Challenges and Opportunities for Seawater Electrolysis: A Mini-Review on Advanced Materials in Chlorine-Involved Electrochemistry
2022-08-10BaihuaCuiYiShiGenLiYananChenWeiChenYidaDengWenbinHu
Baihua Cui , Yi Shi , Gen Li , Yanan Chen ,*, Wei Chen ,4, Yida Deng ,5,*, Wenbin Hu 1,,*
1 Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University,Fuzhou 350207, China.
2 Department of Chemistry, National University of Singapore, Singapore 117543, Singapore.
3 School of Materials Science and Engineering, Tianjin University, Tianjin 300350, China.
4 Department of Physics, National University of Singapore, Singapore 117542, Singapore.
5 School of Materials Science and Engineering, Hainan University, Haikou 570228, China
Abstract: Hydrogen (H2) is an important component in the framework of carbonneutral energy, and the scalable production of H2 from seawater electrolysis offers a feasible route to address global energy challenges. With abundant seawater reserves, seawater electrolysis, especially when powered by renewable electricity sources, has great prospects. However, chloride ions (Cl-) in seawater can participate in the anodic reaction and accelerate the corrosion of electrode materials during electrolysis. Although the oxygen evolution reaction (OER) is thermodynamically favorable, the chlorine evolution reaction is highly competitive because fewer electrons are involved (2e-). These two problems are compounded by the dearth of corrosion-resistant electrode materials, which hinders the practical applications of seawater electrolysis. Therefore, intensive research efforts have been devoted to optimizing electrode materials using fundamental theories for practical applications. This review summarizes the recent progress in advanced electrode materials with an emphasis on their selectivity and anti-corrosivity. Practical materials with improved selectivity for oxygen generation, such as mixed metal oxides, Ni/Fe/Co-based composites, and manganese oxide (MnOx)-coated heterostructures, are reviewed in detail. Theoretically, alkaline environments (pH > 7.5) are preferred for OER as a constant potential gap (480 mV) exists in the high pH region. Nevertheless, corrosion of both the cathode and anode from ubiquitous Cl- is inevitable. Only a few materials with good corrosion resistance are capable of sustained operation in seawater systems; these include metal titanium and carbon-based materials. The corrosion process is usually accompanied by the formation of a passivated layer on the surface, but the aggressive penetration of Cl- can damage the whole electrode. Therefore, the selective inhibition of Cl- transport in the presence of a robust layer is critical to prevent continuous corrosion. Advances in anti-corrosion engineering, which encompasses inherently anti-corrosive materials,extrinsically protective coating, and in situ generated resistive species, are systematically discussed. Rational design can impart the material with good catalytic activity, stability, and corrosion resistance. Finally, we propose the following opportunities for future research: 1) screening of selective and anti-corrosive materials; 2) mechanism of competitive reactions and corrosion; 3) evaluation of anti-corrosive materials; 4) industrial-scale electrolysis with high current density;5) optimization of experimental conditions; and 6) development of integrated electrolyzer devices. This review provides insights for the development of strategies aimed at tackling chlorine-related issues in seawater electrolysis.
Key Words: Seawater electrolysis; Chlorine chemistry; Anti-corrosion; Selective electrocatalyst
1 Introduction
Hydrogen is one of the most promising energy carriers to substitute fossil fuel for tackling the global warming effect in the future. To date, over 80% of the world’s energy obtain from nonrenewable fuels, such as oil, coal, and natural gas that dominate the CO2emission on earth1. Alternatively, hydrogen production from water electrolysis powered by renewable energies can provide a new approach for the current energy framework without compromising the long-term environmental sustainability2. Most water electrolysis systems adopt distilled freshwater with different pH over the past decades3-6, which is not an ideal approach in the regions with deficient freshwater supply but plenty of available seawater. From a practical point of view, direct seawater electrolysis has tremendous advantages owing to the abundant storage of seawater on earth and the equal capability to produce high-purity hydrogen.
In fact, there are still some problems to be solved in direct use of seawater for electrolysis. Natural seawater is a complex system including diverse inorganic ions (e.g., Cl-, Br-, SO24-,Na+, K+, Mg2+, and Ca2+) and marine microorganisms1, which are quite different from high-purity distilled freshwater.Specifically, the concentration of chloride ions, the major component in seawater, is as high as ~3.5%. The seawater electrolysis comprises two main half-reactions: hydrogen evolution reaction (HER) at the cathode and oxygen evolution reaction (OER) at the anode. It is difficult to evaluate the influence of all the constituents on the catalytic reactions up to now7,8. Although a large number of Cl-can act as charge carriers to improve the conductivity of the electrolyte9,10, the resulting CER will compete with the OER10-12. Hence, materials with high selectivity for OER are desirable preferred at the anode in seawater electrolysis. Besides, the aggressive chloride products(e.g., Cl2, HClO, or ClO-) could damage the electrolyte environment and cause a negative impact on the reaction system13,14.Both electrode and catalyst materials would suffer inevitable and strong corrosion from complex chlorides and their lifetime severely shortened. Different from conventional anti-corrosion studies on materials that are used in the ocean, those employed in seawater electrolyzer should have high catalytic activity,selectivity and good corrosion resistance. Most reported active materials are unlikely to sustain in highly corrosive solutions for a long period. The capable ones tend to show dissatisfactory catalytic performance, which is far away from the practical application of seawater electrolysis. As a result, the selection and design of the eligible materials for seawater electrolysis are of great importance in dealing with impairment from chlorine chemistry in the saline system.
Herein, we summarized three strategies for the design of highly selective and anti-corrosive materials employed in seawater electrolysis (Fig. 1). Firstly, some selective materials for the OER were discussed: a) transition metal-doped noble metal oxides; b) Ni/Fe/Co-based composites (e.g., oxides,boride, nitride, and sulfide); c) MnOx-coated heterostructure. In addition, the competition from CER could be reduced by controlling the applied potential and pH. Secondly, three approaches for the design of the anti-corrosive materials were outlined: a) inherently anti-corrosive materials (e.g., Ti, noble metal, and carbon-based materials); b) active materials coated with an extrinsically protective layer; c) in situ generated resistive species (e.g., SO42-, oxy/hydroxide). Particularly,manipulation of electrolytes with low Cl-concentration can be another viable way to ease corrosion and increase selectivity.This review ends up with ideas about the challenges and opportunities of seawater electrolysis, aiming to shine a light on future research.
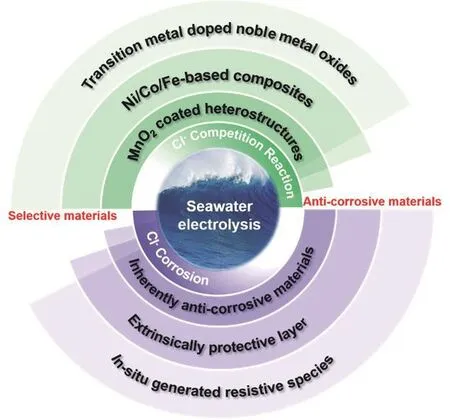
Fig. 1 Strategies for fabrication of the selective and anti-corrosive materials toward seawater electrolysis.
2 Selective materials in seawater splitting
2.1 Fundamental understanding of competitive reactions at the anode
Chlorine-involved oxidation is a complex reaction varies with the reaction conditions, such as pH value, applied potential and temperature. Strasser et al.15constructed the Pourbaix diagram(Fig. 2) where various chlorine-contained production formed at a wide pH range. To compare the chloride and water oxidation,the corresponding reactions (Eqs. 1-5) at two pH ends are shown as follows11,16,17,
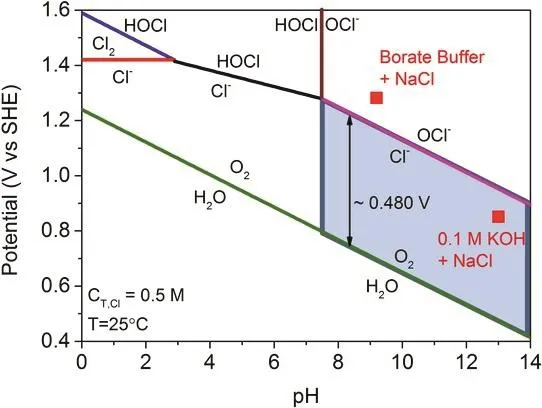
Fig. 2 Pourbaix diagram for artificial seawater model 15.

Fig. 3 The selective materials for seawater splitting. (a) TEM image of hexagonal NiFe LDH nanoplates and smaller FeOx particles, (b) Faradaic efficiency and current density of NiFe LDH on carbon support for OER 15; (c) the estimated amounts of Cl2 generated during 12 h of continuous electrolysis in neutral-buffered seawater electrolyte; (d, e) DFT calculation results of gas evolution reactions on metal hexacyanometallates 44.

According to the Pourbaix diagram, OER is more thermodynamically favorable than CER over the entire pH range. A constant potential gap up to 480 mV was found in the high pH region, which means the alkaline environment (pH >7.5) is desirable for selective oxygen generation in seawater electrolysis. In practical operation, it usually requires large current densities (500 or 1000 mA·cm-2at industrial level)within the overpotentials of 480 mV without hypochlorite generation. The drawbacks of the high pH lie in the formation of insoluble precipitates (e.g., Mg(OH)2, Ca(OH)2) that contribute to poisoning of catalytic sites in natural seawater system18.Although the application of diaphragm on electrolyzers with independent electrolyte feeds is a possible strategy for this dilemma, the cost would increase accordingly and the reaction kinetics could be impaired by the membrane17,19. To alleviate those tricky issues, the design and fabrication of materials with superior activity and selectivity should be considered to maximize the operational potential difference of those two reactions.
Compared to the CER, OER is more sluggish process involving four-electron transfer. The well-accepted OER mechanism20is described in Eqs. 6-9,

And a possible Volmer-Heyrovsky mechanism of CER on RuO2surfaces was proposed in 2016 by Exner et al. (Eqs. 10,11), where only two electrons and a single intermediate participate in the reaction21.
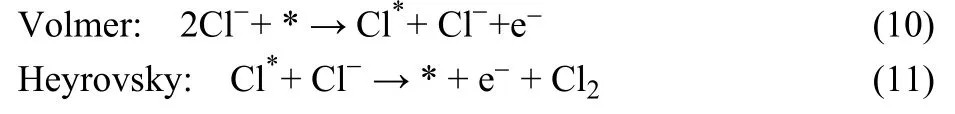
Moreover, the disproportionation reaction of Cl2could take place in water solution, generating hypochlorous acid (pH <7.46) or/and hypochlorite ions (pH > 7.46)22-26. Some highly selective materials have been investigated and the catalytic mechanism has been unraveled.
2.2 Materials with high OER selectivity
The competition between OER and CER exists not only in the seawater electrolysis but also the Chlor-alkali process27.Different from seawater electrolysis, CER is the preferred reaction rather than the OER. The precious metal (such as Ru and Ir) oxides are known to be good catalyst for the CER28-30as well as for the OER31-34catalysts. Although potential of the two reactions overlaps at a certain region, the reaction path in the catalytic sites differs depending on the materials35,36. For example, Zn-doped RuO2features with high selectivity toward OER due to the tailored structure of the active sites37. Lead ruthenate pyrochlore (Pb2Ru2O7-x) with abundant oxygen vacancies was synthesized and employed in neutral/alkaline seawater system, which displays superior OER activity and high selectivity (~99%) at pH = 13 than that in neutral solution17. The better performance compared to RuO2was attributed to the rich high-valence Ru(V) and the oxygen vacancies that exist in Pb2Ru2O7-xnanostructure. Doping has proved to be an effective strategy for improving selectivity of noble metal oxides to the OER.
Those well-studied OER catalysts, Ni/Co/Fe-based composites, can be regarded as potential alternatives for seawater electrolysis owing to their outstanding activity38-41.Early in 2016, Strasser and his co-workers15proposed the criterion for operating conditions of seawater electrocatalysis.NiFe layered double hydroxide, for the first time, achieves nearly 100 % oxygen selectivity with an overpotential < 480 mV in the seawater-mimicking electrolyte (pH = 13), as shown in Fig. 3a, b. This study offers powerful guidance from experimental and theoretical aspects for the following research in this field13,42,43. In 2018, Hsu et al.44found that CER is more favorable on noble-metal-based materials in comparison to OER, but transition metal hexacyanometallates are promisingly electrocatalysts for OER with high selectivity based on density functional theory (DFT) calculations (Fig. 3c-e). The core-shell nanoarchitecture consists of catalytic Co2[Fe(CN)6] and conductive cobalt carbonate, which facilitates the fast charge transport during the OER. A solar cell was employed to drive seawater splitting at neutral pH solution, delivering near 100%Faradaic efficiency and extraordinary efficiency of 17.9% for solar-hydrogen transformation. More and more practicable candidates are explored for highly efficient and selective OER.Bimetallic oxy-boride (Co-Fe-O-B) was composed of Co3O4core and Co2B-shell, with a 100% selectivity to the OER45.However, the Faradaic efficiency is only ~74% ± 4% at high current densities of 100 mA·cm-2. The situation tends to even worse in neutral solution owing to the competitive CER. The excellent selectivity to OER of those transition metal-based catalysts can be attributed to the favored thermodynamics and facilitated kinetics at the reaction sites46.
Building a blocking layer outside the active catalysts can prevent the Cl-ions from catalytic sites on the surface. MnOxdeposited IrOxwas found to exhibit a strong tendency (nearly 100% selectivity) to the OER in the acidic saline water47. MnOxbased upper layer effectively blocked the transport of Cl-but allowed the permeation of H2O and O2, facilitating the OER on under catalytic layer48,49. It is feasible to extend the blocking effect to other catalyst systems.
3 Anti-corrosive materials in seawater electrolysis
3.1 Mechanism of the chlorine-involved corrosion process
Chlorination is a complex and challenging process in the seawater electrolysis50. The competitive CER may be suppressed significantly or even eliminated by the rational design of materials and the control of electrolyte and applied potential. Nevertheless, the corrosion on the electrodes or catalysts is inevitable due to the ubiquitous and aggressive Clin the seawater (Fig. 4a), which this situation is particularly serious for materials with poor corrosion resistance. It is widely accepted that the passivation layer forms on the surface under Cl-involved oxidizing conditions51. Because of strong adsorption and fierce penetration of Cl-, the passivating part tends to degrade into soluble chlorides51,52, and continuous dissolution or even disappearance of the metal body could take place eventually (Fig. 4b).

Fig. 4 (a) Corrosion on the metal-based electrode before and after passive layer formation;(b) destroy of passive layer and underlying metal by fierce corrosion 51.
The chlorine corrosion mainly consists of two typical steps(Eqs. (12) and (13), M: metal substrate)53,

Many factors can affect the corrosion rate, such as Clconcentration, pH of electrolyte, operational temperature,applied potential, and type of passivation layer. Fabricating materials with high corrosion resistance is a prime and critical task in seawater electrolysis. Low-cost and active materials are widely used in the electrolysis systems54-57, but most of them cannot withstand in highly corrosive seawater for a long run. We have summarized some fundamental strategies for the design of anti-corrosive materials used in seawater electrolysis, which have hardly been reviewed before.
3.2 Design of anti-corrosive materials
To fabricate the highly anti-corrosive materials, the following aspects should be taken into consideration: 1) materials with inherently high resistance to corrosion (such as metal Ti, carbonbased substrate), they can be well applied in the electrolysis of seawater by combination with high-performance catalysts; 2)materials with a protective layer, the coating can protect internal parts from the Cl-corrosion. selectively to avoid contact with.3) in situ generated anti-corrosive species, they are active species for specific reactions sometimes.
3.2.1 Inherent anti-corrosive materials
The high potential makes anode more vulnerable to corrosion in the high saline environment. Transition metals (like Ti and Ru) show good resistance to corrosion in the seawater, enabling them to maintain a robust structure during long cycling58,59. An alloyed Pt-Ru-M (M = Cr, Fe, Co, Ni, Mo) on Ti mesh was reported to show significantly enhanced catalytic activity for HER and desirable stability over 172 h in comparison to Pt or Pt-Ru electrodes (Fig. 5a, b)60. Alloying the guest M with Pt-Ru species not only boosts the catalytic activity but also enhances the anti-corrosive capability. Good stability derives from the thermodynamic advantage of the guest M species over Pt-based counterparts in competitive dissolution reactions. Moreover,RuCoMoxdeposited Ti foil electrode was found to own good stability during continuous operation over 12 h61. Despite the unsatisfactory activity presented in those works, the concept of alloying non-noble metals with noble metals provides a good way to design stable alloy electrodes at low cost.
Three-dimensional carbon-based substrates are suitable choices as electrodes owing to their high conductivity and good resistance to chlorine ions that guarantees the overall system efficiency. Kim et al.62constructed carbon-coated sodium cobalt-iron pyrophosphate (Na2Co1-xFexP2O7/C, 0 ≤ x ≤ 1)nanoparticles on the carbon cloth as the anode for alkaline seawater electrolysis (Fig. 5c). As-prepared sample exhibited optimized activity by controlling Co/Fe ratio and maintained good stability without Cl-corrosion or chlorine oxidation (Fig.5d, e). A newly-emerged fancy 2D transition metal carbides/nitrides, MXene, present great potential in resisting corrosion. Qiu et al.18reported a multilevel hollow MXene tailored low-Pt catalyst synthesized by ultrafast aerosol drying method (Fig. 5f). The novel structure endows sufficient active sites and robust frameworks with high conductivity,synergistically improving the performance of the electrolysis(Fig. 5g). It presented a long lifetime of 250 h with nearly 100%Faradaic efficiency (Fig. 5h), which was much higher than that of commercial Pt/C (8 h) and other recorded electrocatalysts(10-100 h). Considering the scarcity of Pt and the feasibility of MXene-based catalyst in the seawater system, Qiu group substituted precious metals with low-cost CoxMo2-xC@NG composites integrated with MXene63. The catalyst delivered superior HER performance to Pt/C for HER in natural seawater,and their remarkable durability (225 h) was 64 times better than that of Pt/C with 98% Faradaic efficiency. The inferior stability was observed in the MXene-free counterpart for only several hours, highlighting the important role of MXene in enhancing the robustness of the hybrid catalysts.
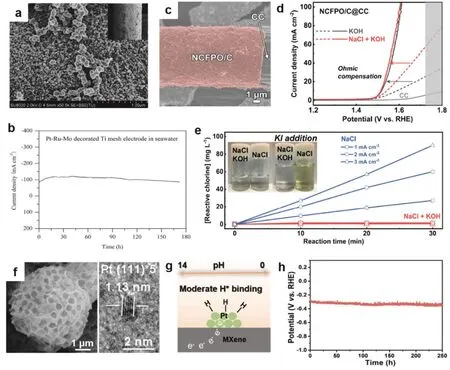
Fig. 5 The materials with inherently high resistance to seawater corrosion. (a) SEM images of Pt-Ru-Mo-decorated Ti mesh and (b) corresponding long-term stability for HER in seawater 60; (c) FESEM images of NCFPO/C@CC; (d) polarization curves of NCFPO/C@CC at a scan rate of 5 mV·s-1 (the gray area denotes the generation of hypochlorite ions); (e) the time-dependent reactive chlorine concentration profiles of NCFPO/C in the NaCl and NaCl + KOH electrolyte 62; (f) SEM and HRTEM images of Pt nanocrystallites on mh-3D MXene; (g) illustration of multilevel hollow MXene tailored low-Pt catalyst with multifunctional catalytic interface and its benefit in promoting the HER under multi-pH conditions; (h) chronopotentiometric response of 2.4% Pt@mh-3D MXene at a current density of 10 mA·cm-2 18.
3.2.2 Active catalysts coated with an extrinsically protective layer
Surface coating is a commonly-used and effective method to protect materials from corrosion64. Ir-based materials are highperformance catalysts for OER and CER especially in acidic electrolyte65-68. MnOxis known as a low-cost OER catalyst but not an excellent one69. Interestingly, MnOx/IrOxcomposite was reported to have a high selectivity toward OER but CER was significantly suppressed48. Systematic study elucidated that the MnOxfilm functions as a permeable layer that hinders the transport of Cl-but allows H2O, H+, and O2onto IrOx(Fig. 6a,b). Selective transport of ions a promising approach to protect both cathode and anode from Cl-fierce corrosion70. Another promising alternative for the protective layer is carbon-based materials, such as carbon nanotube71,72, graphene and their derivatives. Their attractive merits in conductivity, chemical stability, mechanical strength, hydrophilic nature make them popular in electrocatalysis73. Graphene coating can effectively prevent the corrosive species from the underlying electrode owing to their outstanding blocking effect. CoMoP coated by a few-layer N-doped carbon shell is a superior catalyst for HER in seawater splitting74. CoMoP@C exhibited better activity than commercial Pt/C among a wide pH range with high Faradaic efficiency of 92.5% in real seawater. The stability was proved to be remarkably enhanced by the carbon coating in comparison to carbon-free counterpart (Fig. 6e, f). The carbon shell not only protects CoMoP from corrosion but also optimizes the absorption behavior of intermediates during the electrolysis.Chauhan et al.75made a summary on plenty of graphene coatings, for instance, single-/multi-layered graphene coatings were employed to protect the vulnerable matrix. This versatile strategy can be easy to scale up in the industrial process since we already have mature graphene synthesis technology. In addition,graphene coating has been used in acidic media76-78, which means the materials can be used in seawater systems within a large pH range.
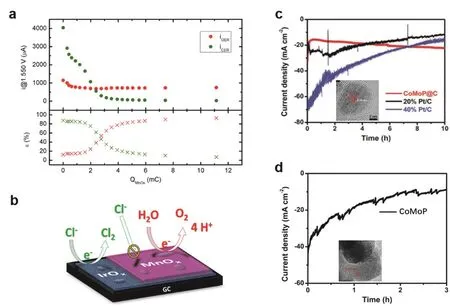
Fig. 6 The anti-corrosive materials with extrinsically protective layer. (a) Top panel: currents for OER (red) and CER (green) at E = 1.55 V, lower panel: corresponding selectivities toward OER (red) and CER (green); (b) illustration of the protecting mechanism of MnOx 48. (c) Time-dependent current density curve of CoMoP@C, 20% and 40% Pt/C in seawater; (d) time-dependent current density curve of CoMoP in seawater 74.
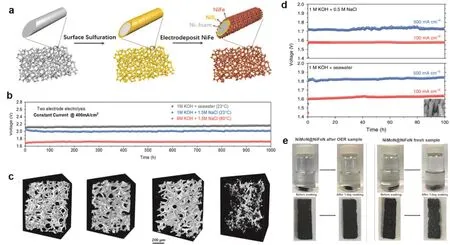
Fig. 7 The anti-corrosive materials with in situ generated anti-corrosive layer. (a) Fabrication and structure of the dual-layer NiFe/NiSx-Ni foam anode for seawater splitting; (b) durability tests (1000 h) recorded at a constant current of 400 mA·cm-2 of the seawater-splitting electrolyzer;(c) 3D X-ray micro tomography of anode: before seawater splitting and no activation, after 1000 h stability test in 1 mol·L-1 KOH + real seawater(activation carried out in 1 mol·L-1 KOH and 1 mol·L-1 KOH + 0.5 mol·L-1 NaCl), after 300 h stability test in 1 mol·L-1 KOH + 2 mol·L-1 NaCl(activation carried out in 1 mol·L-1 KOH and 1 mol·L-1 KOH + 2 mol·L-1 NaCl), Ni foam with electrodeposited NiFe (activated in 1 mol·L-1 KOH)after 8 h of stability test in 1 mol·L-1 KOH + 2 mol·L-1 NaCl 53; (d) durability tests of the electrolyzer at constant current densities of 100 and 500 mA·cm-2 in different electrolytes at 25 °C; (e) corrosion resistance mechanism 12.
3.2.3 In situ generated resistive species
Noting that the exotic protective layer may increase mass transfer resistance of the catalytic reaction when exerting blocking effect. In contrast, the in situ generated species on the electrodes will be more desirable. Surface oxidation79,80and reconstruction81,82are often accompanied by the formation of new species or structures especially at the anode where positive bias was applied. Dai et al.54grown NixFey(OH)z/NiSxmultilayer on conductive Ni foam to serve as the anode in solardriven alkaline seawater electrolysis (Fig. 7a). This system can sustain over 1000 h with negligible degradation in continuous operation (Fig. 7b), reaching the industrial requirement for current densities (0.4 to 1 A·cm-2). The active NiFe electrocatalyst layer delivers a high selectivity of ~100% toward OER in seawater. Compared to the anode without NiSx,NixFey(OH)z/NiSxexhibited excellent robustness of the whole skeleton in after the long-term electrolysis (Fig. 7c). Such superior corrosion resistance was attributed to the chloride repelling of in situ generated oxidation products (SO24-and) in passivating layers. Transition metal nitrides are also good candidates for seawater splitting owing to their high corrosion resistance and conductivity83,84. Nitrogen-rich Mo5N6nanosheets were reported as the HER catalyst for seawater electrolysis83. The origin of the high stability of Mo5N6in seawater was resulted from higher nitrogen content in metal nitride. Ren et al.12synthesized a core-shell structured NiFeN/NiMoN catalyst supported on Ni skeleton, which afforded an excellent activity and durability toward OER in the alkaline seawater electrolysis (Fig. 7d). It only required 1.709 V to achieve current densities of 1000 mA·cm-2at 60 °C with a Faradaic efficiency of around 97.8%. NiFe oxy(hydroxide)amorphous layers in situ evolved from anode surface during the OER (Fig. 7e), which not only take the responsibility for desirable OER performance but benefit the superior anticorrosion feature. However, the anti-corrosive mechanism in NiFeN/NiMoN system needs in-depth study. Similarly, Ni5P4nanosheets hybridized with amorphous nickel hydr(oxy)oxide[Ni2+δOδ(OH)2-δ] was proved to be a good HER catalyst for seawater splitting. A 3-nm amorphous Ni2+δOδ(OH)2-δlayer not only facilitates good electronic interaction between P-H but promotes the stability in the electrolysis85. As a result,hydr(oxy)oxide indeed has a protective effect for electrode materials to some extent. Beyond that, Xie et al.86found N/S co-doped carbon nanosheets modified rhodium (Rh)nanoparticles is a high-performance HER catalyst in seawater.Strong interaction between metal and carbon provides a safe circumstance of metal to the resistance of corrosion. The above studies offer different perspectives on the rational selection and design of advanced materials with good activity, stability and corrosion resistance.
In addition, optimization of the external conditions, such as temperature, pH, and concentration of Cl-, could also alleviate both chlorine corrosion and competitive reactions simultaneously. Sun and co-workers87employed NiCoFe phosphide as a bifunctional electrode in a simulative seawaterelectrolysis system. This electrode afforded a current density of 500 mA·cm-2for over 100 h, the Faradaic efficiency of which was approximately 100%. Such good performance mainly derived from the common-ion effect that halved the concentration of Cl-during the continuous electrolysis in real seawater. Strasser and his co-workers19,42made a great effort in the design of highly efficient seawater electrolyzers. They directly fed neutral seawater at the cathode but circulated pure KOH electrolyte at anode, achieving better cell performance than that of symmetric alkaline seawater electrolytes. The Faradaic efficiency and durability of NiFe-LDH for OER remarkably outperformed commercial IrOx-based catalysts.Although the catalytic mechanism of CER suppression in NiFe-LDH system remains unclear, the proposed concept of the asymmetric electrolyzer has great potential for long-term splitting of the seawater with high current densities.
4 Conclusion and outlook
Seawater electrolysis is a promising method for large-scale hydrogen production. However, the chlorine corrosion and the chlorine oxidation caused by Cl-in the seawater seriously hinder the development of this technology. Therefore, researchers have carried out a lot of studies on the materials modification and structure optimization of electrodes and catalysts. In this account, we summarized the advanced materials used in seawater splitting with high selectivity to OER and introduced three effective strategies for the fabrication of anti-corrosive structure. It can be confirmed that materials with excellent performance can be obtained through reasonable material selection and exquisite structural design. Despite the achievements, there remains some important tasks and opportunities for the future research. a) Screening of selective and anti-corrosive materials. Previous studies provide us a lot of data for materials screening to find potential candidates by artificial intelligence (AI) technology-enabled simulations or theoretical calculation. b) Mechanism of competitive reactions and corrosion. Advanced operando techniques are needed to probe the real reaction situation on the active sites. Besides, the effect of impurities on the reaction is unclear. c) Evaluation of anti-corrosive materials. Conventional electrochemical/physical techniques are well-developed for the investigation of the corrosion process, which is applicable to the sweater electrolysis. The systematic and accurate measurements of corrosion process could provide more clues for the design of anti-corrosive materials. d) Industrial-scale electrolysis. Limited by selectivity and corrosion, seawater electrolysis at a high current density remains a great challenge in the long-term operation. e) Optimization of the experimental conditions. The impact of the local microenvironment (e.g., local cations, pH,magnetic fields) on the catalytic process may offer new insights into optimized catalytic performance. f) Novel integrated electrolyzer devices. Good electrolyzer design is likely to improve the overall reaction efficiency. In a word, the research on this field is not well-investigated up to now, deep exploration of rationally-designed materials is particularly important for chlorine-involved electrolysis.
杂志排行
物理化学学报的其它文章
- Strategies to Improve the Energy Density of Non-Aqueous Organic Redox Flow Batteries
- 一体化电极电催化二氧化碳还原研究进展
- 锂离子电池氧化亚硅负极结构优化和界面改性研究进展
- 锂离子电池隔膜的功能化改性及表征技术
- 三维大孔/介孔碳-碳化钛复合材料用于无枝晶锂金属负极
- NH2-MIL-125 (Ti) Derived Flower-Like Fine TiO2 Nanoparticles Implanted in N-doped Porous Carbon as an Anode with High Activity and Long Cycle Life for Lithium-Ion Batteries
