Baicalin inhibits inflammation of lipopolysaccharide-induced acute lung injury via toll like receptor-4/myeloid differentiation primary response 88/nuclear factor-kappa B signaling pathway
2022-07-28ZHUChangleFENGCuilingFENGFengYaoXiaoqinWANGGuishuSHILiangtianZHENGJiakun
ZHU Changle,FENG Cuiling,FENG Feng,Yao Xiaoqin,WANG Guishu,SHI Liangtian,ZHENG Jiakun
ZHU Changle,WANG Guishu,SHI Liangtian,ZHENG Jiakun,Department of respiration,Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine,Beijing 100000,China
ZHU Changle,FENG Cuiling,Department of traditional Chinese Medicine,Peking University People's Hospital,Beijing,100000,China
Yao Xiaoqin,Department of Traditional Chinese Medicine,Peking University International Hospital,Beijing 100000,China
Abstract OBJECTIVE:To explore the effect and mechanism of baicalin in the treatment of acute lung injury (ALI) by in vivo and in vitro experiments.METHODS:ALI was induced by instilling 10 mg/mL lipopolysaccharide (LPS) into the airway of rats.Different doses of baicalin (50 and 100 mg·kg-1·d-1) were administered by gavage one day before modeling.RESULTS:Baicalin significantly reduced the permeability of the alveolocapillary membrane,alleviated tissue injury and inflammatory infiltration,and inhibited the secretion of inflammatory factors and the infiltration of neutrophils.The decline in these inflammations was related to the inhibition of the toll like receptor-4 (TLR4)/myeloid differentiation factor 88 (MyD88)/nuclear factor-kappa B(NF-κB)/nod-like receptor pyrin containing 3 (NLRP3)signaling pathway and the mitogen-activated protein kinase (MAPK) signaling pathway.CONCLUSIONS:Baicalin inhibits the secretion of inflammatory factors by inhibiting the TLR4-MyD88-NFκB/NLRP3 pathway and the MAPK signaling pathway.Thus,it reduces lung bronchial epithelial layer,alveolar damage,and pulmonary edema as detected in the in vivo and in vitro experiments.Therefore,baicalin may be a potential preventive and therapeutic drug for ALI.
Keywords:baicalin;acute lung injury;epithelial cells;lipopolysaccharides;inflammation
1.INTRODUCTION
Acute lung injury (ALI) is a common clinical inflammatory syndrome caused by various pathogenic factors,such as infection,trauma,and inhalation of harmful gases,which affects >200 000 people in the US each year,with a mortality rate of 30%-40%.1The clinical manifestations of ALI are hypoxemia and bilateral pulmonary infiltration.The early pathological manifestations of ALI are alveolar-capillary membrane damage,increased permeability,the accumulation of neutrophils into the capillary wall,and the infiltration of inflammatory cells into the lung interstitial and alveoli.2,3Inflammation activates innate and adaptive immunity to eliminate invading pathogens.However,if a body’s inflammatory response is not regulated,the immune system attacks the host’s normal cells,tissues,and organs,necessitating the prompt suppression of the amplification of the inflammatory response.4Lipopolysaccharide (LPS) is a structural component of cell walls in Gram-negative bacteria.The effect of LPS is manifested by the toll-like receptor (TLR) on the cell membrane surface of the host cell and an effective inducer of inflammation.5Moreover,LPS induces a series of complex inflammatory responses by binding to the cell membrane TLR,and the response is involved in a variety of transcription signals,cytokines,and complex regulatory networks.6,7TLRs play a critical role in the innate immune system and can identify pathogenassociated molecular patterns.Toll like receptor-4(TLR4) is one of the most important pathogenic pattern recognition receptors in the TRL family8and a primary receptor that recognizes LPS.Interestingly,LPS interaction with TLR4 activates the TLR4 signaling pathway.In addition,TLR4 activates the myeloid differentiation factor 88 (MyD88)-dependent and tollreceptor-associated activator of interferon (TRIF)-dependent pathways.The phosphorylation leads to the degradation of IκB protein and promotes the nuclear translocation and activation of the nuclear factor-kappa B (NF-κB) cascade.Nod-like receptor pyrin containing 3 (NLRP3) inflammasome is a critical signaling molecule downstream of the TLR4 signaling pathway.TAK1,downstream of the signaling pathway,triggers mitogen-activated protein kinase (MAPK) activating the MAPK signaling pathway.9NF-κB and MAPK signaling pathways play a major role in TLR4-mediated inflammation.The activation of these signaling pathways promotes the expression of related genes,proteins,and proinflammatory cytokines,such as tumor necrosis factor (TNF),chemokines,and interleukins.The release of proinflammatory cytokines further activates the signaling pathways,thereby exaggerating the inflammatory response and releasing an excess of inflammatory factors,such as interleukin (IL)-8/ C-X-C motif chemokine ligand 1 (CXCL1),IL-6,IL-1β,and monocyte chemotactic protein 1 (MCP-1),which trigger a cytokine storm.10,11
The diagnostic criteria for an animal model of ALI include tissue damage,alveolar-capillary barrier damage,inflammatory response,and physiological dysfunction.ALI model can be diagnosed by satisfying three of the four manifestations.12Moreover,lung injury is most severe 24 h after the stimulation when LPS infusion is used for model establishment.13
Previous studies have shown that clarithromycin (CAM)reduces mortality,shortens the time of discontinuation of mechanical ventilation,and reduces the recruitment of neutrophils to the alveolar cavity.14,15Therefore,it is used as a positive control to verify the sensitivity of the test.
Although many drugs can prevent inflammation and ALI in animal studies,clinically effective drug replacement to prevent ALI has not been found.Therefore,new drugs are an urgently requisite.3,16Baicalin (PubChem CID:64982) is a natural small molecule compound of flavonoids,with a high content (about 8%) in Scutellaria baicalensis.17It has a variety of pharmacological effects,including antioxidant,anti-inflammatory,anti-cancer,anti-viral,anti-hyperglycemia,and cancer cell apoptosis effects.18-20
The present study aimed to explore drugs for the prevention and treatment of ALI,especially with respect to the therapeutic effect and improvement of baicalin on LPS-induced ALI and inflammation.The TLR4 and MAPK signaling pathways are under intensive focus in this study.
2.MATERIAL AND METHODS
2.1.Animals
In this study,male Wistar rats (Beijing Charles River Laboratories,China) aged 6-8 weeks were used as experimental objects,maintained in a specific pathogenfree (SPF) laboratory,housed under optimal laboratory conditions [12 h light/ dark cycle,temperature (23 ±2) ℃,humidity 50% ± 5%] and provide unlimited water and food.All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals,and all the procedures were approved by the Biomedical Ethics Committee for animal use and protection of Peking University.
2.2.Reagents
LPS (055:B5) was purchased from Sigma-Aldrich(Louis,MO,USA).Dimethyl sulfoxide (DMSO) was purchased from Lonza (USA).Baicalin (purity ≥ 99%)was obtained from Chengdu Must Biotechnology Co.Ltd.(Chengdu,China).CAM was purchased from Livzon (product specifications:0.25 g/pill).
2.3.Experimental protocols
A total of 100 rats were randomly and equally divided into five groups and administered by gavage 1 d before modeling.The rats in the low-dose baicalin (L-baicalin),high-dose baicalin (H-baicalin),and CAM groups were administered baicalin 50 mg·kg-1·d-1,baicalin 100 mg·kg-1·d-1,and CAM 45 mg·kg-1·d-1by gavage,respectively,as described previously.13,21The control and model groups were administered normal saline 0.1 mL·kg-1·d-1by gavage.13On the second day,the model and the drug groups were administered LPS 10 mg/mL(100 μL) by airway instillation.Rats were anesthetized by inhaling isoflurane,and blood was collected through the abdominal aorta after 24 h of modeling.
2.4.Determination of wet/dry lung ratio
The lung edema and alveolar capillary permeability were evaluated by measuring the wet/dry ratio of the lung lobes.Then,the wet lungs were weighed before baking the tissues in an oven at 60 ℃ for 48 h.The wet/dry ratio of lung tissue=W/D.
2.5.Collection of bronchoalveolar lavage fluid (BALF)and determination of protein concentration
A volume of 10 mL of phosphate-buffered saline (PBS)was injected into the lungs from the trachea.An equivalent volume was slowly injected and repeatedly aspirated five times.The recovery of the lavage fluid was >80%.21
Enzyme-linked immunosorbent assay (ELISA) of BALF BALF supernatants were evaluated using Quantikine™ELISA Kit (R&D Systems,Minneapolis,MN,USA) to determine the level of cytokines released.The selected cytokines included TNF-α,IL-1β,IL-6,CXCL1,and myeloperoxidase (MPO).
2.6.Collection of serum and ELISA
After the intervention,blood was collected from the abdominal aorta,and the supernatant was extracted by centrifugation at 4 ℃ and stored at-80 ℃.The concentration of TNF-α,IL-1β,IL-6,CXCL1,and MPO of each group was detected using the ELISA kit.
2.7.Alcian blue-periodic acid schiff (AB-PAS) staining of lung tissue
The mucin level of lung tissues was detected with the AB-PAS staining kit (Solarbio Life Science).The tissue containing neutral and acidic mucin may be stained bluepurple.
2.8.Histopathological analysis and neutrophil count of lung tissue
The lung lobes perfused with PBS were immersed in 4%paraformaldehyde and fixed for 24 h.After dehydration,the lung tissues were embedded in paraffin.Then,the blocks were sliced into 3-μm-thick sections and stained with hematoxylin and eosin (HE).The pathological changes in bronchi,alveoli,and neutrophil infiltration were observed under a high-power microscope.The number of neutrophils in the alveoli and interstitial were counted under high magnification (× 400),and a minimum of ten fields was observed randomly.
2.9.Immunohistochemical assays of lung tissue
The paraffin sections were dewaxed with xylene and ethanol according to standard procedures.For immuneohistochemistry (IHC) analysis,the sections were incubated with primary antibodies,anti-TLR4 (1∶500,Cat.No.sc-293072,Santa Cruz Biotechnology,Dallas,TX,USA),anti-MyD88 (1∶800,Cat.No.AF5195,Affinity Biosciences,Cincinnati,OH,USA),anti-p-NF-kB p65(Ser536) Ab [1 ∶800,Cat.No.3033,Cell Signaling Technology (CST),USA],anti-NLRP3 Ab (1∶300,Cat.No.ab214185,Abcam,Cambridge,MA,USA),anti-PERK1/2 (Thr202/Tyr204) (1∶300,Cat.No.AF1015,Affinity Biosciences),anti-p-p38 MAPK (Thr180/Tyr182)(1∶1000,Cat.No.AF4001,Affinity Biosciences),and anti-P-JNK1/2/3 (Thr183+Tyr185) (1∶800,Cat.No.AF3318,Affinity Biosciences) and horseradish peroxidase(HRP)-conjugated secondary antibodies (1 ∶ 100,Invitrogen,Grand Island,NY,USA),according to standard procedures.The images were captured using the NanoZoomer 2.0 system (Hamamatsu,Japan).
2.10.Protein isolation and Western blot (WB) analysis of lung tissue
According to the manual,lung tissues were lysed with radioimmunoprecipitation (RIPA) buffer (Solarbio,Beijing,China) to extract total proteins.Denatured protein samples were separated by 8% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes.Subsequently,the membrane was blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween 20(TBST) for 2 h,probed with primary antibodies,such as anti-TLR4 (1∶1000,Cat.No.sc-293072,Santa Cruz Biotechnology),anti-MyD88 (1∶1000,Cat.No.AF5195,Affinity Biosciences),anti-TRIF (1∶1000,Cat.No.4596,CST),anti-NF-κB p65 (1∶2000,Cat.No.8242,CST),anti-p-NF-kB p65 (Ser536) (1∶800,Cat.No.3033,CST),anti-NLRP3 Ab (1∶300,Cat.No.ab214185,Abcam),anti-p44/42 MAPK (Erk1/2) (1∶1000,Cat.No.9102,CST),anti-P-ERK1/2 (Thr202/Tyr204) (1∶300,Cat.No.AF1015,Affinity Biosciences),anti-P38 MAPK Ab (1∶1000,Cat.No.9212,CST),anti-p-p38 MAPK(Thr180/Tyr182) (1∶1000,Cat.No.AF4001,Affinity Biosciences),anti-SAPK/JNK (1∶1000,Cat.No.9252,CST),anti-P-JNK1/2/3 (Thr183+Tyr185) (1∶800,Cat.No.AF3318,Affinity Biosciences),and GAPDH (1∶10000,Cat.No.60004-1-Ig,Proteintech,USA),at 4 ℃overnight,followed by incubation with HRP-conjugated goat anti-mouse IgG (H+L) (1∶5000,Cat.No.SA00001-1,Proteintech) secondary antibody for 1 h at room temperature.Finally,the immunoreactive bands were scanned with a ChemiDoc chemiluminescence imaging system (Bio-Rad,USA) and analyzed using the Image J system.
2.11.BEAS-2B cell culture
BEAS-2B (ATCC®CRL9609TM),a type of normal human bronchial epithelial cells,were selected for the present study.LPS was used to stimulate the cells to induce acute epithelial cell injury.Baicalin was applied to explore the effect of the molecule on the relief of acute epithelial cell injury.
LPS was solubilized in PBS to prepare 5 mg/mL stock solution and stored at 4 ℃.Clarithromycin is used as a positive control to verify the sensitivity of the test.In the treatment of lung diseases,CAM at 10 μg/mL is reported as the average therapeutic concentration and the highest non-lethal concentration.22,23When the baicalin concentration was 5 and 10 μg/mL,the cellviability was >90%.LPS (50 μg/mL) is a positive stimulant for acute cell injury,as detected before.CAM 10 μg/mL,Lbaicalin 5 μg/mL,and H-baicalin 10 μg/mL is the therapeutic concentration for acute cell injury by diluting the solution with BEGM.23LPS and drug intervention were performed when the cells enter the logarithmic growth phase,and the cytokine secretion was detected at confluency.
The cells reached the logarithmic growth phase on day 3.Before LPS intervention,the medium was changed to BEBM without a growth factor for 24 h.On day 5,except for the control group,the cells in the other groups were exposed to LPS (50 μg/mL) for 24 h.On day 6,the cells reached confluency and were at a similar growth stage.The model group was continually exposed to LPS(50 μg/mL).The medication group was exposed to LPS(50 μg/mL) and treated with low-dose baicalin (L-baicalin,5 μg/mL),high-dose drugs (H-baicalin,10 μg/mL),and CAM (10 μg/mL),respectively.The culture supernatants were harvested at 6,18,and 24 h after the treatment for Meso Scale Discovery (MSD) assay.BEAS-2B cells were also harvested for WB and qPCR analysis at 24 h after the treatment with LPS and drugs.The experimental procedures were as follows.
2.12.Determination of cytokines in the supernatant by MSD
The MSD cytokine assay is a rapid and convenient method for measuring the levels of multiple factors in a small-volume sample.Multiplex assays were carried out using the V-PLEX Human Cytokine 8-Plex Kit (Meso Scale Diagnostics,Rockville,MD,USA) to estimate the levels of the following cytokines:GM-CSF,IL-8,IL-6,TNF-α,interferon-γ (IFN-γ),IL-1β,IL-10,IL-4,IL-2,and IL-12.The levels were determined using a MESOTM QuickPlex SQ 120 (Meso Scale Diagnostics).
2.13.Cell protein isolation and WB analysis
After drug intervention,the cell culture supernatant was collected,and the cells were washed two times with 2 mL of pre-chilled PBS.The protein concentration of the cell lysate was detected by the DC protein assay kit and separated on 8% SDS-PAGE.The WB of the lung tissue was carried out as per the standard procedure.The expression of TLR4,TRIF,MyD88,NF-κB,p-NF-κB,and NLRP3 proteins was detected using the following primary antibodies at 4 ℃ overnight:anti-TLR4 (1∶1000,Cat.No.sc-293072,Santa Cruz Biotechnology),anti-MyD88 (1 ∶1000,Cat.No.AF5195,Affinity Biosciences),anti-TRIF (1∶1000,Cat.No.4596,CST),anti-NF-κB p65 Ab (1∶2000,Cat.No.8242,CST),antip-NF-kB p65 (Ser536) (1∶800,Cat.No.3033,CST),anti-NLRP3 Ab (1∶300,Cat.No.ab214185,Abcam),and GAPDH (1 ∶10000,Cat.No.60004-1-Ig,Proteintech).
2.14.Determination of mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR)
E.Z.N.A.®Total RNA Kit I (Omega,BioTek,USA) was used to extract total RNA from BEAS-2B cells,according to the manufacturer’s instructions.ReverTra Ace qRT-PCR Kit (Toyobo,Japan) was used to synthesize the cDNA template for Real-time PCR according to the instruction manual.Single-stranded cDNA products were used as templates for PCR.GADPH was the normalized internal reference gene.The primers are listed in Table 1.
2.15.Evaluation of IL-8 chemotactic ability by Transwell assay
BEAS-2B cells were seeded in 12-well plates (Corning,NY,USA) at a density of 3 × 104cells/cm2.At the logarithmic growth phase,the cells were intervened with LPS (LPS 50 μg/mL) for 24 h and then treated with Lbaicalin (5 μg/mL),H-baicalin (10 μg/mL),and CAM(10 μg/mL) for 24 h.
Peripheral blood neutrophils were isolated from healthy adult volunteers using a human peripheral blood neutrophils separation kit (Solarbio,USA).The migration experiment was performed with a Transwell insert (Corning) with 3-μm pore diameter,6.5-mm diameter,and 10-μm-thick porous membrane.Neutrophils (20 × 104in 200 μL) were added to the upper chamber and allowed to migrate towards the lower chamber for 30 min at 37 ℃ and 5% CO2.Then,the number of neutrophils that migrated to the lower chamber was counted using a hemocytometer.
2.16.Statistical analysis
SPSS 25.0 (SPSS Inc.,Chicago,IL,USA) was used for statistical analysis.The data were represented as mean ±standard deviation ().When normality and homogeneity of variance assumptions are satisfied,a one-way analysis of variance (ANOVA) was applied;otherwise,the equivalent non-parametric test was used.Pairwise comparisons were carried out using the least significant differencet-test.P<0.05 indicated statistically significant difference.
3.RESULTS
3.1.Alterations in the permeability of the alveolocapillary membrane
The wet/dry ratio of lung weight was upregulated significantly in the rats treated with LPS (Table 2),and the ratio was higher than the control,baicalin,and CAM groups (P<0.05).Moreover,compared to the L-baicalin group,H-baicalin remarkably reduced the weight of wet lungs.The protein concentration of BALF is upregulated in the rat treated with LPS (Table 2),and the concentration is higher than that of the control,baicalin,and CAM groups (P<0.05).According to visual observation,a large number of protein fragments were detected in the alveolar cavity in the LPS group (the green arrow points to the protein fragments),and baicalinreduced protein infiltration.Our results showed that baicalin reduces the permeability of the alveolocapillary membrane of ALI and alleviates pulmonary edema.

Table 1 RT-PCR primer sequences
Table 2 Alterations in the permeability of the alveolocapillary membrane ()

Table 2 Alterations in the permeability of the alveolocapillary membrane ()
Notes:L-baicalin:low-dose baicalin group,H-baicalin:high-dose baicalin group,CAM:Clarithromycin group,Model group:Lipopolysaccharide(LPS).The rats in the low-dose baicalin (L-baicalin),high-dose baicalin (H-baicalin),and CAM groups were administered baicalin 50 mg·kg-1·d-1,baicalin 100 mg·kg-1·d-1,and CAM 45 mg·kg-1·d-1 by gavage,respectively,as described previously.The control and model groups were administered normal saline 0.1 mL·kg-1·d-1 by gavage.On the second day,the model and the drug groups were administered LPS 10 mg/mL(100 μL) by airway instillation.aP <0.01,bP <0.05,compared to the model group.
3.2.Tissue injury and inflammatory infiltration
LPS instillation triggered lung tissue damage and epithelial edema,while baicalin remarkably reduced the damage.Moreover,the treatment of baicalin in rats alleviates cilia adhesion and lodging and loss of epithelial cells from rats with ALI (Supplementary Figure 1A).LPS instillation intervention had an apparent effect on the severity of ALI.Conversely,baicalin markedly reduced mucus secretion and improved lung damage and lung edema (Supplementary Figure 1B).Compared to the LPS group,baicalin and CAM reduced the thickness of the alveolar wall and protein fragments in the alveolar cavity.The rats intervened with LPS showed a large amount of neutrophil granulocyte migration to the alveolar interstitial and alveolar space,while baicalin inhibited the migration of neutrophil granulocyte (the green arrow points to the protein fragments) (Supplementary Figure 1C,Figure 1).Taken together,the pathological results showed that baicalin is the key drug for treating lung tissue injury and inflammatory infiltration in rats with ALI.
3.3.Baicalin inhibits inflammatory factor secretion and the infiltration of neutrophils in ALI
The inflammatory factors,CXCL1,IL-6,IL-1β,TNF-α,and MPO,were upregulated by LPS intervention in BALF and serum.Baicalin significantly reduces the secretion of inflammatory factors and MPO in a dosedependent manner (Tables 3,4).Although how baicalin relieves inflammation is not yet clarified,it reduces the severity of ALI with respect to inflammatory factor secretion.
Table 3 Infiltration of inflammatory factors and MPO in BALF ()

Table 3 Infiltration of inflammatory factors and MPO in BALF ()
Notes:L-baicalin:low-dose baicalin group,H-baicalin:high-dose baicalin group,CAM:Clarithromycin group,Model:Lipopolysaccharide group,BALF:Bronchoalveolar Lavage Fluid.The rats in the low-dose baicalin (L-baicalin),high-dose baicalin (H-baicalin),and CAM groups were administered baicalin 50 mg·kg-1·d-1,baicalin 100 mg·kg-1·d-1,and CAM 45 mg·kg-1·d-1 by gavage,respectively,as described previously.The control and model groups were administered normal saline 0.1 mL·kg-1·d-1 by gavage. On the second day,the model and the drug groups were administered LPS 10 mg/mL (100 μL) by airway instillation.Changes in CXCL1 (Chemokine Ligand 1),IL-6 (Interleukin 6),IL-1β(interleukin-1β),TNFα (tumor necrosis factor-α),and MPO (myeloperoxidase) concentration in the unstimulated control group and LPS group in response to LPS,L-baicalin group in response to LPS and baicalin,CAM group in response to LPS and CAM in BALF from rats.aP<0.01,bP <0.05,compared to the model group for each group.
Table 4 Secretion of inflammatory cytokines and MPO in the serum ()

Table 4 Secretion of inflammatory cytokines and MPO in the serum ()
Notes:L-baicalin:low-dose baicalin group,H-baicalin:high-dose baicalin group,CAM:Clarithromycin group,Model group:Lipopolysaccharide.The rats in the low-dose baicalin (L-baicalin),high-dose baicalin (H-baicalin),and CAM groups were administered baicalin 50 mg·kg-1·d-1,baicalin 100 mg·kg-1·d-1,and CAM 45 mg·kg-1·d-1 by gavage,respectively,as described previously.The control and model groups were administered normal saline 0.1 mL·kg-1·d-1 by gavage.On the second day,the model and the drug groups were administered LPS 10 mg/mL (100 μL) by airway instillation.CXCL1 (C-X-C motif chemokine ligand 1),IL-6 (interleukin-6),IL-1β (interleukin-1β),TNF-α(tumor necrosis factor-α),and MPO (Myeloperoxidase).Changes in CXCL1,IL-6,IL-1β,TNF-α,and MPO production in the serum from rats.aP <0.01,bP <0.05,compared to the model group for each group.
3.4.Baicalin alleviates the inflammation of ALI via TLR4-TRIF/MyD88-NF-κB signaling pathway and NLRP3
The current results showed that baicalin significantly reduced the phosphorylation of NF-κB and the expression of TLR4 and MyD88 proteins (Figure 2,3).Combined with the results of BALF and serum inflammatory factor detection,baicalin relieves the secretion of inflammatory factorsviathe TLR4 signaling pathway.Since the effect of baicalin on TRIF is not obvious,it is speculated that baicalin mainly transmits the inflammatory stimulation through the MyD88 adaptor.

Figure 1 Tissue injury and inflammatory infiltration
The findings demonstrated that baicalin remarkably suppressed the expression of NLRP3 protein (Figure 2E).Thus,baicalin is capable of regulating the NF-κB and NLRP3 proteins to modulate IL-1β production.
3.5.Baicalin alleviates the inflammation of ALI via MAPK signaling pathway
The current study showed that baicalin treatment inhibits ERK and p38 phosphorylation and has no effect on JNK phosphorylation (Figures 4,5),suggesting that baicalin may play a role in the MAPK signaling pathway.Combining the results of inflammatory factors detection,we speculated that baicalin inhibits the secretion of inflammatory factors,such as IL-6 and TNF-α throughthe MAPK signaling pathway,wherein p-ERK and p-p38 play a significant role but JNK does not inhibit inflammation significantly.
3.6.Effects on cell morphology
At 400 × high-power microscopes,compared to the control group,the epithelial cells stimulated by LPS for 48 h showed edema,cell death,and acute damage.However,cell edema and death are remarkably reduced after the treatment of baicalin and CAM for 24 h(Supplementary Figure 2).
3.7.Baicalin inhibits cytokine secretion in the cell culture supernatant
The cytokines,IL-8,IL-6,TNF-α,IFN-γ,IL-1β,and GM-CSF,were upregulated by LPS treatment in BEAS-2B cells at 8,16,and 24 h.Compared to the model group,baicalin and CAM inhibit the secretion of IL-8,IL-6,TNF-α,IFN-γ,IL-1β,and GM-CSF at 16 and 24 h,respectively (Supplementary Figure 3).Among these,the H-baicalin group has the best impact on inhibiting IL-6 and TNF-α secretion,and CAM and H-baicalin had the best effect on inhibiting IL-8 secretion.Due to the limitation of the detection limit of the assay kit,we cannot detect the concentration of IL-10,IL-4,IL-2,and IL-12.
3.8.Baicalin inhibits TLR4-TRIF/MyD88-NF-κB/NLRP3 protein expression in epithelial cells
The results demonstrated that LPS-induced epithelial cell injury increases the expression of TLR4,MyD88,p-NF-κB,and NLRP3 proteins,whereas baicalin and CAM inhibit the expression of these proteins to varying degrees,thereby reducing epithelial cell injury (Figure 6).

Figure 2 Effects of baicalin on inhibiting the protein expression of TLR4-TRIF/MyD88-NF-κB pathway signaling and NLRP3 in lung tissues detected by Western blotting

Figure 3 Effects of baicalin on inhibiting the expression of TLR4-TRIF/MyD88-NF-κB pathway and NLRP3 protein in lung tissues detected by immune-ohistochemistry analysis

Figure 4 Effects of baicalin on inhibiting MAPK pathway protein expression in lung tissues detected by Western blotting

Figure 5 Effects of baicalin on inhibiting MAPK pathway protein expression in lung tissues detected by immunohistochemical
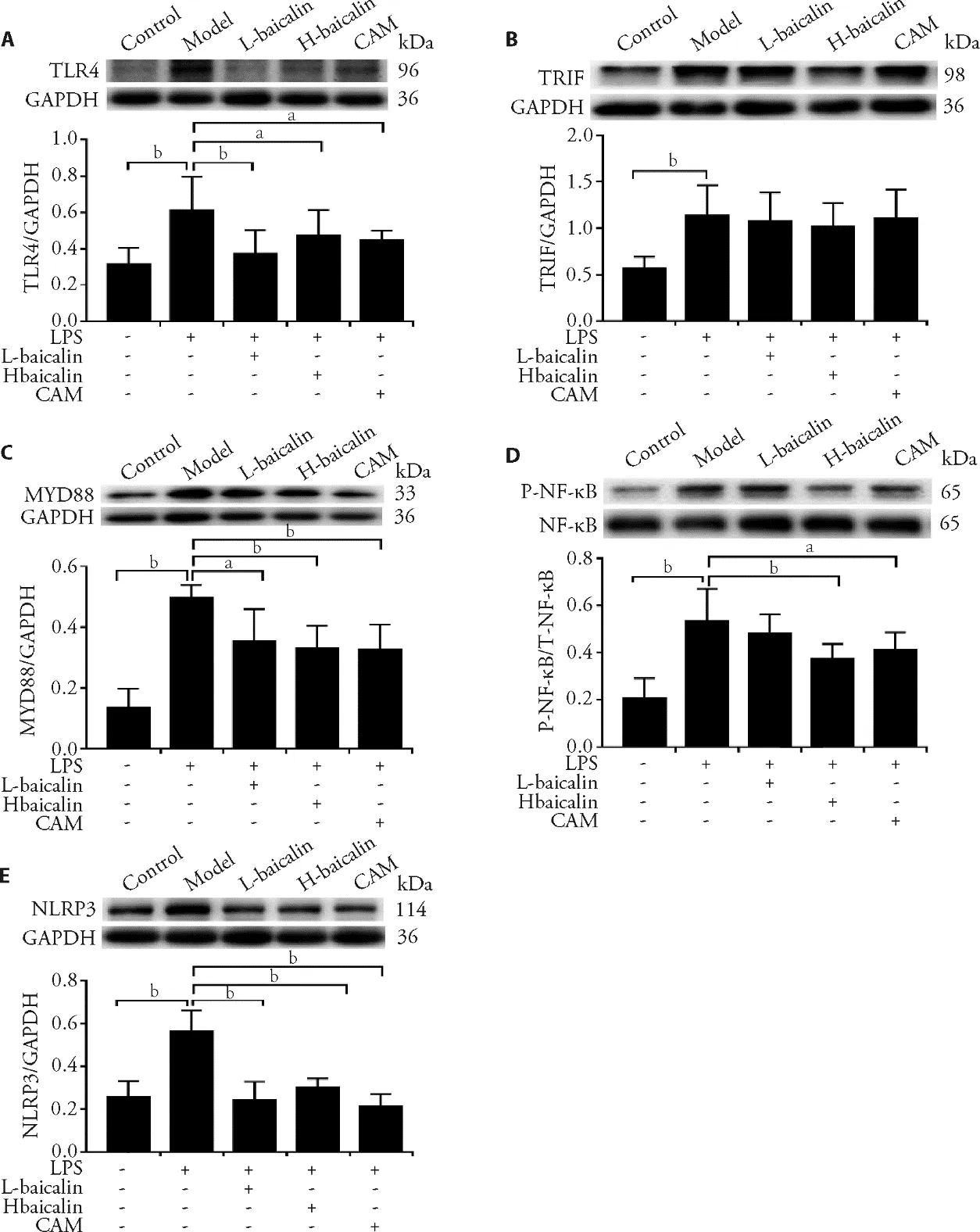
Figure 6 Effects of baicalin on inhibiting TLR4-TRIF/MyD88-NF-κB/NLRP3 pathway protein expression in epithelial cells
3.9.Baicalin inhibits TLR4-MyD88-NF-κB pathway mRNA expression in epithelial cells
The data showed that the expression levels of TLR4 and MyD88 in LPS-stimulated cells are significantly upregulated,while NF-κB gene expression is significantly reduced (Table 5).Baicalin reduced the expression of TLR4 and MyD88 genes and increased the expression of the NF-κB gene.This phenomenon is consistent with the results of protein detection.Baicalin inhibits the expression of TLR4 and MyD88 genes and proteins at the same time and inhibits NF-κB phosphorylation but has no obvious inhibitory effect on the TRIF gene.
Table 5 Effects of baicalin on inhibiting TLR4-MyD88-NF-κB pathway mRNA expression in LPS-induced epithelial cells ()

Table 5 Effects of baicalin on inhibiting TLR4-MyD88-NF-κB pathway mRNA expression in LPS-induced epithelial cells ()
Notes:control:epithelial cells cultured with BEGM for 48 h.Model:epithelial cells treated with LPS (Lipopolysaccharide) 50 μg/mL for 48 h.L-baicalin:low-dose baicalin group,epithelial cells treated with LPS 50 μg/mL for 48 h and baicalin 5 μg/mL for 24 h.H-baicalin:high-dose baicalin group,epithelial cells treated with LPS 50 μg/mL for 48 h and baicalin 10 μg/mL for 24 h.CAM:clarithromycin group,epithelial cells treated with LPS 50 μg/mL for 48 h and CAM 10 μg/mL for 24 h.TLR4:toll like receptor-4;TRIF:toll-receptor-associated activator of interferon;MyD88:myeloid differentiation factor 88;NF-κB:nuclear factor-kappa B.Expression of TLR4,TRIF,MyD88,and NF-κB genes of the epithelial cells in the control group,LPS group,L-baicalin group,H-baicalin group,and CAM group.aP <0.01,bP <0.05,compared to the model group for each group.
3.10.Baicalin inhibits IL-8 to chemotactic neutrophils in LPS-induced epithelial cells
The data indicated that LPS induces epithelial cell damage and produces abundant chemokines,such as IL-8.The results showed that the epithelial cells stimulated by LPS chemotactic more IL-8 like 8.24 × 104,and the difference compared with control group was significant(P <0.01).Baicalin and CAM reduce the secretion and inhibit the chemotaxis of the inflammatory factor IL-8.High-dose baicalin inhibited the migration of IL-8 like 3.15 × 104,and the difference compared with model group was significant (P <0.01).
4.DISCUSSION
Eliminating pulmonary edema and reducing lung inflammation are essential treatments for alleviating ALI.In this study,we investigated the effects of baicalin on LPS-induced ALI in thein vivoandin vitroexperiments.As expected,baicalin significantly alleviated the permeability of the alveolocapillary membrane of ALI and reduced the protein concentration in BALF.As a result,baicalin remarkably reduced pulmonary edema.The activation of neutrophil infiltration could be inferred by measuring the activity of MPO.24Penget al25showed that in chickens administered baicalin remarkably alleviated LPS-induced MPO production in serum and BALF.Previous studies have shown that suppressing the amount of polymorphonuclear neutrophil leukocytes(PMN) can alleviate ALI.26In addition,baicalin alleviates the expression of inflammatory factors and MPO in LPS-stimulated ALI to avoid exaggerating inflammation and the activation of PMN.Also,baicalin reduces LPS-induced lung bronchial epithelial damage and mucus secretion,thereby decreasing the invasion and retention of substances such as exogenous bacteria and viruses.Simultaneously,baicalin reduces LPS-induced alveolar damage,alveolar wall thickening,and protein debris deposition in the alveolar cavity.Taken together,baicalin significantly inhibited the migration of PMN into the alveolar stroma and alveolar cavity,inhibited the secretion of inflammatory factors and the occurrence of inflammatory storms,and reduced lung bronchial epithelial cells and alveolar-capillary membrane damage.
Corresponding to the experimentsin vivo,baicalin reduces cell edema,death,and the secretion of LPSinduced inflammatory factors,such as IL-6,IL-8,GMCSF,INF-γ,IL-12,and TNF-α.This phenomenon was consistent with the effect of baicalin inhibiting LPSinduced airway epithelial cell injury and inflammatory factor secretion in rats.Donget al.indicated that baicalin significantly reduces cell damage and inflammatory factor secretion of LPS-stimulated bronchial epithelial cells.27In addition,IL-8 is a critical neutrophil chemokine.28The results indicated that LPS induces epithelial cell damage and produces a large number of chemokines,such as IL-8 and GM-CSF.Conversely,baicalin inhibits the chemotaxis of IL-8,thereby reducing the damage of LPS to epithelial cells and inhibiting the outbreak of inflammatory storms.This phenomenon is consistent with baicalin reducing the infiltration of inflammatory cells into the alveolar interstitial and alveolar spacein vivoexperiments.
LPS binds to TLR4 and activates the TLR4 signaling pathway.The downstream pathway of TLR4 mainly includes two branches:MyD88-and TRIF-dependent signaling pathways,which activate the downstream factors.Among these,the transcription factor NF-κB is one of the most critical effects.Based on the activation of this pathway,NF-κB is phosphorylated and translocated into the nucleus,leading to the release of proinflammatory cytokines,such as TNF-α and IL-8.Subsequently,the release of proinflammatory cytokines further strengthens the activation of the TLR4 signaling pathway,prompting the pathway to activate additional effectors,such as the JNK,p38,and ERK,which in turn triggers the secretion of multiple proinflammatory cytokines,such as IL-6,IL-1β,and MCP-1.The further activation of the signaling pathway effectuated an inflammatory factor storm and exaggerated the inflammatory response.29,30
The present study showed that LPS stimulation elevates MyD88 expression in rats,indicating that MyD88 plays a critical role in the inflammatory outbreak of LPSinduced ALI.The intervention of baicalin remarkably inhibited the expression of MyD88.These results are consistent with those from previous studies.For example,Oshikawaet al31indicated that LPS stimulation increases the MyD88 expression in thein vivoandin vitroexperiments,and baicalin relieves the damage of rat microglia and the production of proinflammatory cytokines caused by oxygen-glucose deprivation.TRIF is mainly involved in the regulation of TRIF-dependent pathways in the TLR4 signaling pathway.In this study,baicalin significantly altered the expression of TRIF.
IκB binds to NF-κB under physiological conditions.Under pathological conditions,the dissociation of IκB and NF-κB leads to phosphorylation and translocation of NF-κB to the nucleus,causing the production of a large number of cytokines.32This study indicated that baicalin remarkably inhibits phosphorylation and nuclear translocation of NF-κB.
The NLRP3 inflammatory body is a crucial downstream effector in the TLR4-mediated inflammatory pathway and a key factor inducing the secretion of proinflammatory cytokines.33When the TLR4 signaling pathway is activated,NF-κB activates the NLRP3 inflammatory bodies and causes a large amount of IL-1β production.34The results of the current study indicated that baicalin intervention inhibits the increase in LPSinduced NLRP3 expression.The correspondingin vivoexperiments revealed the therapeutic effect of baicalin on LPS-induced bronchial epithelial cell injury.The findings showed that baicalin significantly inhibited the expression of TLR4,MyD88,p-NF-κB,and NLRP3 proteins in LPS-induced epithelial cells,reducing epithelial cell damage.Consistent with protein expression,baicalin inhibits the increase in TLR4 and MyD88 gene expression without remarkably affecting the expression of TRIF gene.These findings are consistent with recent reports,35in which baicalin blocks the TLR4 signaling pathway,inhibits the expression of TLR4 and MyD88 proteins and genes,and mitigates inflammatory responses in rat periodontitis.In summary,these results showed that baicalin blocks the expression of effector proteins and genes by inhibiting TLR4/MyD88/NF-κB/NLRP3 signaling pathway activation.Thus,it suppresses the outbreak of inflammation,reduces cell damage and edema,and relieves LPS-induced ALI.
The MAPK pathway is one of the signaling cascades downstream to the TLR4-mediated inflammation pathway,which mainly includes three proteins,like ERK,p38,and JNK.36Some studies have shown that baicalin relieves the severity of arteriosclerosis in mice by reducing the phosphorylation levels of JNK,p-38,and in the MAPK signaling pathway.37Our data suggested that baicalin relieves LPS-induced inflammation by inhibiting ERK and p38 phosphorylation.Typically,baicalin intervention inhibited the TLR4-MyD88-NF-κB/NLRP3/MAPK signaling pathway,thereby inhibiting ALI inflammation.
In summary,this study showed that baicalin inhibits the secretion of inflammatory factors and neutrophil infiltration by inhibiting the TLR4-MyD88-NF-κB/NLRP3 pathway,as assessed in thein vivoandin vitroexperiments.It also inhibits the MAPK signaling pathway,which is related to the ERK and P38 proteins,thereby reducing lung bronchial epithelial and alveolar damage and decreasing pulmonary edema.Therefore,baicalin has the potential to treat ALI.
5.ACKNOWLEDGMENTS
The authors acknowledge the assistance of the Central Laboratory and the Animal Laboratory of Peking University People’s Hospital.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Acupoint application therapies for essential hypertension:a systematic review and Meta-analysis
- Biosynthesis of titanium dioxide nanoparticles using Hypericum perforatum and Origanum vulgare extracts and their main components,hypericin and carvacrol as promising antibacterial agents
- Protective effect of resveratrol on rat cardiomyocyte H9C2 cells injured by hypoxia/reoxygenation by regulating mitochondrial autophagy via PTEN-induced putative kinase protein 1/Parkinson disease protein 2 signaling pathway
- Efficacy of aqueous extract of flower of Edgeworthia gardneri (Wall.)Meisn on glucose and lipid metabolism in KK/Upj-Ay/J mice
- Effect of manipulation on cartilage in rats with knee osteoarthritis based on the Rho-associated protein kinase/LIM kinase 1/Cofilin signaling pathways
- Modified Gexiazhuyu decoction (膈下逐瘀汤加减方) alleviates chronic salpingitis via p38 signaling pathway
