligand regulation, which results from the molar ratio of Co2+/Co3+of NiCo2O4nanowires. The as prepared NiCo2O4NWs-MOFB exhibits the most excellent catalytic activity with small overpotential both for OER and HER at current of 10 mA·cm-2, which are 280 and 83 mV respectively. The optimal NiCo2O4NWs-MOFB is an efficient bifunctional electrocatalyst, only 2.1 V at the current density of 100 mA·cm-2, which can maintain 90 percent catalytic activity within 15 h.
XRD patterns of the group of surfaces modified NiCo2O4nanowires via organic ligand shown in Fig. 1a. There are NiCo2O4NWs-MOFA, NiCo2O4NWs-MOFB, NiCo2O4NWs-MOFC and NiCo2O4NWs from up to bottom. For the series of samples, MOFs grown on NiCo2O4NWs contain its peak position and peak amount. NiCo2O4NWs-MOFA, NiCo2O4NWs-MOFC and NiCo2O4NWs-MOFB their XRD patterns shown in Fig. S1 (Supporting Information). The hybrid structure contains cubic NiCo2O4with the lattice constants a = b = c =0.811 nm and a space group of F*3 (JCPDS card NO. 20-781).NiCo2O4NWs-MOFB contains peaks of Ni-MOF-74 and Co-MOF-74 in low degree area. The surface compositions and chemical states of materials were analyzed by X-ray photoelectron spectroscopy (XPS). The fitting analysis (Fig. 1b)revealed the coexistence of Ni3+and Ni2+. The binding energies at 855.8 and 873.36 eV correspond to Ni2+2p1/2and 2p3/215,22.In the original spinel structure, Ni adopts a tetrahedral field coordination fashion, which valence state is +2. After ligand modification, Ni2+is extracted from the original phase by the ligand and partially converted to Ni3+, which helps to enhance the catalytic activity of water splitting reaction. High-resolution of Co 2p XPS spectra (Fig. 1c) of NiCo2O4NWs-MOFA,NiCo2O4NWs-MOFB, NiCo2O4NWs-MOFC and NiCo2O4NWs is in the graph left, and in the right of graph is the scatter diagram of Co2+2p3/223-25. A bar graph of molar ratio of Co2+/Co3+(Fig. 1d), the binding energies at 796 and 780 eV correspond to Co 2p1/2and 2p3/2. The highest binding energy of Co 2p is NiCo2O4NWs-MOFB (B) among the group of samples.It was confirmed by IR test (Fig. S2) that before and after MOFs grows, for all as prepared hybrids, after modification characteristic peaks of organic functional group of the ligand could be found26,27. Modification by the ligand can change the ratio of Co2+/Co3+. Specifically, the Co3+occupying the octahedral coordination in spinel structure, will transform into a tetrahedral coordination state, thus moving toward low field. For the best we know, Co2+with a tetrahedral field coordination has excellent catalytic performance for OER. In line with the peak area, the ratio of Co2+to Co3+of NiCo2O4NWs-MOFB is the highest in the series of samples. Through organic ligand regulation, the peak positions of Co 2p had changed slightly. It can be concluded that the growth of MOFs has little effect on the position of the whole peak. A little of lattice of NiCo2O4NWs were broken, and then metal ions are pulled out by the ligand of MOFs during the reaction. The bonding state between the original O in NiCo2O4and the surrounding metal will also be affected, which will further promote the dissolution of the metal ions on the surface of NiCo2O4NWs. Fig. 1e is the highresolution O 1s XPS spectra of the series of samples. Fig. 1f shows the molar ratio of O-vacancy versus the whole of O.Comparing the four groups of materials, binding energy of O 1s of NiCo2O4NWs-MOFB is 530.89 eV, not the highest of all samples. Meanwhile, its O-vacancy is not the lowest one11,28.For our research system, O-vacancy is not the most important factor for catalytic activity, the valence state of Co ion is the key factor for improvement of catalytic activity.
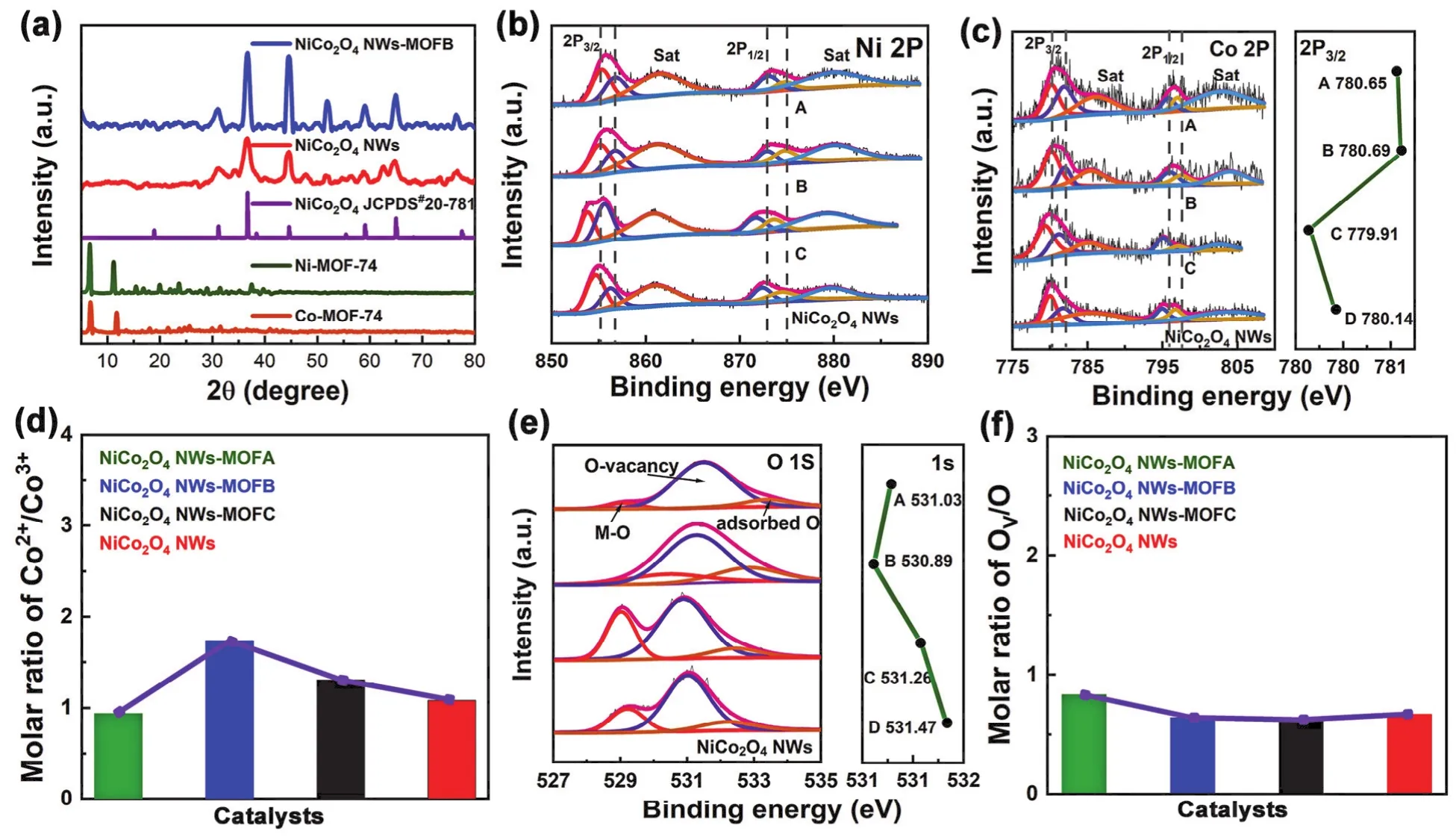
Fig. 1 (a) The XRD shown NiCo2O4 NWs-MOFA, NiCo2O4 NWs-MOFB, NiCo2O4 NWs-MOFC and NiCo2O4 NWs from up to bottom; (b) Ni 2p XPS spectra; (c) high-resolution Co 2p XPS spectra of NiCo2O4 NWs-MOFA, NiCo2O4 NWs-MOFB, NiCo2O4 NWs-MOFC and Ni-Co2O4 NWs;(d) molar ratio of Co2+/Co3+; (e) high-resolution O 1s XPS spectra of the series of samples; (f) molar ratio of O-vacancy/the whole of O.
Typical scanning electron microscopy (SEM) images show that all of samples are equipped with the original morphology of NiCo2O4NWs, like a sea urchin (Fig. 2a). Admitting the MOFs grow on its surface, they also hold much difference among each other. NiCo2O4NWs-MOFA (Fig. 2b) was synthesized by utilizing the most mass concentration of ligand, there are at least one or two types of prismatic MOF particles on NiCo2O4NWs.NiCo2O4NWs-MOFB (Fig. 2c) is also grown two or three kinds spheroidal MOF particles on NiCo2O4NWs. However, NiCo2O4NWs-MOFC (Fig. 2d) is prepared by least among of ligand, but it shows the most distinctive features among the series covered with many finely-divided particles on the NiCo2O4NWs, which looks like a tube override it. Whereas the samples covered by MOF particles are still keeping the morphology as a sea urchin.There is more detail information of the sample in Transmission electron microscopy (TEM). As Fig. 2e shows, there are many small Ni-MOF-74 and Co-MOF-74 particles grow on the NiCo2O4NWs, from TEM we can find interface between NiCo2O4NWs and Ni-MOF-74 and Co-MOF-74. Subsequently,HRTEM patterns (Fig. 2f) of NiCo2O4NWs-MOFB. The clear continuous lattice bands were observed on the side of NiCo2O4,but near the Ni-MOF-74 and Co-MOF-74, lattice bands become dim, which suggests plenty of defects. During MOFs growing on, the superficial Co2+and Ni2+of the initial NiCo2O4dissolved in solution, and they will be caught by the free ligand in solution,then gave rise to the production of Ni-MOF-74 and Co-MOF-74 on the surface of NiCo2O4NWs. In the MOFs region we can further finding out another two lattice fringes, it is different from the lattice fringes of NiCo2O4. We also measuring their lattice space as CoO (0.210 nm) and NiO (0.208 nm) from up to bottom.During MOFs generating on the NiCo2O4NWs, the Co2+and Ni2+inside of NiCo2O4NWs were pulled out, a large number of defects were produced at the same time, as indicated by circles in Fig. 2f. The mapping test patterns we can get more information: i) the morphology of initial NiCo2O4NWs in the NiCo2O4NWs-MOFB have not been broken altogether. ii) Ni,Co and O are still distributing evenly, after the introduction of ligand 2,5-dihydroxyterephthalic acid, the sparse distribution of C can be seen on the sample, while only in the particle of MOFs the concentration of C is the highest. iii) In the process of reaction, the MOFs are growing on the surface of NiCo2O4NWs.DMF is a good dipolar aprotic solvent in the solvent thermal reaction the NiCo2O4NWs because of its structural defects, it will be easier to dissolve the Co2+and Ni2+. Fig. 2g, h shows the energy-dispersive X-ray (EDX) elemental mapping of NiCo2O4NWs-MOFB, illustrating the homogeneous spatial distributions of Ni, Co, O and C in crystal lattice. Linear scanning patterns of NiCo2O4NWs-MOFB (Fig. 2i) has confirmed that a high distribution of C in the MOFs region. Although air containing CO2would have an impact, only the presence of MOFs can make the C signal so strong. Our study demonstrate that we can introduce the ligand in the reaction solution enclosing the wellprepared nanomaterial, resulting in MOFs growing on NiCo2O4NWs in situ.

Fig. 2 SEM patterns of (a) NiCo2O4 NWs, (b) NiCo2O4 NWs-MOFA, (c) NiCo2O4 NWs-MOFB and (d) NiCo2O4 NWs-MOFC; (e) TEM patterns of NiCo2O4 NWs-MOFB, there is interface between the NiCo2O4 NWs and MOFs; (f) HRTEM patterns of NiCo2O4 NWs-MOFB; (g, h) energydispersive X-ray (EDX) elemental mapping patterns of Ni-Co2O4 NWs-MOFB; (i) linear scanning patterns of NiCo2O4 NWs-MOFB.
As we all know Ir as a catalyst with electrocatalytic water splitting property which is the best element for OER, owing to its very low overpotential and very low Tafel slope in alkaline media. The series of as prepared NiCo2O4NWs-MOFs samples act as the catalyst for overall water splitting and their OER performance will be described in detail. As shown in Fig. 3a,NiCo2O4NWs-MOFB, shows the lowest overpotential of 280 mV at the current density of 10 mA·cm-2. Compared with the NiCo2O4NWs-MOFA (300 mV), NiCo2O4NWs-MOFC (320 mV) and NiCo2O4NWs (320 mV). Similarly, we compared NiCo2O4NWs-MOFB performance with the known material with excellent properties Ir/C (20%). Shown as Fig. 3i, under the same measure station to test NF/Ir/C, NiCo2O4NWs-MOFB and bare Ni foam. When under the same potential of 1.55 V, the current of NiCo2O4NWs-MOFB is 54.2 mA·cm-2, 4.8 and 46.8 times higher than that for NF/Ir/C and bare NF, respectively. By calculating the corresponding Tafel slopes of all samples (Fig.3b), we can get NiCo2O4NWs-MOFB has the best reaction kinetic reaction, its Tafel slope is 31 mV·dec-1. Comparing with the control samples: Tafel slope of NiCo2O4NWs-MOFA,NiCo2O4NWs-MOC and NiCo2O4NWs respectively are 56, 77,and 58 mV·dec-1. In addition, we use the chronopotentiometry(CP) function to test its durability from 10 mA·cm-2to 100 mA·cm-2(Fig. 3c) without iR correction only by the same sample and shown a long rang durability.
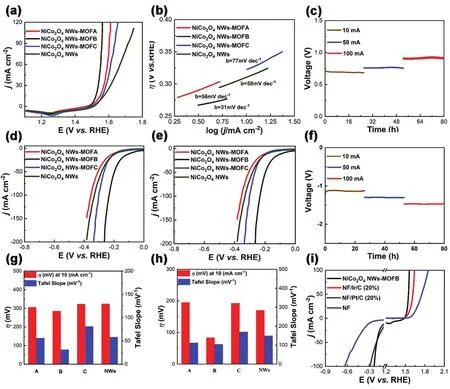
Fig. 3 (a) LSV curves of NiCo2O4 NWs-MOFs for the OER; (b) Tafel slope of NiCo2O4 NWs-MOFs for the OER; (c) durability of NiCo2O4 NWs-MOFB for the OER; (d) LSV curves of NiCo2O4 NWs-MOFs for the HER; (e) Tafel slope of NiCo2O4 NWs-MOFs for the HER; (f) durability of NiCo2O4 NWs-MOFB for the HER; (g) overpotential and Tafel slope at j = 10 mA·cm-2 for NiCo2O4 NWs-MOFA (A), NiCo2O4 NWs-MOFB (B),NiCo2O4 NWs-MOFC (C) and NiCo2O4 NWs; (h) overpotential and Tafel slope at j = 10 mA·cm-2 for NiCo2O4 NWs-MOFA (A), NiCo2O4 NWs-MOFB(B), NiCo2O4 NWs-MOFC (C) and NiCo2O4 NWs; (i) LSV curves of NiCo2O4 NWs-MOFB, NF/Ir/C, NF/Pt/C and NF for the OER and HER.
Except the properties of OER, the HER behavior of the series of samples under alkaline condition is also good that will be described in detail below. As we shown (Fig. 3d-f)), the NiCo2O4NWs-MOFB has the best HER catalytic activity and the lowest overpotential of 83 mV at 10 mA·cm-2out of the series. Similarly, we also compared the excellent HER sample with Pt/C (20%), and drop Pt/C on Ni foam for HER testing,respectively are NF/Pt/C, NF/NiCo2O4NWs-MOFB and bare NF. Under the same potential of -0.2 V, the current of NiCo2O4NWs-MOFB is 53.52 mA·cm-2, 1.1 and 11.1 times higher than that for NF/Pt/C and bare NF. By calculating the corresponding Tafel slope of all samples (Fig. 3e), we can get NiCo2O4NWs-MOFB has a better reaction kinetic, 104 mV·dec-1. Resulting in faster HER kinetics than NiCo2O4NWs-MOFA, NiCo2O4NWs-MOFC and NiCo2O4NWs. In terms of alkaline HER stability,we also used the piece of sample to test its stability by CP, from 10 to 100 mA·cm-2(Fig. 3f) without iR-correction.Subsequently, we plot the overpotential of the OER process and the HER process at 10 mA·cm-2(Fig. 3g, h). In the histogram,we can compare to the catalytic activity of NiCo2O4NWs-MOFB in the series more intuitively. For the overall water splitting, the LSV curves in the potential range of -1 to 1.8 V29.By comparing several sets of materials (Fig. 3i), it can be more intuitive to get a comparison of their properties. Chiefly NiCo2O4NWs-MOFB has the best performance both in OER and in HER and better than those compared noble metal catalysts.
After the long-range stability testing, the XPS was used to test their change24,27. As shown in Fig. 4, the elements are comparing the initial with after OER and HER. For the OER testing, Co 2p and Ni 2p have not occurred obvious displacement. After analyzing, their +2 and +3 valence state ratios changed slightly after testing. O 1s the overall movement to the low field can reduce the binding energy. Similarly, after HER process both of Co 2p and Ni 2p are also have not occurred obvious displacement. This indicates that the Co and Ni remain keep the same valence before and after catalysis. The overall peak position of O remained unchanged, and the oxygen vacancy was still dominant. However, the enhancement of metal oxygen bond indicated that the bond between oxygen and metal was closer after testing. In the meantime, the morphology of the samples which had been tested were observed by SEM (Fig. S3). It shows that the morphology of the samples can be maintained after a long time of stability testing, and there is still a clear and discernible nanowire structure28-30. Although the sample with MOFs growing on has become blurred, the original can still recognize the nanowires’ morphology. The two phases are tightly integrated, and it is also reconfirmed those MOFs particles still grows on nanowires. Meanwhile, Fig. S4 shows the XRD patterns after OER and HER testing. After the long-range testing, NiCo2O4NWs-MOFB can keep its characteristic peaks and the position of peaks.

Fig. 4 (a, b) Co 2p, (c, d) Ni 2p and (e, f) O 1s XPS spectra of NiCo2O4 NWs-MOFB.

Fig. 5 (a) Changing current test by the function of amperometric i-t curve and (b) calculating the quantity of electricity;(c) scheme of working condition; (d) test of change temperature; (e) stability text for two-electrode configuration;(f) scatter plot of working voltage of current density at 10 mA·cm-2 and time.
Given the excellent bifunctional performance, the NiCo2O4NWs-MOFB was used as a bifunctional catalyst to further assess its performance in an electrolytic cell at ambient environment.In Fig. 5a, similarly, we make the sample in a changing current test by the function of i-t Curve. Then we calculate the quantity of electricity (Fig. 5b). Evidently shown, it can still be competent for such tests. In Fig. 5c, from the scheme shows the working condition of electrolytic cell, electrolyzation in the two-electrode system with the NiCo2O4NWs-MOFB as both the anode and cathode were tested in an alkaline aqueous electrolyte solution.During the electrolysis, electrochemical working station provided electrical power to generate H2at the cathode and O2at the anode31-33. Fig. 5d for the test of change temperature,along with temperature rising, catalytic activity rise. Then, in the durability test (Fig. 5e) at current density at 100 mA·cm-2, it can remain durability for 15 h, and only attenuates to 95% of the initial. Meanwhile, we found the durability data of some similar materials in the two-electrode system by investigating the literature. And then summarize the scatter plot of working voltage of current density at 10 mA·cm-2and time (Fig. 5f).29
On the basis of these data, we further investigated the effect of the concentration of the ligands in the system on the properties of the final material. For this reason, a series of samples can be synthesized by changing the ligand concentration. At the same time, the best combination of NiCo2O4with Co-MOF-74 and Ni-MOF-74 for spinel structure in this system is also explored. We set the concentration of the ligands involved in the preparation of NiCo2O4NWs-MOFA as the unit, normalized the other samples and added 1/2, the five groups of samples. After testing their OER properties and HER properties, we constructed the volcano plot as shown in Fig. S5. It can be seen from the diagram that ‘0.1’ corresponds to B mentioned in this paper. NiCo2O4NWs-MOFB is at the top of the volcanic map in both OER and HER aspects, and is the best proportion.
3 Conclusions
An efficient surface modified NiCo2O4Nanowires via organic ligand for overall water splitting was successfully created, it exhibits excellent catalytic activity for both OER and HER processes under alkaline condition. Owing to the increased ratio of valence of Co2+/Co3+on the surface layer of NiCo2O4NWs, and the newly formed NiO is also a good water supplementary decomposition catalyst. Thus, the abovementioned synergy effects significantly enhance the catalytic performance. To drive 10 mA·cm-2water-splitting current, the NiCo2O4NWs-MOFB based two-electrode water electrolyze needs a cell voltage of only 1.60 V in 1.0 mol·L-1KOH. The study opens up a new avenue of thinking about synthesis materials, by growing MOFs on the surface of nanomaterials in situ, to achieve the purpose of causing bulk metal ions coordination deficiency, thereby improving the catalytic performance of fully decomposition water.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
