Lymphangiogenesis contributes to exercise-induced physiological cardiac growth
2022-07-29YihuaBeiZhenzhenHuangXingFengLinLiMengWeiYujiaoZhuShuqinLiuChenChenMingmingYinHuiminJiangJunjieXiao
Yihua Bei,Zhenzhen Huang,Xing Feng,Lin Li,Meng Wei,Yujiao Zhu,Shuqin Liu,Chen Chen,Mingming Yin,Huimin Jiang,Junjie Xiao,*
a Cardiac Regeneration and Ageing Lab,Institute of Geriatrics(Shanghai University),Affiliated Nantong Hospital of Shanghai University(The Sixth People’s Hospital of Nantong),School of Medicine,Shanghai University,Nantong 226011,China
b Shanghai Engineering Research Center of Organ Repair,School of Life Science,Shanghai University,Shanghai 200444,China
c Clinical Laboratory Center,Beijing Hospital of Traditional Chinese Medicine,Beijing 100010,China
Abstract Background:Promoting cardiac lymphangiogenesis exerts beneficial effects for the heart.Exercise can induce physiological cardiac growth with cardiomyocyte hypertrophy and increased proliferation markers in cardiomyocytes.However,it remains unclear whether and how lymphangiogenesis contributes to exercise-induced physiological cardiac growth.We aimed to investigate the role and mechanism of lymphangiogenesis in exercise-induced physiological cardiac growth.Methods: Adult C57BL6/J mice were subjected to 3 weeks of swimming exercise to induce physiological cardiac growth. Oral treatment with vascular endothelial growth factor receptor 3(VEGFR3)inhibitor SAR131675 was used to investigate whether cardiac lymphangiogenesis was required for exercise-induced physiological cardiac growth by VEGFR3 activation. Furthermore, human dermal lymphatic endothelial cell(LEC)-conditioned medium was collected to culture isolated neonatal rat cardiomyocytes to determine whether and how LECs could influence cardiomyocyte proliferation and hypertrophy.Results:Swimming exercise induced physiological cardiac growth accompanied by a remarkable increase of cardiac lymphangiogenesis as evidenced by increased density of lymphatic vessel endothelial hyaluronic acid receptor 1-positive lymphatic vessels in the heart and upregulated LYVE-1 and Podoplanin expressions levels.VEGFR3 was upregulated in the exercised heart,while VEGFR3 inhibitor SAR131675 attenuated exercise-induced physiological cardiac growth as evidenced by blunted myocardial hypertrophy and reduced proliferation marker Ki67 in cardiomyocytes,which was correlated with reduced lymphatic vessel density and downregulated LYVE-1 and Podoplanin in the heart upon exercise.Furthermore,LEC-conditioned medium promoted both hypertrophy and proliferation of cardiomyocytes and contained higher levels of insulinlike growth factor-1 and the extracellular protein Reelin, while LEC-conditioned medium from LECs treated with SAR131675 blocked these effects.Functional rescue assays further demonstrated that protein kinase B(AKT)activation,as well as reduced CCAAT enhancer-binding protein beta (C/EBPβ) and increased CBP/p300-interacting transactivators with E (glutamic acid)/D (aspartic acid)-rich-carboxylterminal domain 4(CITED4),contributed to the promotive effect of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation.Conclusion:Our findings reveal that cardiac lymphangiogenesis is required for exercise-induced physiological cardiac growth by VEGFR3 activation, and they indicate that LEC-conditioned medium promotes both physiological hypertrophy and proliferation of cardiomyocytes through AKT activation and the C/EBPβ-CITED4 axis. These results highlight the essential roles of cardiac lymphangiogenesis in exercise-induced physiological cardiac growth.
Keywords: Cardiac lymphatics;Exercise;Physiological cardiac hypertrophy;Proliferation;VEGFR3
1. Introduction
The lymphatic vessels form a specialized network that controls tissue-fluid homeostasis, immunosurveillance, and lipid transport.1,2Recently, meningeal lymphatic vasculature has also been identified to regulate immune response and brain tumor drainage.3,4The heart has an extensive network of lymphatic vessels that are heterogeneous in origin.5,6Increasing evidence has indicated the potential roles of cardiac lymphatics in cardiovascular development and diseases.7,8Therapeutics targeting cardiac lymphatics are believed to be promising strategies to maintain cardiovascular homeostasis and limit cardiovascular injuries.9,10
Lymphangiogenesis is a process of lymphatic growth majorly driven by proliferation, migration, and differentiation of lymphatic endothelial cells(LECs)through activation of vascular endothelial growth factor receptor 3 (VEGFR3).11,12It is known that a dysfunctional cardiac lymphatic network can be induced upon myocardial infarction, while promoting cardiac lymphangiogenesis exerts beneficial effects that reduce cardiac edema and inflammation and improve cardiac function.13-15Improving cardiac lymphangiogenesis also protects against atherosclerosis through reverse cholesterol transport.16-18In addition to these pathological conditions, lymphangiogenesis can also respond to exercise training.19However, little is known about whether lymphangiogenesis is involved in exerciseinduced myocardial adaptations of the heart.
Exercise-induced adaptive cardiac growth is a physiological process that is largely related to the increase in cardiomyocyte size,accompanied by increased protein synthesis and protective metabolic changes.20-22Meanwhile,exercise is able to increase proliferation markers in cardiomyocytes.23Due to the fact that adult mammalian hearts have very limited regenerative capacity, exercise-induced cardiomyocyte proliferation can play an important role in myocardial protection.24-26Accumulating evidence indicates that molecules that contribute to exerciseinduced physiological cardiac growth have the potential to brake bad hypertrophy.27,28Despite the increasing research interest, it remains unclear whether lymphangiogenesis contributes to exercise-induced physiological cardiac growth.
In the present study, we first observed that exercise-induced physiological cardiac growth was associated with cardiac lymphangiogenesis. Using the selective VEGFR3 inhibitor SAR131675, we demonstrated that cardiac lymphangiogenesis was required for exercise-induced physiological cardiac growth by VEGFR3 activation.Furthermore,we cultured LECs and neonatal rat cardiomyocytes (NRCMs) and found that LEC-conditioned medium promoted both hypertrophy and proliferation of cardiomyocytes through protein kinase B (AKT) activation and the CCAAT enhancer-binding protein beta (C/EBPβ)-CBP/p300-interacting transactivators with E (glutamic acid)/D(aspartic acid)-rich-carboxylterminal domain 4(CITED4)axis.
2. Material and methods
2.1. Animals
Male C57BL/6J mice aged 8-10 weeks old were purchased from Beijing Vital River Laboratory Animal Technology Co.Ltd.(Beijing,China)and maintained in the specific pathogenfree laboratory animal facility of Shanghai University(Shanghai, China). To evaluate whether gender differences could influence lymphangiogenesis during exercise-induced physiological cardiac growth, female C57BL/6J mice aged 8-10 weeks old were also purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China) and maintained as described above.
2.2. Ethics statement
All procedures involving animals were conducted according to the guidelines on the use and care of laboratory animals for biomedical research published by National Institutes of Health(No.85-23,revised 1996)and were approved by the committee on the Ethics of Animal Experiments of Shanghai University.
2.3. Animal model and treatment
To induce physiological cardiac growth, mice were randomly divided into the exercise group (3 weeks of swimming exercise) and the sedentary control group. The protocol has been described in detail previously.24-26In brief,for the exercise group, mice were subjected to 5 min of adaptive swimming before the first day of swimming exercise. On the first day, swimming began with 10 min twice a day and increased by 10 min per day until reaching 90 min twice a day.The total duration of swimming exercise lasted for 3 weeks.To investigate the role of VEGFR3 in exercise-induced cardiac growth,mice were treated daily with selective VEGFR3 tyrosine kinase inhibitor SAR131675 (M2078; AbMole, Houston, TX,USA; 100 mg/kg/d) or vehicle controls by gavage29and were either subjected to swimming exercise or remained sedentary for 3 weeks. In detail, SAR131675 powders (AbMole) were dissolved with 0.6%methylcellulose (M0512;Sigma-Aldrich,St.Louis,MO,USA;0.6 g/100 mL ddH2O,w/v)on the day of use and sonicated for 30-40 min to prepare a 10 mg/mL SAR131675 solution. After 3 weeks of swimming exercises with SAR131675 treatment or without,heart tissues were harvest. The body weight (BW), heart weight (HW), and tibia length (TL) of the mice were measured. The HW/BW ratio and HW/TL ratio were calculated for evaluation of cardiac growth. Heart tissues were embedded into Tissue-Tek OCT(4583; Sakura Finetek, Torrance, CA, USA) or snap frozen and conserved at-80 ˚C for further examinations.
2.4. NRCM isolation and culture
Hearts from neonatal Sprague-Dawley rats (Beijing Vital River Laboratory Animal Technology Co. Ltd., Beijing,China) aged 1-3 days old were harvested, minced, and digested with Collagenase II (17101015; Gibco, Waltham,MA, USA) and Pancreatin from porcine pancreas (P3292;Sigma-Aldrich). Using differential velocity adherent technique,Percoll(17-0891-01;GE healthcare,Chicago,IL,USA)treatment,and centrifugation,NRCMs were isolated and purified as previously reported.30NRCMs were maintained in Dulbecco’s modified Eagle’s medium (DMEM; 10-013-CVR;Corning, Corning, NY, USA) containing 4.5 g/L glucose supplemented with 10%horse serum(16050-122;Gibco)and 5%fetal bovine serum (04-001-1ACS; BioInd, Kibbutz Beit Haemek,Israel)before further treatment.
2.5. LEC-conditioned medium
Human dermal LECs(HDLECs;C-12217;Promocell,Heidelberg, Germany) were cultured in endothelial cell growth media(EGM-MV2) (C-22121; Promocell) and passaged by accutase solution (C-41310; Promocell) up to Passage 6. Immunofluorescent stainings for lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE-1) (ab14917; Abcam, Cambridge, UK; 1:100 dilution) and Podoplanin (ab11936; Abcam; 1:50 dilution) were carried out to confirm the purity of cultured LECs.After growing to 70%confluence,LECs were washed with 1×phosphate-buffered saline (B548117; Sangon, Shanghai, China) and then cultured with EGM-MV2 for 24 h for collection of LECconditioned medium. The EGM-MV2 without culturing LECs was used as control medium. To investigate whether VEGFR3 influenced the effect of LEC-conditioned medium, LECs were treated with VEGFR3 inhibitor SAR131675 (S2842; Selleck,Houston,TX,USA)at 10 nM or 100 nM for 24 h,and LEC-conditioned medium was collected after treatment.
2.6. NRCM treatment
To investigate the effect of LEC-conditioned medium on cardiomyocytes, NRCMs were starved and then treated with either LEC-conditioned medium or control medium for 48 h.To examine whether AKT activation contributed to the effect of LEC-conditioned medium, NRCMs were treated with AKT inhibitor MK2206(S1078;Selleck)at 10 nM during the first 24 h of treatment and then cultured with either control medium or LEC-conditioned medium for 48 h as described above. To examine the involvement of the C/EBPβ-CITED4 axis in the effect of LECconditioned medium, NRCMs were infected with C/EBPβ expression lentivirus using Polybrene (40804ES76; YEASEN,Shanghai, China) or transfected with CITED4 small interfering RNA using Lipofectamine 2000 (11668-019; ThermoFisher,Waltham, MA, USA) during the 48-h treatment of control medium or LEC-conditioned medium. At the end of treatment,total RNA was extracted from cardiomyocytes for reverse transcription quantitative polymerase chain reactions (RT-qPCRs),and immunofluorescent stainings for Ki67/α-Actinin or 5-ethynyl-2’-deoxyuridine (EdU)/α-Actinin were carried out to determine cardiomyocyte hypertrophy and proliferation.
2.7. Immunofluorescent stainings for cardiac tissues
Cryosections of cardiac tissues were fixed with 4% paraformaldehyde (PFA; P6148; Sigma-Aldrich), permeabilized with 0.5% TritonX-100 (T8787; Sigma-Aldrich), and blocked with 5% bovine serum albumin (BSA; KGY00850; KeyGEN, Nanjing, China). LYVE-1 and wheat germ agglutinin (WGA)co-immunofluorescent staining was performed to evaluate cardiac lymphatics density and myocardial hypertrophy.Briefly,cryosections were incubated with primary antibody LYVE-1(ab14917; Abcam; 1:100 dilution) at 4 ˚C overnight, and then incubated with Cy3-AffiniPure goat anti-rabbit IgG antibody(111-165-144; Jackson ImmunoResearch, West Grove, PA,USA;1:200 dilution)for 2 h at room temperature.Next,cryosections were stained with WGA (L4895; Sigma-Aldrich; 1:100 dilution).Nuclei were counterstained with Hoechst(62249;ThermoFisher, Waltham, MA, USA). Myocardial surface area, density of cardiac LYVE-1 positive lymphatics(lymphatics/mm2,as calculated by the number of LYVE-1 positive lymphatics per mm2), and lymphatic-to-cardiomyocyte ratio (lymphatics/CM ratio,as calculated by the ratio of the number of LYVE-1 positive lymphatics to the number of cardiomyocytes per field) were determined in order to evaluate cardiac hypertrophy and lymphatic vessel density,respectively.
For evaluation of proliferation marker Ki67 in cardiomyocytes,cryosections were incubated with primary antibodies Ki67(ab16667; Abcam; 1:100 dilution) and α-Actinin (A7811;Sigma-Aldrich; 1:200 dilution) at 4 ˚C overnight. After incubation with Cy3-AffiniPure goat anti-rabbit IgG antibody(111-165-144;Jackson ImmunoResearch;1:200 dilution)and Alexa Fluor®488-AffiniPure donkey anti-mouse IgG antibody (715-545-150;Jackson ImmunoResearch;1:200 dilution)secondary antibodies,nuclei were counterstained with Hoechst (ThermoFisher). The percentage of Ki67-positive and α-Actinin labeled cardiomyocytes in comparison to total α-Actinin labeled cardiomyocytes was determined in order to evaluate cardiomyocyte proliferation.Investigators were blinded to treatment group during image acquisition and analysis. All images were photographed with a fluorescence microscope (Zeiss Axio Imager 2; Zeiss, Oberkochen,Germany)and analyzed with ImageJ Software 1.50i(NIH,Bethesda,MD,USA).
2.8. Immunofluorescent stainings for cardiomyocytes
Cardiomyocyte hypertrophy and proliferation were determined by co-immunofluorescent stainings for Ki67/α-Actinin and EdU/α-Actinin. Briefly, NRCMs were fixed with 4% PFA(Sigma-Aldrich),permeabilized with 0.5% TritonX-100(Sigma-Aldrich),and blocked with 5%BSA(KeyGEN).Cells were then incubated with primary antibodies Ki67(Abcam,1:100 dilution)and α-Actinin(Sigma-Aldrich,1:200 dilution)at 4 ˚C overnight,followed by incubation with correspondent secondary antibodies Alexa Fluor®488-AffiniPure goat anti-rabbit IgG antibody(111-545-003; Jackson ImmunoResearch; 1:200 dilution) and Cy3-AffiniPure donkey anti-mouse IgG antibody(715-165-151;Jackson ImmunoResearch;1:200 dilution).Finally,nuclei were counterstained with Hoechst(ThermoFisher).For EdU staining, cells were pre-incubated with EdU working solution A for 24 h according to kFluor488 Click-iT EdU Kit manuals (KGA331;KeyGEN). At the end of treatment, NRCMs were stained with α-Actinin(Sigma-Aldrich)followed by EdU staining.Investigators were blinded to treatment groups during image acquisition and analysis.Cells were photographed with a fluorescence microscope(Leica DMi8;Leica,Wetzlar,Germany)for determinations of cell surface area and the percentage of Ki67(or EdU)positive cardiomyocytes in comparison to total cardiomyocytes using ImageJ software 1.50i(NIH).
2.9. Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed to measure the levels of insulin-like growth factor-1 (IGF-1) and the extracellular protein Reelin(RELN) in LEC-conditioned medium with or without pretreatment of VEGFR3 inhibitor SAR131675 in cultured LECs.Quantitative measurement of IGF-1 was performed in LEC culture supernatants using human IGF-1 ELISA Kit(ab108873; Abcam) according to manufacturer’s manuals. To measure the relative RELN levels in LEC-conditioned medium, sandwich ELISA was performed according to another previously reported method.31In brief,MaxiSorp Flat-Bottom 96-well plate (Nunc, Rochester, NY, USA) was precoated with LEC culture supernatants overnight at 4˚C and blocked with 0.3% (w/v) gelatin in 1× phosphate-buffered saline buffer (Sangon) at room temperature for 2 h. Plates were then incubated with RELN primary antibody(ab281278;Abcam; 1:100 dilution) for 2 h, followed by incubation with goat anti-rabbit IgG antibody horseradish peroxidase (HRP)antibody (ab6721; Abcam; 1:1000 dilution) for 45 min. After washing and adding the substrate solution composed of 0.325% ortho-phenylenediamine dihydrochloride (Sigma-Aldrich)and 0.085%H2O2(Sigma-Aldrich)in 0.3 M Tris-citrate buffer(Sigma-Aldrich;pH 6.0),reactions were stopped by adding 2 M H2SO4(Sigma-Aldrich).Finally,plates were measured for optical density within 30 min at 490 nm using an ELISA plate reader(Bio-Rad,Hercules,CA,USA).
2.10. Western blot analysis
Mouse heart tissues were homogenized and lysed with radioimmunoprecipitation assay lysis buffer (KeyGEN Bio-TECH,Nanjing,China)supplemented with 1%phenylmethylsulfonyl fluoride (36978; ThermoFisher) and Pierce protease and phosphatase inhibitor (88668; ThermoFisher). Denatured proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene fluoride membranes. After being blocked with 5% nonfat milk,the membranes were incubated overnight at 4 ˚C with primary antibodies against LYVE-1(ab14917;Abcam),Podoplanin (ab11936; Abcam), VEGFR3 (A10971; ABclonal,Wuhan,China),and IGF-1(28530-1-AP;Proteintech,Wuhan,China) from 1:500 to 1:1000 dilution. The membranes were then incubated with HRP-conjugated secondary antibodies,and protein blots were developed with an enhanced chemiluminescence kit. Glyceraldehyde-3-phosphate dehydrogenase(GAPDH) (AP0063; Bioworld, Nanjing, China; 1:1000 dilution) and β-actin (AC026; Abclonal; 1:1000 dilution) were used as loading controls. Finally, protein band densities were quantified using ImageJ software(NIH).
2.11. RT-qPCR
Total RNA was extracted from mouse heart tissues or NRCMs with a Trizol RNAiso Plus kit (9109; TaKaRa,Kusatsu, Japan). After cDNA synthesis using RevertAid First Strand cDNA Synthesis Kit (K1622; ThermoFisher), RTqPCRs were conducted using TaKaRa SYBR Premix Ex Taq(RR420A; TaKaRa) on Roche LightCycler480 PCR System(Roche Diagnostics,Rotkreuz,Switzerland).The primers(forward and reverse,5′-3′)used for RT-qPCR analyses are listed in Supplementary Table 1.
2.12. Statistical analysis
Statistical analysis was carried out with SPSS software Version 20.0(IBM Corp.,Armonk,NY,USA)or GraphPad Software Version 8.3.0 (GraphPad Software, San Diego, CA,USA).Results were presented as mean±SD using GraphPad Software Version 8.3.0 (GraphPad Software). An independent-sample t test(two-tailed)was used for statistical comparisons between the 2 groups. One-way analysis of variance(ANOVA)followed by Bonferroni’s or Dunnett T3’s multiple comparison test, or two-way analysis of variance followed by the Tukey post hoc test were used for statistical comparisons between multiple groups.A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Cardiac lymphangiogenesis is increased during exerciseinduced physiological cardiac growth
Compared to sedentary mice, swimming exercised mice showed significant increase in HW, HW/BW ratio, and HW/TL ratio(Fig.1A).We carried out WGA and LYVE-1 immunofluorescent staining to assess cardiac lymphangiogenesis upon exercise training (Fig. 1B). WGA staining showed that exercise significantly enlarged the cell surface area of cardiomyocytes (Fig. 1C). In comparison to sedentary control mice,the density of cardiac LYVE-1 positive lymphatics, as calculated by the number of LYVE-1 positive lymphatics per mm2,increased 1.38-fold in exercised mice (Fig. 1D). The lymphatics/CM ratio, as calculated by the ratio of the number of LYVE-1 positive lymphatics to the number of cardiomyocytes per field,saw an even larger increase,with a 2.15-fold in exercised mice.This may be due partly to the enlarged cardiomyocyte size and reduced cardiomyocyte number per field in the exercise group(Fig.1E).
Next, we assessed the expression levels of lymphangiogenic markers LYVE-1 and Podoplanin. LYVE-1 was increased at both the mRNA and protein levels in exercised hearts (Fig. 1F and 1G). Exercise also upregulated Podoplanin at the protein level in heart tissues (Fig. 1G). Meanwhile, swimming exercise also induced physiological cardiac growth and increased cardiac lymphangiogenesis in female adult mice, as demonstrated by increased HW and enlarged myocardial cross-sectional area as well as by the increased density of LYVE-1 positive lymphatic vessels in the heart and upregulated LYVE-1 and Podoplanin expression levels(Supplementary Fig.1).This indicates that there is no gender difference in cardiac lymphangiogenesis induced by exercise training. Collectively, immunofluorescent staining and molecular analysis consistently provide strong evidence that cardiac lymphangiogenesis is increased during exercise-induced physiological cardiac growth.
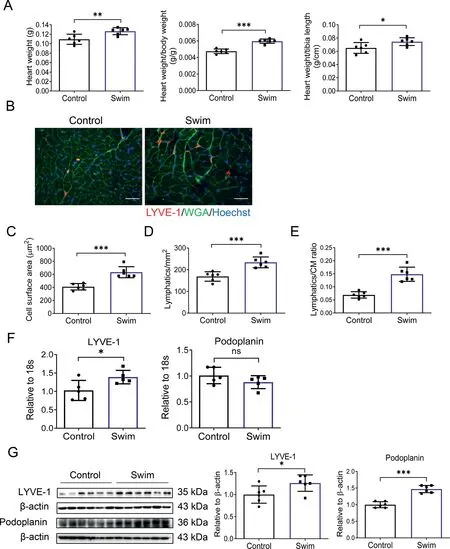
Fig.1. Cardiac lymphangiogenesis is increased during exercise-induced physiological cardiac growth. (A) Heart weight, heart weight/body weight ratio,and heart weight/tibia length ratio were measured after 3 weeks of swimming exercise(n=6);(B)Representative images of immunofluorescent staining for LYVE-1 and WGA in heart tissues from control and swim mice(scale bar=25 μm); (C) Cell surface area of myocardium; (D) LYVE-1 positive lymphatic density; (E) LYVE-1 positive lymphatics/CM ratio based on immunofluorescent staining for LYVE-1 and WGA (n=6); (F) RT-qPCR for LYVE-1 and Podoplanin in heart tissues from control and swim mice (n=5); (G) Western blot for LYVE-1 and Podoplanin in heart tissues from control and swim mice(n=6).Independent-sample t test(two-tailed)was used for statistical comparisons between 2 groups.*p <0.05;**p <0.01;***p <0.001.18s=18s ribosomal RNA; CM=cardiomyocyte; LYVE-1=lymphatic vessel endothelial hyaluronic acid receptor 1; ns=not significant; RT-qPCR=reverse transcription quantitative polymerase chain reaction;WGA=wheat germ agglutinin.
3.2. Cardiac lymphangiogenesis is required for exerciseinduced physiological cardiac growth by VEGFR3 activation
Vascular endothelial growth factor C (VEGFC), Vascular endothelial growth factor D (VEGFD), and VEGFR3 are important lymphangiogenic regulators. We assayed their expression levels in cardiac samples and found that VEGFR3 was significantly upregulated in exercised hearts (Fig 2A and 2B). Meanwhile, exercise also induced VEGFC and VEGFD expression levels in heart tissues (Fig. 2C). Based on these observations,we treated mice with a selective VEGFR3 inhibitor SAR131675 (100 mg/kg/d) by gavage, as previously reported,29and subjected mice to 3 weeks of swimming exercise(Fig.2D)to investigate whether inhibiting VEGFR3 could alter exercise-induced cardiac lymphangiogenesis,thus attenuating exercise-induced physiological cardiac growth.
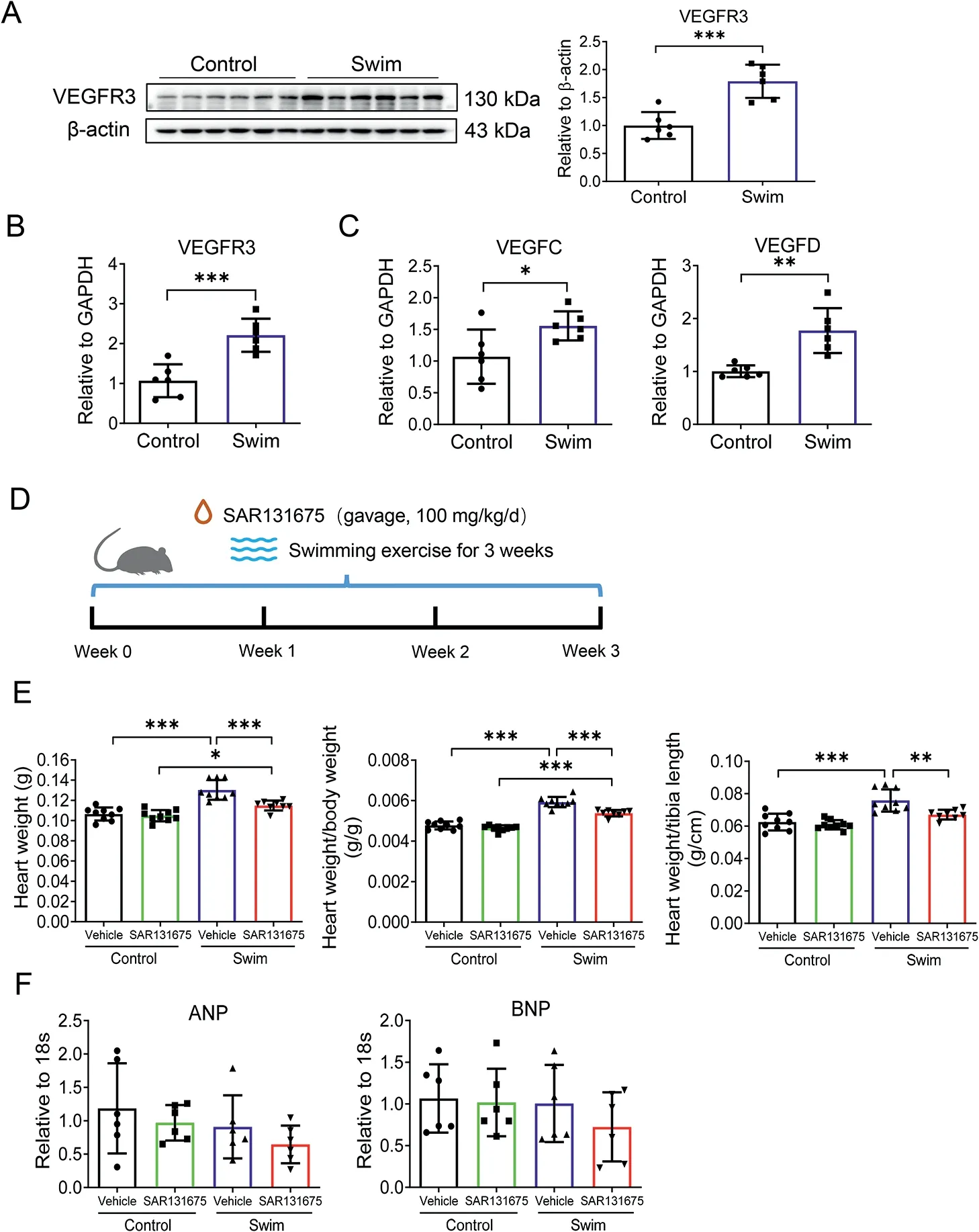
Fig. 2. VEGFR3 inhibition attenuates exercise-induced physiological cardiac growth.(A)Western blot for VEGFR3 in heart tissues from control and swim mice (n=6); (B) RT-qPCR for VEGFR3 in heart tissues from control and swim mice (n=6); (C) RT-qPCR for VEGFC and VEGFD in heart tissues from control and swim mice (n=6); (D) Schematic diagram showing that mice were gavaged by SAR131675(100 mg/kg/d)or vehicle controls during 3 weeks of swimming exercise; (E) Heart weight, heart weight/body weight ratio,and heart weight/tibia length ratio were measured after 3 weeks of swimming exercise(n=8-9);(F)RT-qPCR for ANP and BNP in heart tissues from control and swim mice(n=6).Independent-sample t test(two-tailed)was used for statistical comparisons between 2 groups.Two-way ANOVA followed by Tukey post hoc test was used for statistical comparisons between multiple groups. * p <0.05; ** p <0.01; *** p <0.001. ANOVA=analysis of variance; ANP=atrial natriuretic peptide; BNP=brain natriuretic peptide;GAPDH=glyceraldehyde-3-phosphate dehydrogenase; RT-qPCR=reverse transcription quantitative polymerase chain reaction;VEGFC=vascular endothelial growth factor C; VEGFD=vascular endothelial growth factor D;VEGFR3=vascular endothelial growth factor receptor 3.
After 3 weeks of swimming exercise, we observed that exercise was effective to induce physiological cardiac growth,as evidenced by increased HW, HW/BW ratio, and HW/TL ratio,which was partially attenuated by SAR131675 treatment(Fig. 2E). Meanwhile, we observed no regulation of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP)expression levels in the heart tissues (Fig. 2F), excluding the occurrence of pathological cardiac hypertrophy. Next, we evaluated expression levels of VEGFR3 and lymphangiogenic markers in heart tissues. We found that the upregulation of VEGFR3, as well as LYVE-1 and Podoplanin expression levels in exercised hearts, were clearly blocked by SAR131675 treatment (Fig. 3A and 3B). These data suggest that cardiac lymphangiogenesis is required for exercise-induced physiological cardiac growth by VEGFR3 activation.
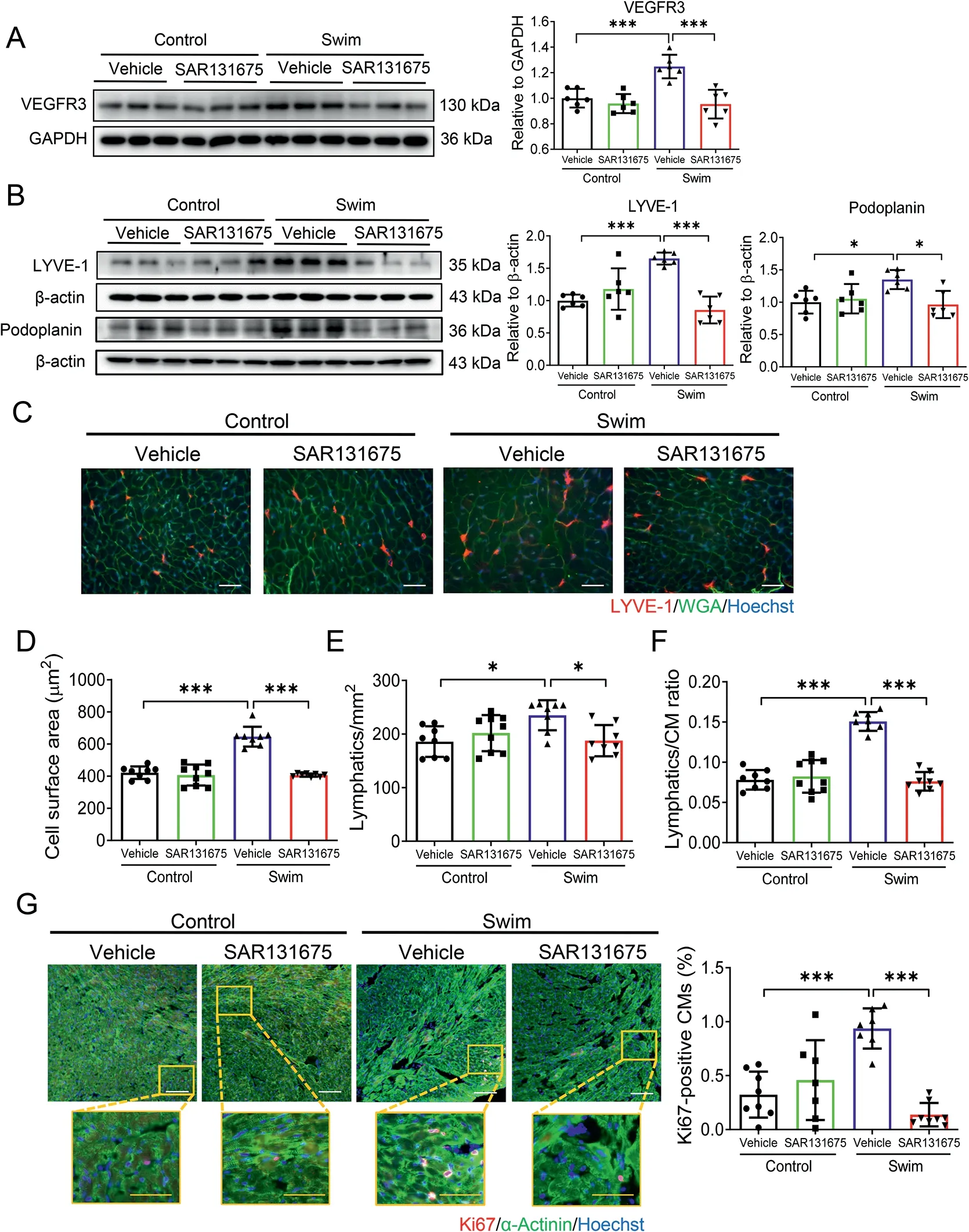
Fig. 3. VEGFR3 inhibition attenuates exercise-induced lymphangiogenesis and cardiomyocyte hypertrophy and proliferation in the heart. (A) Western blot for VEGFR3 in heart tissues from control and swim mice treated with SAR131675 (100 mg/kg/d) or vehicle controls (n=6); (B) Western blot for LYVE-1 and Podoplanin in heart tissues from control and swim mice treated with SAR131675 or vehicle controls (n=6); (C) Representative images of immunofluorescent staining for LYVE-1 and WGA in heart tissues from control and swim mice treated with SAR131675 or vehicle controls(scale bar=25 μm); (D) Cell surface area of myocardium; (E) LYVE-1 positive lymphatic density; (F) LYVE-1 positive lymphatics/CM ratio based on immunofluorescent staining for LYVE-1 and WGA (n=8-9); and (G) Immunofluorescent staining for Ki67 and α-Actinin in heart tissues from control and swim mice treated with SAR131675 or vehicle controls (n=7-8). White scale bar=100 μm. Yellow scale bar=50 μm. Two-way ANOVA followed by Tukey post hoc test was used for statistical comparisons between multiple groups.*p <0.05;***p <0.001.ANOVA=analysis of variance;CM=cardiomyocyte; GAPDH=glyceraldehyde-3-phosphate dehydrogenase; LYVE-1=lymphatic vessel endothelial hyaluronic acid receptor 1;RT-qPCR=reverse transcription quantitative polymerase chain reaction;VEGFR3=vascular endothelial growth factor receptor 3;WGA=wheat germ agglutinin.
3.3. Exercise-induced cardiomyocyte hypertrophy and proliferation are altered by VEGFR3 inhibition
Exercise-induced physiological cardiac growth is presented with both enlarged cardiomyocyte size and increased proliferation markers in cardiomyocytes. Here, we first carried out co-immunofluorescent stainings for WGA and LYVE-1 to assess cardiomyocyte hypertrophy as well as lymphatic density in exercised mice with SAR131675 treatment(Fig.3C).WGA staining showed that exercise-induced enlargement of cardiomyocyte size was attenuated by SAR131675 (Fig. 3D).Meanwhile, the increased density of cardiac LYVE-1 positive lymphatics and the lymphatics/CM ratio were also attenuated by SAR131675 in exercised hearts(Fig.3E and 3F).Next,we performed Ki67 and α-Actinin co-immunolabeling and found that SAR131675 also inhibited exercise-induced increase of proliferation marker Ki67 in cardiomyocytes (Fig. 3G). Collectively,these data indicate that exercise-induced cardiomyocyte hypertrophy and proliferation are both altered by VEGFR3 inhibition with a blunted cardiac lymphangiogenesis.
3.4. LECs promote cardiomyocyte hypertrophy and proliferation in a VEGFR3-dependent manner
It was recently reported that LECs can promote cardiac growth and repair through lymphangiocrine signals.31To further determine whether and how LECs could influence proliferation and/or hypertrophy of cardiomyocytes, human dermal LECs were cultured, and LEC-conditioned medium was collected to culture isolated NRCMs. First, immunofluorescent labeling for lymphatic endothelium markers demonstrated that LECs were positive for both Podoplanin and LYVE-1, confirming the purity of the LECs used in our in vitro experiments (Fig. 4A). Next, we cultured NRCMs with either LEC-conditioned medium or control medium for 48 h and then measured and compared cardiomyocyte area and proliferation using Ki67/α-Actinin or EdU/α-Actinin co-immunofluorescent stainings. We observed that LEC-conditioned medium was able to induce both hypertrophy and proliferation of cardiomyocytes(Fig.4B and 4C).
As VEGFR3 was upregulated in cardiac tissues upon exercise, we further treated LECs with VEGFR3 inhibitor SAR131675 to examine whether VEGFR3 inhibition could prevent the pro-hypertrophic and pro-proliferative effects of LEC-conditioned medium on cardiomyocytes. Although SAR131675 itself did not influence the hypertrophy and proliferation of cardiomyocytes when treated with control medium (Supplementary Fig. 2), LEC-conditioned medium from SAR131675 pre-treated LECs significantly attenuated cardiomyocyte hypertrophy and proliferation in a dosedependent manner (Fig. 4D). These data support that VEGFR3 was necessary to mediate the effects of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation.
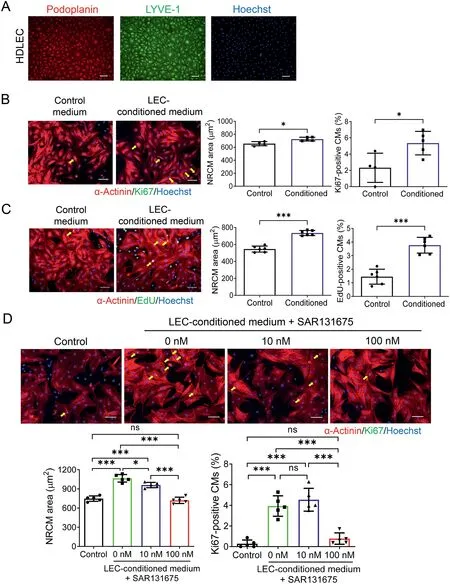
Fig. 4. LEC-conditioned medium promotes hypertrophy and proliferation of cardiomyocytes in a VEGFR3-dependent manner. (A) Representative images of Podoplanin and LYVE-1 immunofluorescent labelings in cultured HDLECs(scale bar=100 μm); (B) Immunofluorescent staining for Ki67 and α-actinin in isolated NRCMs cultured with control medium or LEC-conditioned medium for 48 h (n=4-5; scale bar=50 μm);(C) Immunofluorescent staining for EdU and α-actinin in NRCMs cultured with control medium or LECconditioned medium for 48 h (n=6; scale bar, 50 μm); (D) Immunofluorescent staining for Ki67 and α-Actinin in NRCMs cultured with control medium or LEC-conditioned medium in the absence or presence of SAR131675(0 nM,10 nM,100 nM)for 48 h(n=5,scale bar=50 μm).Independent-sample t test (two-tailed) was used for statistical comparisons between 2 groups.One-way ANOVA test followed by Bonferroni’s comparison test was used for statistical comparisons between multiple groups. * p <0.05; *** p <0.001.ANOVA=analysis of variance; EdU=5-ethynyl-2’-deoxyuridine;HDLEC=human dermal lymphatic endothelial cell; LEC=lymphatic endothelial cell; LYVE-1=lymphatic vessel endothelial hyaluronic acid receptor 1; NRCM=neonatal rat cardiomyocyte; RT-qPCR=reverse transcription quantitative polymerase chain reaction.
3.5. IGF-1 and RELN serve as potential mediators in the crosstalk between LECs and cardiomyocytes
To explore molecular players mediating the effects of LECconditioned medium upon VEGFR-3 manipulation in cultured cardiomyocytes,we evaluated any potential mediators in LECconditioned medium that may promote cardiomyocyte hypertrophy and proliferation;we also measured their expression levels in heart tissues from swimming exercised models in the presence or absence of VEGFR3 inhibitor SAR131675. We first measured the IGF-1, a canonical growth factor in response to exercise that can bind to IGF-1 receptors and activate the phosphatidylinositol 3-kinase(PI3K)-AKT signaling to generate exercise-induced physiological cardiac growth.32Meanwhile, we measured the RELN, which has recently been identified as a lymphangiocrine signal in LEC-conditioned medium that increases cardiomyocyte proliferation and survival.31Interestingly,in our exercised model, we demonstrated that both IGF-1 and RELN were significantly upregulated in heart tissues from swimming exercised mice(Fig.5A and 5B),while VEGFR3 inhibitor SAR131675 significantly blocked the upregulation of IGF-1 and RELN in the exercised heart tissues(Fig. 5C and 5D). Next, we measured IGF-1 and RELN levels in LEC-conditioned medium using ELISA. Our results showed that LEC-conditioned medium contained higher levels of IGF-1 and RELN compared to control medium. This was, however,attenuated by the culture medium from VEGFR3 inhibitortreated LECs (Fig. 5E and 5F). Indeed, these data demonstrate that exercise can upregulate IGF-1 and RELN expression levels in the hearts, while VEGFR3 inhibition blocks these changes.IGF-1 and RELN may serve as potential mediators in the crosstalk between LECs and cardiomyocytes.
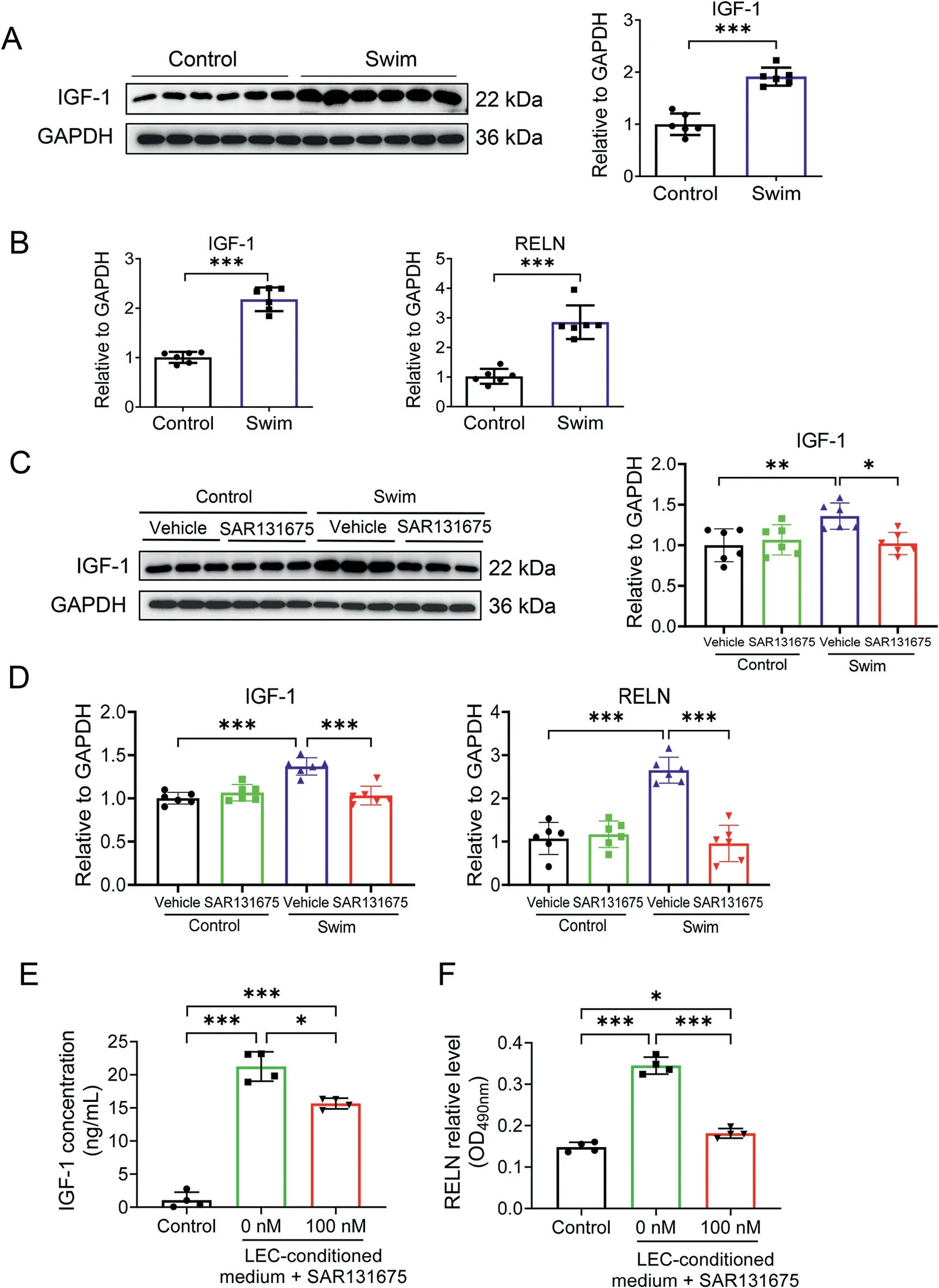
Fig.5. IGF-1 and RELN as potential mediators in the crosstalk between LECs and cardiomyocytes. (A) Western blot for IGF-1 in heart tissues from control and swim mice (n=6); (B) RT-qPCR for IGF-1 and RELN in heart tissues from control and swim mice(n=6);(C)Western blot for IGF-1 in heart tissues from control and swim mice treated with SAR131675(100 mg/kg/d) or vehicle controls (n=6); (D) RT-qPCR for IGF-1 and RELN in heart tissues from control and swim mice treated with SAR131675(100 mg/kg/d)or vehicle controls (n=6); and ELISA for (E) IGF-1 and (F) RELN levels in LEC-conditioned medium pre-treated with VEGFR3 inhibitor SAR131675 (100 nM) or not(n=4).Independent-sample t test(two-tailed)was used for statistical comparisons between 2 groups. One-way ANOVA test was used for statistical comparisons among 3 groups.Two-way ANOVA followed by Tukey post hoc test was used for statistical comparisons among 4 groups.*p <0.05;**p <0.01; *** p <0.001. ANOVA=analysis of variance; GAPDH=glyceraldehyde-3-phosphate dehydrogenase; IGF-1=insulin-like growth factor-1;LEC=lympathic endothelial cell;OD=optical density; RELN=the extracellular protein Reelin;RT-qPCR=reverse transcription quantitative polymerase chain reaction.
3.6. AKT activation and the C/EBPβ-CITED4 axis contribute to the effect of LEC-conditioned medium on cardiomyocytes
The activation of PI3K-phosphoinositide-dependent protein kinase 1 (PDK1)-AKT signaling pathway plays a key role in physiological cardiac growth.21,33,34Here we first observed that cardiomyocytes treated with LEC-conditioned medium had no expression change in pathological hypertrophy markers including ANP and BNP (Fig. 6A), supporting the hypothesis that cardiomyocytes developed physiological hypertrophy after LEC-conditioned medium treatment. Then we investigated the potential mechanisms with respect to how LEC-conditioned medium promoted the hypertrophy and proliferation of cardiomyocytes.Our results showed that although PI3K expression level was not significantly regulated, PDK1 was markedly upregulated in cardiomyocytes after LEC-conditioned medium treatment (Fig. 6B). By treating cardiomyocytes with AKT inhibitor MK2206, we observed that the promotive effect of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation was obviously attenuated by AKT inhibition (Fig. 6C). This part of functional rescue assay data indicates that AKT activation is necessary for the effect of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation.
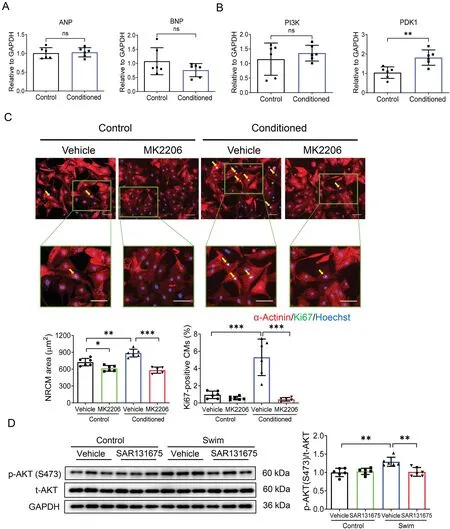
Fig.6. AKT activation contributes to the effects of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation.(A)RT-qPCR for ANP and BNP in NRCMs cultured with control medium or LEC-conditioned medium for 48 h(n=6);(B)RT-qPCR for PI3K and PDK1 in NRCMs cultured with control medium or LEC-conditioned medium for 48 h (n=6); (C) Immunofluorescent staining for Ki67 and α-Actinin in NRCMs cultured with control medium or LEC-conditioned medium in the presence or absence of AKT inhibitor MK2206(n=5-6;scale bar=50 μm);and(D)Western blot for AKT phosphorylation level in heart tissues from control and swim mice treated with SAR131675(100 mg/kg/d)or vehicle controls(n=6).Independent-sample t test(two-tailed)was used for statistical comparisons between 2 groups.Two-way ANOVA followed by Tukey post hoc test was used for statistical comparisons between multiple groups.*p <0.05;** p <0.01; *** p <0.001. AKT=protein kinase B; ANOVA=analysis of variance; ANP=atrial natriuretic peptide; BNP=brain natriuretic peptide;GAPDH=glyceraldehyde-3-phosphate dehydrogenase;LEC=lymphatic endothelial cell;NRCM=neonatal rat cardiomyocyte;ns=not significant;PDK1=phosphoinositide-dependent protein kinase 1;PI3K=phosphatidylinositol 3-kinase;RT-qPCR=reverse transcription quantitative polymerase chain reaction.
Meanwhile,we examined the C/EBPβ-CITED4 axis,which is known for its important contribution to aspects of exerciseinduced physiological cardiac growth.23,35We found that cardiomyocytes treated with LEC-conditioned medium showed downregulation of C/EBPβ and upregulation of CITED4(Fig. 7A). We further conducted functional rescue assays by treating NRCMs with C/EBPβ expression lentivirus or CITED4 small interfering RNA (Supplementary Fig. 3). We observed that the effect of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation was significantly attenuated by C/EBPβ overexpression(Fig.7B)and CITED4 downregulation(Fig. 7C). These data indicate that the C/EBPβ-CITED4 axis contributes to the effect of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation.
Interestingly, we also observed an increased phosphorylation level of AKT in mice hearts upon exercise, which was attenuated by SAR131675 (Fig. 6D). Meanwhile, exerciseinduced downregulation of C/EBPβ and upregulation of CITED4 were also attenuated by SAR131675 in vivo(Fig.7D).These in vivo findings highly support that AKT activation and the C/EBPβ-CITED4 axis contribute to the promotive effects of lymphatics on cardiomyocyte hypertrophy and proliferation.
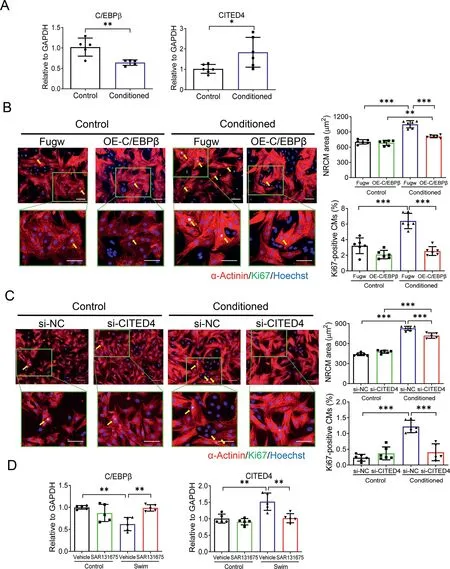
Fig. 7. The C/EBPβ-CITED4 axis is involved in the effects of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation. (A) RTqPCR for C/EBPβ and CITED4 in NRCMs cultured with control medium or LEC-conditioned medium for 48 h(n=5-6);(B)Immunofluorescent staining for Ki67 and α-Actinin in NRCMs cultured with control medium or LEC-conditioned medium in the presence or absence of C/EBPβ expression lentivirus infection(n=6;scale bar=50 μm);(C)Immunofluorescent staining for Ki67 and α-Actinin in NRCMs cultured with control medium or LEC-conditioned medium in the presence or absence of CITED4 small interfering RNA transfection (n=5-6; scale bar=50 μm); and (D) RT-qPCR for C/EBPβ and CITED4 in heart tissues from control and swim mice treated with SAR131675 or vehicle controls(n=5).Independent-sample t test(two-tailed)was used for statistical comparisons between 2 groups. Two-way ANOVA followed by Tukey post hoc test was used for statistical comparisons between multiple groups. * p <0.05; ** p <0.01; *** p <0.001. ANOVA=analysis of variance; C/EBPβ=CCAAT enhancer-binding protein beta; CITED4=CBP/p300-interacting transactivators with E(glutamic acid)/D(aspartic acid)-richcarboxylterminal domain4; GAPDH=glyceraldehyde-3-phosphate dehydrogenase;LEC=lymphatic endothelial cell;NC=negative control;NRCM=neonatal rat cardiomyocyte; OE=overexpression; RT-qPCR=reverse transcription quantitative polymerase chain reaction;si=small interfering.
4. Discussion
In this study, we demonstrated that exercise-induced cardiac adaptive growth was accompanied by a remarkable increase of cardiac lymphangiogenesis. Notably, cardiac lymphangiogenesis was necessary for exercise-induced physiological cardiac hypertrophy and cardiomyocyte proliferation through activation of VEGFR3. We further demonstrated that LEC-conditioned medium promoted both physiological hypertrophy and proliferation of cardiomyocytes and contained higher levels of IGF-1 and RELN, while LEC-conditioned medium from LECs treated with VEGFR3 inhibitor did not have these effects.Finally,we provided evidence that the effects of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation were associated with AKT activation and modulation of the C/EBPβ-CITED4 axis.
Increasing evidence indicates the contributions of impaired lymphangiogenesis to insufficient cardiac lymphatic drainage,chronic myocardial edema and inflammation, and adverse remodeling in different myocardial injuries,36,37while promoting lymphangiogenesis is beneficial in limiting these modifications that occur in cardiovascular pathology.13,31,38,39It was previously reported that exercise, especially eccentric training that can improve cardiac function in patients with cardiovascular disease,40led to increased cardiac lymphatic vessel density in a rat model of downhill running exercise.19Chronic exercise training can induce physiological cardiac growth, which may exert beneficial effects in cardiovascular pathology and diseases.28,41,42First, we were interested to know whether lymphangiogenesis is involved in exerciseinduced physiological cardiac growth. Using a mouse model of swimming exercise meant to induce physiological cardiac growth,we observed a significant increase of lymphangiogenesis, as evidenced by increased density of LYVE-1 positive lymphatic vessels in the heart as well as upregulation of lymphatic markers including LYVE-1 and Podoplanin.These data provide essential evidence showing that exercise-induced physiological hypertrophy is accompanied with increased lymphangiogenesis.
VEGFR3 binds VEGFC and VEGFD,which are key regulators for lymphangiogenesis. The VEGFC/D-VEGFR3 signaling has been reported to be regulated in animal exercise models as well as in human exercise tests.19,43,44In a study of rat downhill treadmill running, a decreased VEGFD mRNA level and lymphatic vessel density were observed in skeletal muscles,while increased VEGFC and VEGFD expression levels and cardiac lymphatic vessels were found in cardiac tissues.19Consistently,we observed upregulated VEGFC and VEGFD as well as VEGFR3 expression levels in the exercised hearts in our study.VEGFC and VEGFD are primary lymphangiogenic factors contributing to lymphatic vascular growth,which can be expressed in most tissues by multiple cell types, including endothelial cells,muscle cells,myofibroblasts,and inflammatory cells.45-47Increasing evidence has shown that VEGFC can be induced in endothelial cells in response to proinflammatory cytokines.48,49Likewise,VEGFD is expressed in the vessel walls and can also be found in damaged muscle fibers and infiltrated macrophages in diabetic skeletal muscles.43Inflammatory cells such as macrophages and dendritic cells can be a rich source of VEGFC and VEGFD.50Additionally, vital cardiomyocytes around the infarct area express VEGFC in myocardial remodeling tissues after infarction.51Indeed, the increased VEGFC and VEGFD expression levels during exercise-induced physiological cardiac growth can be related to multiple cell types,such as endothelial cells, cardiomyocytes, and inflammatory cells. Additionally,VEGFA,via activating VEGFR2 expressed in LECs,have been reported to promote LEC migration and lymphatic vasculature formation in tumors or during wound healing.45,52Although beyond the main objectives of the present study, the role of VEGFA in exercise-induced lymphangiogenesis cannot be excluded. Collectively, our data provide evidence that cardiac lymphangiogenesis with VEGFR3 activation is notably increased during exercise-induced physiological cardiac growth.
To further elucidate whether VEGFR3 activation and lymphangiogenesis contributes to exercise-induced physiological cardiac growth,we applied SAR131675(oral treatment)in the mouse model of swimming exercise. We demonstrated that VEGFR3 inhibition through SAR131675 was able to attenuate exercise-induced cardiac growth. Meanwhile, SAR131675 reduced lymphatic vessel density and downregulated LYVE-1 and Podoplanin expression levels in the heart upon exercise.Interestingly, exercise-induced cardiomyocyte hypertrophy and proliferation were also attenuated in mice treated with SAR131675. Collectively, these data suggest that VEGFR3 activation is necessary for exercise-induced physiological cardiac growth, while VEGFR3 inhibition reduces lymphangiogenesis and attenuates both physiological hypertrophy and proliferation of cardiomyocytes in exercised hearts. These findings propose a critical involvement of cardiac lymphangiogenesis in exercise-induced physiological cardiac growth through activation of VEGFR3.
To advance our understandings of how LECs could regulate cardiomyocyte hypertrophy and proliferation, NRCMs were treated with LEC-conditioned medium. We first observed that LEC-conditioned medium induced a notable hypertrophy and proliferation of cardiomyocytes. It was recently reported that LEC-produced RELN increased the proliferation and survival of cardiomyocytes, while LEC-specific RELN reduction aggravated cardiac remodeling and cardiac dysfunction,which correlated with reduced proliferation and increased apoptosis of cardiomyocytes after myocardial infarction.31Our findings of increased cardiomyocyte proliferation induced by LEC-conditioned medium were consistent with the previous study.Additionally, we demonstrated that LEC-conditioned medium also promoted cardiomyocyte physiological hypertrophy, as evidenced by increased cardiomyocyte area without change of ANP and BNP expression levels. Interestingly, treatment of LECs with VEGFR3 inhibitor abolished the effects of LECconditioned medium on cardiomyocyte hypertrophy and proliferation. These data provide compelling evidence that VEGFR3 is necessary to mediate the effects of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation.
To further explore molecular players mediating the effects of LEC-conditioned medium upon VEGFR-3 manipulation in cultured cardiomyocytes, we evaluated potential mediators in LEC-conditioned medium that may promote cardiomyocyte hypertrophy and proliferation; we also measured their expressions in heart tissues from swimming exercised models in the presence or absence of SAR131675 treatment. Interestingly,we found that IGF-1 (an essential mediator contributing to exercise-induced physiological cardiac growth) and RELN (a recently reported lymphangiocrine factor promoting cardiomyocyte survival and proliferation) were both upregulated in the heart tissues of swimming exercised mice, but that these upregulations were blocked by VEGFR3 inhibitor in the swimming exercised mice. Furthermore, we demonstrated that IGF-1 and RELN levels were higher in LEC-conditioned medium, which was also attenuated by the pre-treatment of LECs with VEGFR3 inhibitor. Indeed, we speculated that exercise-induced increase of IGF-1 and RELN in the heart may mediate the crosstalk between LECs and cardiomyocytes during physiological cardiac growth. Further studies are needed (e.g., studies using LEC-conditional IGF-1 or RELN knockout mice) to provide direct evidence for the roles of IGF-1 and RELN acting as lymphangiocrine factors contributing to exercise-induced physiological cardiac growth.
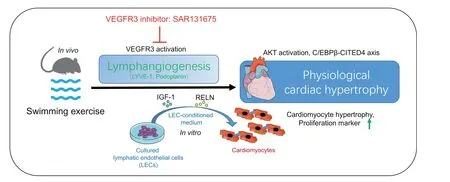
Fig.8. Cardiac lymphangiogenesis is required for exercise-induced physiological cardiac growth by VEGFR3 activation.LEC-conditioned medium promotes both physiological hypertrophy and proliferation of cardiomyocytes through AKT activation and the C/EBPβ-CITED4 axis. AKT=protein kinase B;C/EBPβ=CCAAT enhancer-binding protein beta;CITED4=CBP/p300-interacting transactivators with E(glutamic acid)/D(aspartic acid)-rich-carboxylterminal domain4;IGF-1=insulin-like growth factor-1;LEC=lymphatic endothelial cell;LYVE-1=lymphatic vessel endothelial hyaluronic acid receptor 1;RELN=the extracellular protein Reelin;VEGFR3=vascular endothelial growth factor receptor 3.
AKT activation and the C/EBPβ-CITED4 axis are well known as signaling mechanisms that mediate physiological cardiac growth. Meanwhile, there is crosstalk between AKT and C/EBPβ-CITED4 signaling in regulating cardiomyocyte hypertrophy and proliferation during physiological hypertrophy.53It was previously reported that increased AKT activity significantly reduced C/EBPβ expression in primary cardiomyocytes and regulated its downstream exercise adaptive genes that contribute to the regulation of cardiomyocyte hypertrophy and proliferation.23Moreover, both IGF-1 and RELN have been reported as capable of activating AKT phosphorylation in cardiomyocytes.31,54Here we first examined whether LEC-conditioned medium regulated these signalings in cardiomyocytes.Our results showed that NRCMs treated with LEC-conditioned medium had significantly upregulated PDK1 as well as reduced C/EBPβ and increased CITED4 expression levels. Cardiomyocyte-specific knockdown of PDK1 prevented cardiac hypertrophy upon exercise in mice.33Global or cardiac AKT1 genetic ablation also attenuated exerciseinduced physiological cardiac growth.55,56Additionally,reduction of the transcription factor C/EBPβ and increased CITED4 contributed to exercise-induced cardiac growth and led to physiological cardiac hypertrophy.23,35In our study,we further performed functional rescue assays to examine whether these signalings contribute to the effects of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation. In LEC-conditioned medium culture, NRCMs treated with AKT inhibitor had blunted cell hypertrophy and proliferation.Similar results were seen in NRCMs with C/EBPβ overexpression or CITED4 knockdown. These functional rescue assays provide direct evidence that AKT activation as well as reduced C/EBPβ and increased CITED4 contribute to the promotive effect of LEC-conditioned medium on cardiomyocyte hypertrophy and proliferation.Finally,we observed that in our exercised mice hearts,exercise-induced AKT activation as well as downregulated C/EBPβ and upregulated CITED4 were also attenuated by SAR131675.Thus,these data were integrated to demonstrate that AKT activation and the C/EBPβ-CITED4 axis mediate the promotive effect of lymphatics to physiological cardiac growth aspects including cardiomyocyte hypertrophy and proliferation.
5. Conclusion
Our findings reveal that cardiac lymphangiogenesis is required for exercise-induced physiological cardiac growth by VEGFR3 activation. They also indicate that LEC-conditioned medium promotes both physiological hypertrophy and proliferation of cardiomyocytes through AKT activation and the C/EBPβ-CITED4 axis (Fig. 8). These results highlight the essential roles and molecular mechanisms of cardiac lymphangiogenesis in exercise-induced physiological cardiac growth, and may promote further investigations of the potential contribution of lymphangiogenesis in exercise-mediated myocardial protection.
Acknowledgments
This study was supported by the grants from National Key Research and Development Project (2018YFE0113500 to JX),National Natural Science Foundation of China (82020108002 and 81911540486 to JX,81970335 and 82170285 to YB),Innovation Program of Shanghai Municipal Education Commission(2017-01-07-00-09-E00042 to JX), Science and Technology Commission of Shanghai Municipality (20DZ2255400 and 18410722200 to JX), the “Dawn” Program of Shanghai Education Commission(19SG34 to JX),the Shanghai Rising-Star Program (19QA1403900 to YB), and the Science and Technology Commission of Shanghai Municipality(21SQBS00100 to YB).
Authors’contributions
YB,ZH,XF,LL,MW,YZ,SL,CC,MY,and HJ performed the experiments and analyzed the data;JX conceived the study,participated in its design and coordination,and helped to draft the manuscript.All authors read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Supplementary materials
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.02.005.
杂志排行
Journal of Sport and Health Science的其它文章
- The Journal of Sport and Health Science:Commemorating a decade of publishing milestones and impact
- Prevalence of meeting 24-Hour Movement Guidelines from pre-school to adolescence:A systematic review and meta-analysis including 387,437 participants and 23 countries
- Which psychosocial factors are associated with return to sport following concussion?A systematic review
- Do people with low back pain walk differently?A systematic review and meta-analysis
- Influence of biological sex and exercise on murine cardiac metabolism
- Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p
