热带农业废弃物源生物炭中多环芳烃的分布特征及其生态风险评估
2022-08-06谭华东张晓瑛武春媛赵淑巧
谭华东,张晓瑛,武春媛*,赵淑巧
热带农业废弃物源生物炭中多环芳烃的分布特征及其生态风险评估
谭华东1,张晓瑛2,武春媛1*,赵淑巧1
1. 中国热带农业科学院环境与植物保护研究所,海南海口 571101;2. 中国热带农业科学院试验场,海南儋州 571737
针对热带农业废弃物资源化过程中生物炭安全利用数据不足,以热带农业废弃物[香蕉茎(banana stem, BS)、菠萝叶(pineapple leaves, PL)、荔枝杆(litchi stem, LS)、水稻秆(rice stalk, RS)、椰子壳(coconut shell, COS)、蘑菇渣(mushroom residue, MR)和木薯茎(cassava stem, CAS)]为原料于不同温度(300、500、700℃)下制备生物炭,利用超声辅助提取-QuEChERS-GC-MS测定其中的16种优控多环芳烃(polycyclic aromatic hydrocarbons, PAHs)分布特征,结合原料/生物炭理化因子与制备温度解析PAHs残留影响因素,基于苯丙a芘毒性当量浓度(toxic equivalent concentration of benzo[a]pyrene, TEQ-BaP)和风险熵值法评价生物炭中PAHs的生态风险。结果表明,∑16PAHs处于1217.6~9547.1 μg/g,检出率与浓度最高均为萘(naphthalene, NAP)与菲(PHE),2~4环PAHs是生物炭中主要的PAHs类型;生物炭中∑16PAHs呈现BS>PL>LS>RS>COS>MR>CAS,不同生物炭中PAHs种类与含量不同,与来源及制备温度相关。冗余分析(RDA)结果显示,生物炭中PAHs含量与生物炭的电导率(electric conductivity, EC)、碳氧比(carbon oxygen ratio, C/O)呈正相关,而与碳氢比(hydrocarbon ration, C/H)呈负相关(<0.05),且与温度密切相关。从毒性当量浓度和风险商(risk quotient, RQ)看,生物炭中PAHs的毒性来源于低环PAHs,且致癌毒性效应较小;生物炭中NAP浓度存在生态风险,其他单体PAH的RQ<0.1,无生态风险,但值得注意的是总PAHs的RQ>1,需加以关注。该结果可为今后热带土壤中生物炭安全利用提供科学依据。
农业废弃物;生物炭;多环芳烃;污染特征;风险评估
生物炭是一类经生物质在限氧条件生成的多孔含碳物质[1]。因其具有优异的多孔结构、营养物质缓释和pH缓冲能力,常做土壤改良剂[2-3]。多环芳烃(polycyclic aromatic hydrocarbons, PAHs)是一类典型的持久性有机物污染物,具有“三致效应”和内分泌干扰效应,威胁人体健康[4-5]。生物炭制备过程中不可避免地伴随着PAHs的产生,使得生物炭源输入成为了土壤等产地环境中PAHs重要来源。李阳等[6]已报道玉米秸秆源生物炭暴露小麦种子与幼苗,可分别观察到发芽抑制和氧化应激反应,甚至高浓度暴露下幼苗出现生理损伤,呈现显著的植物毒性效应。因此,制备的生物炭用于改良剂等用途,需对其中PAHs的含量、构成及生态风险进行评估。
近年来,国内外学者对生物炭中PAHs的浓度水平、分布、来源及生态风险等做了研究工作。已有研究表明,生物炭中PAHs含量差异显著,低于0.1 μg/g及超过10 000 μg/g均有报道[7-8]。这造成了研究者极大困扰,一些研究者认为生物炭中PAHs含量过低不会对土壤等造成生态风险[9-10]。如SINGH等[11]报道木头、树叶、造纸厂污泥、家禽垃圾和牛粪来源的(温度400、550℃制备的)生物炭PAHs低于健康水平(小于0.5 μg/g)。然而,不少研究也报道了生物炭中的PAHs会造成土壤系统中的微生物群落改变、动植物出现氧化胁迫反应,甚至出现生殖与发育毒性,因此他们认为生物炭中PAHs污染不可忽略[6, 12-13]。
随着热带农业废弃物资源利用多样化如制备生物炭调控土壤质量[2-3, 14-15],生物炭来源PAHs直接污染土壤等产地环境面临风险,有关热区农业废弃物源生物炭中PAHs残留及生态风险鲜见报道。因此,本研究以我国热区常见农业废弃物为原料的制备生物炭为研究对象,对其中16种优控PAHs质量浓度、组成、污染水平和关键影响因素进行研究,并对其潜在的生态风险进行评价,以期为热带生物炭安全利用提供科学依据。
1 材料与方法
1.1 材料
1.1.1 原料采用热带代表性的农业废弃物—香蕉茎(banana stem, BS)、菠萝叶(pineapple leaves, PL)、荔枝杆(litchi stem, LS)、水稻秆(rice stalk, RS)、椰子壳(coconut shell, COS)、蘑菇渣(mushroom residue, MR)和木薯茎(cassava stem, CAS)制备生物炭。通过热解(300℃、500℃和700℃)产生生物炭,原料基本性质如表1所示(C、O、H、N和S分别表示生物炭制备原料中碳、氧、氢、氮、硫元素含量,P、K、Ca、和Mg分别表示生物炭制备原料中磷、钾、钙、镁元素含量,C/O、C/N为生物炭制备原料中碳氧比与碳氮比)。
1.1.2 试剂与仪器 PAHs标准物质均购自J&K Scientific公司(美国);HPLC级甲醇、乙腈和丙酮,购自Thermo Fisher试剂公司(美国);固相分散剂十八烷基硅烷(octadecylsilane, C18)、N-丙基乙二胺(primary secondary amine, PSA),购自Biocomma生物技术有限公司(深圳,中国)。准确称取PAH单体标准品0.1000 g,用丙酮溶解并定容至100 mL,于4℃冰箱中避光保存。采用正己烷溶液将储备溶液稀释获得0.5~500.0 μg/L单标与混标溶液,待用。TRACE GC 1310 ISQ GC-MS(Thermo Scientific,美国),配有ISQ QD300单级杆MS检测器及TraceGOLD TG-5MS毛细管柱(30 m×0.25 mm×0.25 μm);JL-721DTH型数控超声波清洗器(南京,中国);Centrifuge 5417R台式冷冻离心机(Eppendorf有限公司,德国);XH-B涡旋振荡器(上海汗诺仪器有限公司,中国);OA-sys氮吹仪(Organomation公司,美国)。

表1 生物炭制备原料理化性质
1.2 方法
1.2.1 生物炭理化性质 (1)生物炭制备。自然条件下风干原料,切碎、研磨过60目筛。准确称取10 g原料,在120℃气氛炉中分别升温至300、500、700℃下真空限氧条件(0.07 MPa)裂解1 h制备生物炭,待到冷却后,取出后装袋、编号,待用。
(2)生物炭表征。采用元素分析仪(EA2400-II,PerkinElmer)测定生物炭中C、O、H、N和S含量;P、K、Ca、Mg参考鲍士旦[16]的方法测定;参考GB/T12496.7—1999方法测定pH;参考LY/T 1616—2004测定电导率(electric conductivity, EC);参考HJ889—2017方法测定阳离子交换量(cation exchange capacity, CEC);以生物炭与原料重量比值计算产率;BET比表面积(SSA)采用ZHANG等[17]的方法测定。
1.2.2 GC-MS分析 生物炭中16种PAHs的GC-MS分析参数参考谭华东等[18-19]的方法并有所调整,即超声辅助提取-QuEChERS-GC-MS方法测定生物炭中的PAHs。准确称取样品0.200 g(精确到0.001 g),置于15 mL塑料离心管内,加入10 mL二氯甲烷与正己烷(1∶1,/)混合溶液且超声辅助提取30.0 min,之后以4000 r/min离心10 min,分离所有提取液。重复提取1次,合并2次提取溶液,减压方式旋转蒸发浓缩至5 mL,加入100 mg PSA、100 mg C18净化剂摇匀、涡旋混合1 min,其后以4000 r/min离心10 min,转换溶剂用正己烷定容至1 mL,过0.22 μm有机滤膜,待分析。每分析10个样品含空白、加标和加标平行样品,每个样品重复3次。NAP平均回收率为65.1%~68.1%,其他PAHs为76.4%~110.1%,平行样品标准偏差≤15%,检出限0.5~1.2 ng/g。
1.2.3 生态风险评估 (1)毒性当量法。基于WANG等[20]提出毒性当量因子法(equivalency factor, TEF),以苯并(a)芘(benzo[a]pyrene, BaP)为基准毒性物质,其他PAHs与BaP比值计算单体PAH毒性当量浓度(BEQi,ng/g)及总PAHs毒性当量浓度(toxicity equivalence, TEQ,ng/g),计算公式如下:
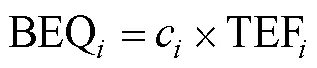

(2)风险熵值法。基于CAO等[21]的关于风险熵值(risk quotient, RQ)法进行生物炭中PAHs生态风险评估。因生物炭施加进入土壤,采用10%生物炭施加量条件下土壤中PAHs浓度评估,RQ以下列公式计算:


1.3 数据处理
采用Canoco 5软件进行冗余分析(RDA)评价原料/生物炭理化因子、温度与生物炭中PAHs的关系。
2 结果与分析
2.1 生物炭理化性质
生物炭热解制备过程中,原料基本理化组成前后发生了显著变化。如表2所示,制备生物炭C、O、H、N和S元素平均百分比含量分别为57.8%、37.5%、3.0%、1.12%和0.56%,P、K、Ca和Mg分别为4.5、54.6、30.0和4.9 mg/g,pH值为7.75~10.18,碳氧比(carbon oxygen ratio,C/O)和碳氢比(hydrocarbon ratio, C/H)比分别为1.86和22.8。其中,生物炭中C占比最大,显示C是生物炭中最丰富元素,主要来源于原料中纤维素、半纤维素、木质素和脂肪族碳热解[24]。对比表1,热解后,生物炭中C元素平均百分比含量增加,而H、N、O和S元素含量下降。外观上,随着热解温度从300℃升高700℃,生物炭颜色从棕色变为深黑色(图1)。随着温度升高,生物炭产率从55%下降到35.1%,且O百分比含量下降,H百分比含量从6%降低1%;pH值、C/H和C/N等物质随着温度升高而增加,且C/O随着温度的升高显著降低,温度升高氧化程度加深。
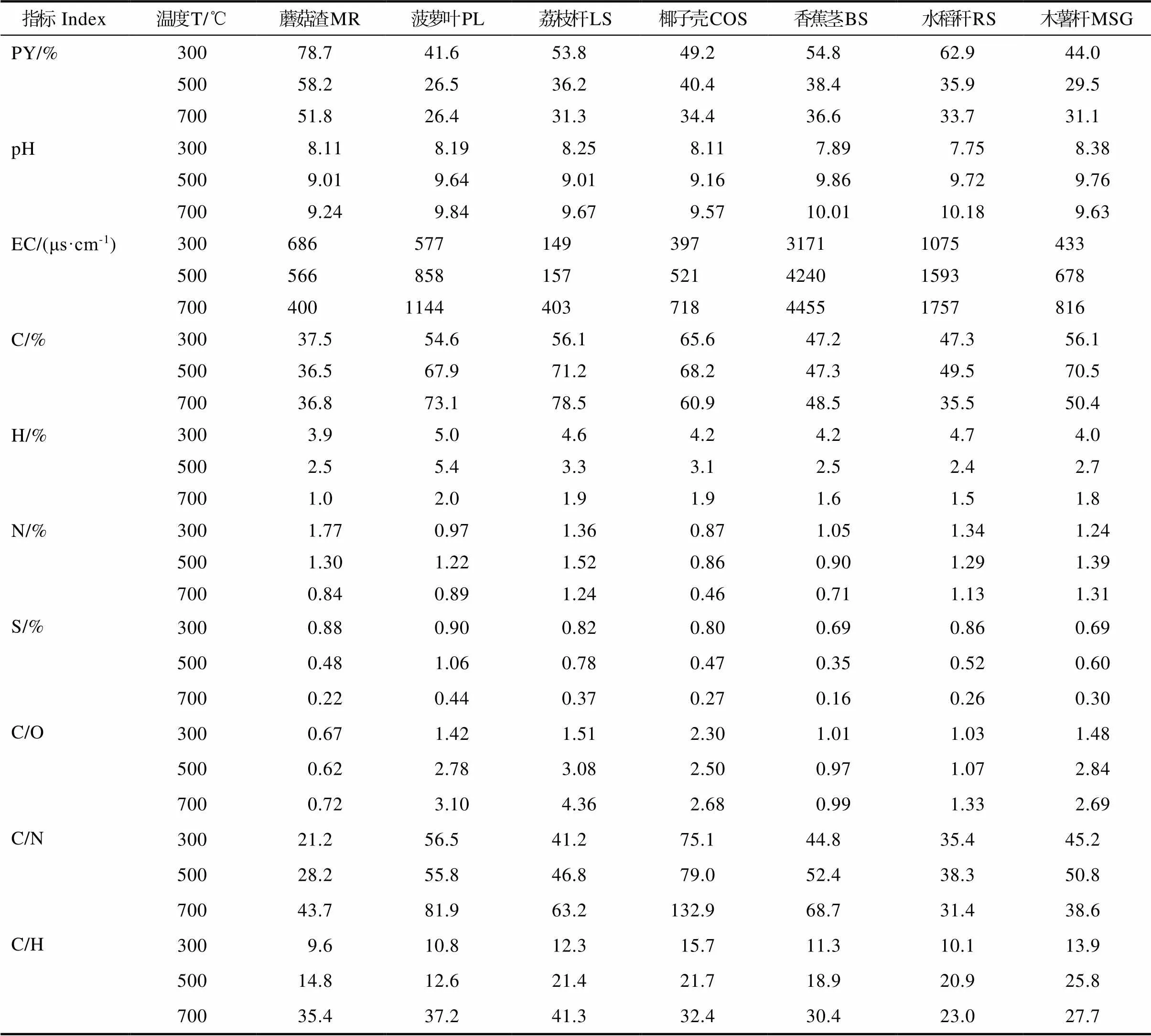
表2 生物炭的理化性质
续表2 生物炭的理化性质
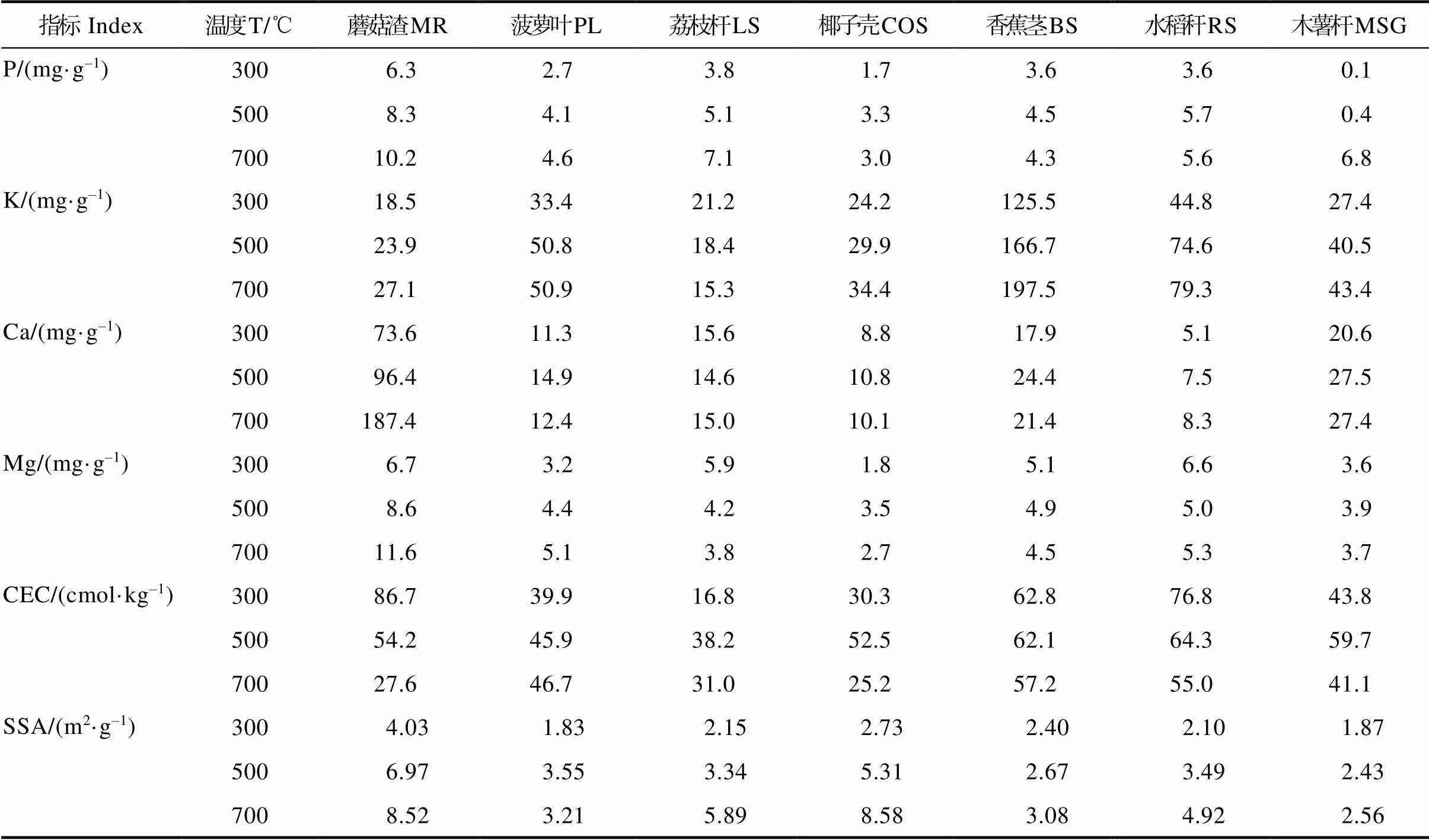
Tab. 2 Physicochemical properties of prepared biochar(continued)
注:PY表示生物炭产率;EC表示电导率;CEC为阳离子交换量;SSA表示BET比表面积。
Note: PY represents the yields of biochar; EC is the conductivity of biochar; CEC represents cation exchange capacity of biochar; SSA is BET specific surface area of biochar.

COS300、COS500、COS700分别表示在300、500、700℃条件下制备的椰子壳源生物炭;LS300、LS500、LS700分别表示在300、500、700℃条件下制备的荔枝杆源生物炭。
2.2 生物炭中PAHs残留特征
制备生物炭中PAHs含量如表3所示。16种PAHs总浓度(∑16PAHs)为1217.6~9547.1 ng/g,平均值为(3466.3±1827.5) ng/g,且随来源不同而不同。这些生物炭中总PAHs浓度处在已报道生物炭浓度范围内[10]。非木本植物源(RS、PL)生物炭中PAHs含量较其他源生物炭(COS、CAS)高15%以上,可能由于这些原料含纤维素、半纤维素较木本原料高,这使得其产生更多PAHs[10, 24]。所制备生物炭中PAHs占主导地位单体PAH是萘(naphthalene, NAP),对总PAHs贡献为55.7%~ 78.6%,其次是菲(phenanthrene, PHE)、芴(fluorene, FLU)和蒽(anthracene, ANT),分别占8.2%、8.1%和5.5%。来源上,生物炭中PAHs总量(∑16PAHs)呈现BS>PL>LS>RS>COS>MR> CAS(表3)。

表3 生物炭中PAHs单体的残留浓度
注:ND表示生物炭中PAHs未检出。
Note: ND represents the PAHs were not detected in biochar.
根据芳香环数对生物炭中PAHs分类。不同环数贡献为2环PAHs(68.0%)>3环PAHs(29.3%)>4环PAHs(2.4%)>5环PAHs(0.3%)。低环(2~3环)PAHs(L-PAHs)总含量(∑L-PAHs)为1198.7~8781.6 ng/g,平均浓度为(3372.8± 1692.6) ng/g,占∑16PAHs含量97.3%。高环(5~6环)PAHs(H-PAHs)总含量(∑H-PAHs)为ND~159.9 ng/g,平均值为(11.2±35.9) ng/g,占∑16PAHs含量的0.3%。其中强致癌性PAH(BaP)含量为ND~159.9 ng/g,平均含量为7.6 ng/g,检出率4.8%,占∑PAHs含量的0.2%。制备生物炭L-PAHs以2环NAP为主,占∑16PAHs含量的68.0%;H-PAHs以5环BaP和DBA为主,占∑16PAHs含量的0.3%,其他H-PAHs未检出。
制备的生物炭中总体PAHs含量呈现500℃> 300℃>700℃(<0.01),这与已报道结果相一致[10],值得关注的是,仅COS300高于COS500和COS700(<0.05);7种不同来源材料在3个温度下制备的生物炭中,PAHs几乎以2~3环PAHs和4环FIR、PYR和CHR为主,但PL、BS、COS在300℃与500℃条件下产生5环的PYR,且LS500产生了强致癌物BaP。值得注意的是,在500℃与700℃条件下BS均有5环DBA产生,而所有的生物炭中均未见产生6环PAH单体。不同来源生物炭的PAHs构成差异显著(<0.05,图2)。
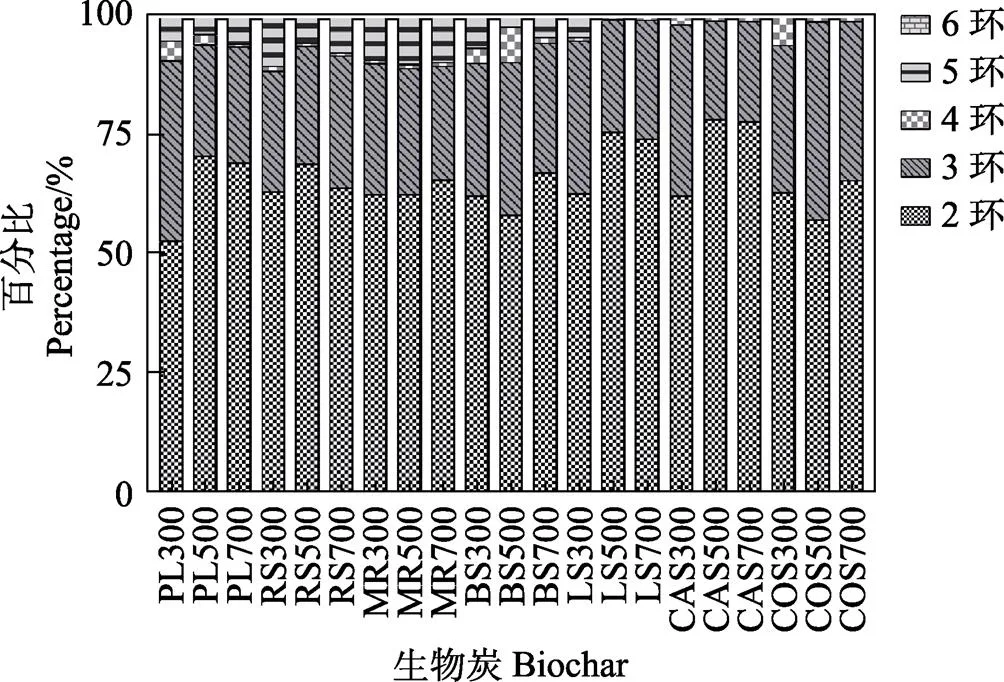
图2 生物炭中PAHs的构成
2.3 冗余分析
生物炭中PAHs生成、残留与原料/本身理化性质、制备温度密切相关[8, 25],本研究通过冗余分析评价它们之间的相互关系。图3所示,原料/生物炭理化性质、制备温度对生物炭中PAHs浓度变化在第一和第二轴总解释量为93.09%。中(4环)、低环PAH单体含量与制备温度呈负相关,而高环BaP、二苯并(a,h)蒽(dibenz[a,h]anthracene, DBA)与温度呈显著正相关;PAHs与生物炭的C/O、EC成正相关,而与C/H呈负相关;生物炭中的低环PAHs与原料的C/H、C/O呈负相关;低环PAH单体之间显著正相关,且与总PAHs呈正相关;生物炭中的低环PAHs与总PAHs高度正相关,显示这2~3环PAHs为主要的PAHs种类。
2.4 生态风险评估
为了量化生物炭中PAHs毒性,采用TEF风险评价法[26]评价其生态风险。表4的结果显示,制备的生物炭中16种PAHs的TEQ-BaP范围为2.24~165.2 ng/g,平均值为22.3 ng/g;7种致癌PAH单体[并(a)蒽(BaA)、Chr、BbF、BkF、BaP、IPY和DBA]的TEQ-BaP为0~159.9 ng/g,平均值为1.65 ng/g,其中Chr、BaP、DBA占总TEQ-BaP比例20%,而其余4种非致癌PAHs(NAP、PHE、FLU和ANT)的TEQ-BaP占比高达74.9%,表明生物炭中PAHs毒性风险主要源于4种非致癌PAH和Chr、BaP、DBA单体。毒性风险呈现BS>LS>PL>MR>RS>COS>CAS,且除了BS(58.80 ng/g)与LS(34.0 ng/g)外,生物炭均呈现较低TEQs值。

实心三角形箭头表示生物炭制备前后的理化性质,空心三角形箭头表示不同的PAH单体。图中含有Y-前缀表示生物炭制备之前生物质的理化性质,无前缀表示制备的生物炭的理化性质。缩写对应含义同表1。
根据RQ值大小可将生态风险分为3类,即RQ>1为高风险,1≥RQ>0.1为中等风险,RQ≤0.1为低风险。PAH单体和∑16PAHs生态风险等级如表5所示,NAP单体在所制备的生物炭中RQ值处于1.25~5.23,均大于1。FLU、PHE和ANT在PL、BS、LS均处于0.1~1。除了FLU、PHE、ANT外,其他PAH单体在生物炭呈现<0.1。总PAHs的RQ值处于1.25~2.27,呈现BS> PL> LS> RS> COS> MR> CAS。

表4 生物炭中PAHs毒性当量浓度(TEQ)
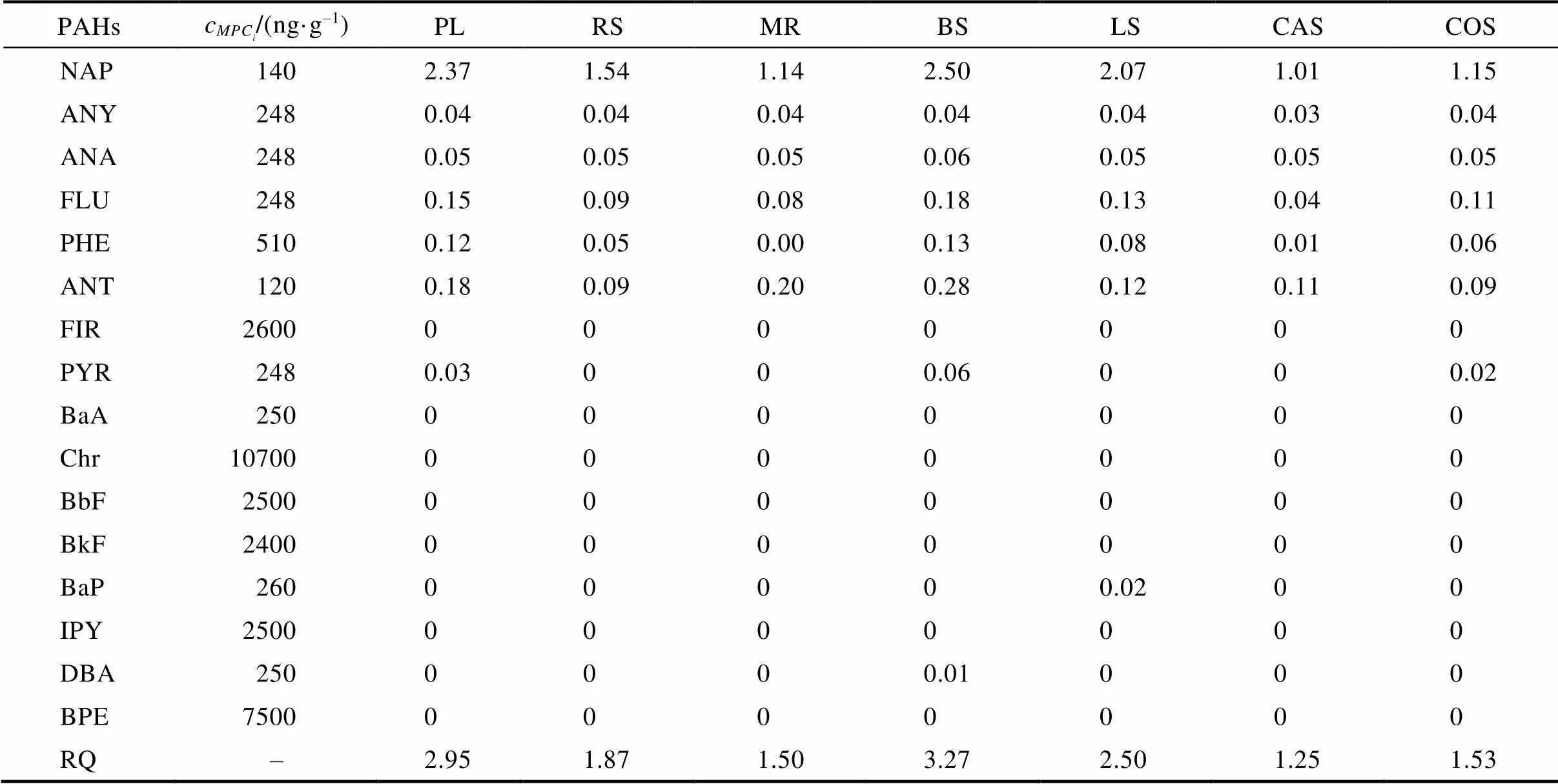
表5 生物炭中单体及总PAHs的风险熵值
3 讨论
3.1 原料对生物炭性质的影响
本研究中生物炭产率、O和H百分比含量随着温度升高而降低,而C百分比含量显著提高,显示升温使得生物质原料发生显著碳化且伴随着其他物质的损失。随着温度升高,生物炭中N占比先增加后略有下降,这归因于热解过程中生物质原料中含肽键物质转化为N杂环碳化合物,而生物炭中酰胺类化合物随着热解温度升高而降低[27-28]。与热解过程中损失C、H、O和N元素变化规律不同,金属元素Na、K、Ca、Mg含量随着温度升高而升高(<0.05),这可能源于温度使得有机结合态元素转化为无机盐等物质[29-30];制备生物炭温度升高pH有所增加,与高温条件下生物炭中金属氧化物、氢氧化物或无机团簇(如碳酸盐、碳酸氢盐和硫酸盐)有关,这些物质含量有助于生物炭中碱度增加[29, 31]。
3.2 生物炭中PAHs的分布特征及影响因素
国际生物炭倡议组织(IBI)将生物炭的总PAHs阈值设定在6~20 μg/g[32],欧洲生物炭认证机构(EBC)将优质与基础级别生物炭中16种PAHs限制在4 μg/g和12 μg/g[33]。本研究中所制备生物炭中PAHs残留水平虽然处于IBI范围内,但部分生物炭(LS、PL)中PAHs浓度高于EBC优质级别限制,显示出部分生物炭中PAHs总量超标。本研究观察到NAP(占比68.7%)和PHE(占比8.2%)均是所制备生物炭中最危险的单体PAH,这归因于较低的制备温度条件(小于500℃)通过直接碳化和芳构化将原料转化低分子量PAHs[8, 34]。FABBRI等[12]测定了NAP最高浓度为1.75~3.36 μg/g,其次是PHE(0.25~3.88 μg/g),这结果与本研究制备的生物炭中主要的单体PAH一致。所制备生物炭中,低温条件下(300℃与500℃)超过97.9%生成低环(2~3)PAHs,仅大于500℃生成BaP、DBA(5环)等高分子PAHs,这与大于500℃时自由基途径生成的活化的低分子多环芳烃通过加成过程形成高分子PAHs密切相关[35]。
本研究观察到∑16PAHs与生物炭中C/H呈显著负相关(<0.01),表明生物炭的碳化程度越高∑16PAHs的含量越低,这与生物炭碳化程度增加(芳香碳含量增加、脂肪碳含量降低)导致生物炭出现更多的微孔结构有关[17, 36]。∑16PAHs、低环PAHs与原料的C/O、C/H、C含量呈显著负相关,说明高C含量不利于制备过程中低环PAHs的生成[7, 37],这与本研究中观察到木本植物源生物炭PAHs含量低结果一致。研究表明生物炭中灰分含量与生物炭PAHs显著正相关,本研究观察到生物炭中PAHs与EC呈显著正相关,这可能归因于灰分对EC有重要贡献。生物炭中的中、低环PAHs单体之间呈显著正相关,而其与高环BaP显著负相关,说明制备的生物炭中的中、低环单体PAHs来源相同,而高环PAHs产生机理和过程显著不同于中、低环PAHs,这与文献中生物炭中的高环与中、低环PAHs产生路径不同报道的结果一致[34-35]。
3.3 生物炭PAHs生态风险评估
本研究中生物炭中PAH单体主要以2~4环为主,占PAHs-TEQ的80%,含有强致癌PAH单体少,因此呈现出较低生物毒性。虽然BaP作为毒性最强的单体之一,其TEQ-BaP范围为0~ 53.3 ng/g,平均值为2.54 ng/g(∑TEQ-BaP/N,N=21),仅仅出现1次(LS),致癌毒性效应可忽略。BbF、BkF、IPY等单体PAH含量可以忽略不计,然而考虑到这些PAHs是相对稳定的、易致癌和易致畸,其中一些(BaP、DBA)在生物炭中的含量仍需加以关注。根据RQ值大小评估生物炭的生态风险大小,其中生物炭中NAP残留浓度极大,其RQ值均超过了高风险标准,呈现高的生态风险。FLU、PHE和ANT在PL、BS、LS处于0.1~1,呈现中等水平生态风险。除了FLU、PHE、ANT外,其他PAH单体的RQ呈现小于0.1,这些PAH单体生态风险较小。虽然生物炭中单体PAHs总体无明显的生态风险,但总PAHs呈现高生态风险,使用生物炭过程中应给予重视。
[1] JERRY ANTAL M, GRØNLI M. The art, science, and technology of charcoal production[J]. Industrial & Engineering Chemistry Research, 2003, 42: 1619-1640.
[2] TROY S M, LAWLOR P G, O'FLYNN C J, O' FLYNN C J, HEALY M G. Impact of biochar addition to soil on greenhouse gas emissions following pig manure application[J]. Soil Biology & Biochemistry, 2013, 60: 173-181.
[3] HERATH H M S K, CAMPS-ARBESTAIN M, HEDLEY M. Effect of biochar on soil physical properties in two contrasting soils: an alfisol and an andisol[J]. Geoderma, 2013, 209-210: 188-197.
[4] TAN H D, LI R L, ZHU Y C, ZHANG Y. In situ quantitative and visual investigation of the retention of polycyclic aromatic hydrocarbons on the root surface ofusing a microscopic fluorescence spectral analysis method[J]. Talanta, 2017, 167: 86-93..
[5] YANG Y Y, ZHOU Y Y, PAN L Q, XU R Y, LI D Y. Benzo[a]pyrene exposure induced reproductive endocrine-disrupting effects via the steroidogenic pathway and estrogen signaling pathway in female scallop Chlamys farreri[J]. Science of the Total Environment, 2020, 726: 138585.
[6] 李 阳, 黄 梅, 沈 飞, 郭海艳, 王 卿. 生物炭对小麦种子萌发与幼苗生长的植物毒理效应[J]. 生态毒理学报, 2017, 12(1): 234-242.
LI Y, HUANG M, SHENG F, GUO H Y, WANG Q. Phytotoxic effects of biochar on seed germination and early growth of wheat[J]. Asian Journal of Ecotoxicology, 2017, 12(1): 234-242. (in Chinese)
[7] WANG C, WANG Y, HERATH H M S K. Polycyclic aromatic hydrocarbons (PAHs) in biochar-their formation, occurrence and analysis: a review[J]. Organic Geochemistry, 2017, 114: 1-11.
[8] KEILUWEIT M, KLEBER M, SPARROW M A, SIMONEIT B R T, PRAHL F G. Solvent-extractable polycyclic aromatic hydrocarbons in biochar: influence of pyrolysis temperature and feedstock[J]. Environmental science & Technology, 2012, 46(17): 9333-9341.
[9] FERNANDES M B, BROOKS P. Characterization of carbonaceous combustion residues: II[J]. Nonpolar Organic Compounds. 2003, 53(5): 447-458.
[10] HALE S E, LEHMANN J, RUTHERFORD D, ZIMMERMAN A R, BACHMANN R T, SHITUMBANUMA V, O’TOOLE A, SUNDQVIST K L,ARP H P H, CORNELISSEN G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars[J]. Environmental Science & Technology, 2012, 46(5): 2830-2838.
[11] SINGH B, SINGH B P, COWIE A L. Characterisation and evaluation of biochars for their application as a soil amendment[J]. Soil Research, 2010, 48(7): 516-525.
[12] FABBRI D, ROMBOLÀ, ALESSANDRO G, TORRI C, SPOKAS K A. Determination of polycyclic aromatic hydrocarbons in biochar and biochar amended soil[J]. Journal of Analytical and Applied Pyrolysis, 2013, 103: 60-67.
[13] TOMCZYK B, SIATECKA A, JĘDRUCHNIEWICZ K, SOCHACKA A, BOGUSZ A, OLESZCZUK P. Polycyclic aromatic hydrocarbons (PAHs) persistence, bioavailability and toxicity in sewage sludge- or sewage sludge-derived biochar-amended soil[J]. Science of the Total Environment, 2020,747: 141123.
[14] 刘 峥, 韦梦琴, 杜玥莹, 冯庆革, 孙 翔. 速生桉树皮基活性炭的制备及吸附特性[J]. 广西大学学报(自然科学版), 2019, 44(6): 1761-1771.
LIU Z, WEI M Q, DU Y Y, FENG Q G, SUN X. Preparation and adsorption property of activated carbons from fast-growing eucalyptus bark[J]. Journal of Guangxi University (Natural Science Edition), 2019, 44(6): 1761-1771. (in Chinese)
[15] 刘跃东, 郑梅迎, 刘 祥, 戴华伟, 王英俊. 海泡石及生物炭对甲霜灵和镉复合污染条件下烟草生长发育和污染物含量的影响[J]. 烟草科技, 2020, 53(7): 1-9.
LIU Y D, ZHENG M Y, LIU X, DAI H W, WANG Y J. Effects of sepiolite and biochar on growth and pollutant content in tobacco under combined metalaxyl and cadmium contamination[J]. Tobacco Science & Technology, 2020, 53(7): 1-9. (in Chinese)
[16] 鲍士旦. 土壤农化分析[M]. 北京: 中国农业出版社, 2008: 265-275.
BAO S D. Soil agro-chemistrical analysis[M]. Beijing: China Agricultural Press, 2008: 265-275. (in Chinese)
[17] ZHANG G, ZHAO Z, GUO X, HAN Z, HE Q, ZHANG F, XU H. Levels of persistent toxic substances in different biochars and their potential ecological risk assessment[J]. Environmental Science and Pollution Research International, 2018, 25(33): 33207-33215.
[18] 谭华东, 张汇杰, 武春媛. GC-MS结合微量QuEChERS法快速测定土壤中16种多环芳烃[J]. 中国测试, 2020, 46(1): 64-70.
TAN H D, ZHANG H J, WU C Y. Rapid determination of 16 polycyclic aromatic hydrocarbons in soil by gas chromatography-tandem mass spectrometry coupled with micro-QuEChERS[J]. China Measurement & Test, 2020, 46(1): 64-70. (in Chinese)
[19] 谭华东, 张汇杰, 武春媛. 超声辅助提取-QuEChERS/GC- MS法快速测定土壤中六六六和滴滴涕[J]. 分析试验室, 2019, 38(11): 1303-1308.
TAN H D, ZHANG H J, WU C Y. Rapid determination of hexachlorocyclohexane and dichlorodiphenyltrichloroethane in soil using ultrasound-assisted QuEChERS/GC-MS[J]. Chinese Journal of Analysis Laboratory, 2019, 38(11): 1303-1308. (in Chinese)
[20] WANG J, XIA K, WAIGI M G, GAO Y, ODINGA E S, LING W, LIU J. Application of biochar to soils may result in plant contamination and human cancer risk due to exposure of polycyclic aromatic hydrocarbons[J]. Environment International, 2018, 121: 169-177.
[21] CAO Z, LIU J, LUAN Y, LI Y, MA M, XU J, HAN S. Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China[J]. Ecotoxicology, 2010, 19: 827-837.
[22] KALF D F, CROMMENTUIJN T, PLASSCHE E J V D. Environmental quality objectives for 10 polycyclic aromatic hydrocarbons (PAHs)[J]. Ecotoxicology & Environmental Safety, 1997, 36(1): 89-97.
[23] SONG J H, KIM D W, KIM H, LEE D S. Need of accurate model prediction of variability of the concentration ratio for testing coherence among environmental quality objectives: A case study of polycyclic aromatic hydrocarbons[J]. Journal of Hazardous Materials, 2014, 266: 34-41.
[24] MCGRATH T E, WOOTEN J B, CHAN W G, HAJALIGOL M R. Formation of polycyclic aromatic hydrocarbons from tobacco: the link between low temperature residual solid (char) and PAH formation[J]. Food & Chemical Toxicology, 2007, 45(6): 1039-1050.
[25] SUN Y N, GAO B, YAO Y, FANG J, ZHANG M, ZHOU Y M, CHEN H, YANG L Y. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties[J]. Chemical Engineering Journal, 2014, 240: 574-578.
[26] LYU H H, HE Y H, TANG J C, HECKER M, LIU Q L, JONES P D, CODLING G, GIESY J P. Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment[J]. Environmental Pollution, 2016, 218: 1-7.
[27] KNICKER H. “Black nitrogen”-an important fraction in determining the recalcitrance of charcoal[J]. Organic Geochemistry, 2010, 41(9): 947-950.
[28] XIAO X, CHEN B, CHEN Z, ZHU L, SCHNOOR J L. Insight into multiple and multi-level structures of biochars and their potential environmental applications: a critical review[J]. Environmental Science & Technology, 2018, 52(9): 5027-5047.
[29] YUAN J H, XU R K, ZHANG H. The forms of alkalis in the biochar produced from crop residues at different temperatures[J]. Bioresource Technology, 2011, 102(3): 3488-3497.
[30] TAG A T, DUMAN G, UCAR S, YANIK J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar[J]. Journal of Analytical & Applied Pyrolysis, 2016, 120: 200-206.
[31] CELY P, GASCÓ G, PAZ-FERREIRO J, MÉNDEZ A. Agronomic properties of biochars from different manure wastes[J]. Journal of Analytical & Applied Pyrolysis, 2015, 111: 173-182.
[32] International Biochar Initiative (IBI). Standardized product definition and product testing guidelines for biochar that is used in soil[S]. 2013.
[33] European Biochar Certificate (EBC). Guidelines for a sustainable production of biochar (Version 6.2E)[S]. 2016.
[34] BROWN R A, KERCHER A K, NGUYEN T H, NAGLE D C, BALLW P. Production and characterization of synthetic wood chars for use as surrogates for natural sorbents[J]. Organic Geochemistry, 2015, 37(3): 321-333.
[35] SULLIVAN R F, BODUSZYNSKI M M, FETZER J C. Molecular transformations in hydrotreating and hydrocracking[J]. Energy & Fuels, 1989, 3(5): 603-612.
[36] ZHANG G X, GUO X F, ZHANG Z H, HE Q S, WANG S F, ZHU Y, YAN Y L, LIU X T, SUN K, ZHANG Y, QIAN T W. Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil[J]. Environmental Pollution, 2016, 218: 513-522.
[37] LIAN F, XING B. Black carbon (biochar) in water/soil environments: molecular structure, sorption, stability, and potential risk[J]. Environmental Science & Technology, 2017, 51(23): 13517-13532.
Distribution and Ecotoxicological Risk of Polycyclic Aromatic Hydrocarbons in Biochar Prepared from Tropical Agricultural Wastes
TAN Huadong1, ZHANG Xiaoying2, WU Chunyuan1*, ZHAO Shuqiao1
1. Environment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Sciences, Haikou, Hainan 571101, China; 2. Chinese Academy of Tropical Agricultural Sciences Proving Ground, Danzhou, Hainan 571737, China
In order to replenish the data on safe biochar utilization as originated from tropical agricultural wastes, biochar was prepared at 300, 500 and 700℃ from typical tropical agricultural wastes, including pineapple leaves (PL), litchi sticks (LS), coconut shells (COS), banana stems (BS), rice straws (RS), cassava stems (CAS) and mushroom residues (MR). The distribution characteristics of 16 polycyclic aromatic hydrocarbons (PAHs) in biochar were determined by QuEChERS, coupled with gas chromatography-mass spectrometry (GC-MS). Furthermore, the key influencing factors of the PAHs were analyzed by redundancy analysis, based on biochar’s physicochemical properties and preparation temperatures. Then, the ecotoxicological risks of the PAHs were evaluated based on risk quotient method (RQ). The results depicted that the concentration of PAHs was 1217.6 to 9547.1 μg/g, with naphthalene (NAP) and phenanthrene (PHE) having the highest detection frequency and level. The arranged concentration level of PAHs was BS>PL>LS>RS>COS>MR>CAS. The main species of the biochar PAHs were significantly different, which was relative to their source and temperature. Redundancy analysis determined that the conductivity (EC), carbon oxygen ratio (C/O), and carbon-hydrogen ratio and temperature had a significant influence on the concentration of PAHs (<0.01). PAH content was positively correlated with electric conductivity (EC) and carbon oxygen ratio (C/O), and was negatively correlated with hydrocarbon ration (C/H) (<0.05). Likewise, it was significantly affected by temperature (<0.01). Risk assessment results depicted that low-ring PAHs contributed to the toxicity of PAHs; thus, the carcinogenic and toxic effects of individual PAHs could be ignored. Except for NAP, the RQ values of the other monomer PAHs were less than 0.1, indicating that no high and medium ecological risks had occurred. However, the total RQ of the PAHs was higher than 1, denoting that greater attention should be paid to the multi-residues of PAHs in biochar. The results of this study could provide a scientific basis for the safe use of biochar in tropical soils.
agricultural waste; biochar; polycyclic aromatic hydrocarbons; pollution characteristics; risk assessment
S432.1
A
10.3969/j.issn.1000-2561.2022.07.023
2021-08-19;
2022-02-20
中国热带作物学会青年托举人才项目(No. CSTC-QN201901);中国热带农业科学院基本科研业务费专项资金(No. 1630042019020);中国热带农业科学院环境与植物保护研究所项目结余经费自主选题专项(No. hzsjy2021001)。
谭华东(1991—),男,硕士,助理研究员,研究方向:有机污染物环境行为及效应。*通信作者(Corresponding author):武春媛(WU Chunyuan),E-mail:chunyuanwu1981@163.com。
