Ultra-transparent nanostructured coatings via flow-induced one-step coassembly
2022-07-26JingjingLiuSoniChvezHoDingMriFrooquiZiliHouShronLinThomsAuriJuliKennedyAnnMrieLChnceLuyiSun
Jingjing Liu ,Soni E.Chvez ,Ho Ding,b ,Mri M.Frooqui ,Zili Hou,b ,Shron Lin ,Thoms D.D'Auri,b,Juli M.Kennedy,b,Ann Mrie LChnce,b,Luyi Sun,b,c,*
a Polymer Program,Institute of Materials Science,University of Connecticut,Storrs,Connecticut,06269,United States
b Department of Chemical & Biomolecular Engineering,University of Connecticut,Storrs,Connecticut,06269,United States
c Department of Biomedical Engineering,University of Connecticut,Storrs,Connecticut,06269,United States
Keywords:Nanocoating Coassembly Polyvinyl alcohol Laponite
ABSTRACT Polyvinyl alcohol (PVA)/laponite (LP) nanocomposite coatings were fabricated via a facile one-step coassembly process.The formed nanocoatings contain a high concentration of LP nanosheets,which can be well aligned along the substrate surface during the coassembly process.Due to the highly orientated structure,the flexible nanocoatings exhibit ultra-high transparency and superior mechanical properties,and can also act as excellent gas barriers.Such nanocoatings can be exceptional candidates for a variety of applications,such as food packaging.
1.Introduction
To address partially the processing and morphology challenges in conventional polymer nanocomposites,polymer/nanosheet nanocomposite coatings(nanocoatings)with a nanoscale structure have been extensively investigated in the past decade and applied for surface protection[27,28],surface modification[29,30],and surface functionalization[31].A desirable nanocoating is one with a well-defined nanostructure that generates unique properties,as well as a sub-micron thickness that brings cost-effectiveness in practical usage.Such nanocoatings with outstanding mechanical[32],barrier[33],optical[34],and flame retardant [33,35] properties have found applications in various fields including food packaging[36,37],flexible electronics[38–40],batteries[41],and solar cells[42,43].
A number of approaches have been developed to fabricate nanocoatings including layer-by-layer (LbL) assembly [28,34,44–46],electrophoretic deposition[47,48],and vacuum-assisted filtration[49,50].Among them,LbL assembly has been intensively studied as a unique coating technique[44]to fabricate high-performance hybrids with a high content of inorganic phase and tunable composition.However,the practical application of LbL assembly has been relatively limited due to the laborious and time-consuming process,which prevents scale-up.Similarly,electrophoretic deposition has weaknesses of low throughput and a less-than-optimal interface,leading to low mechanical properties[47].Although vacuum-assisted filtration is a facile process,defects and a poor alignment are likely to form due to the dynamics of the filtration process caused by the high pressure,high suspension viscosity,and interactions among nanofillers[49].As a result,it is highly desirable to develop a new technique to combine facile nanostructure fabrication and high efficiency for the industry.
A facile one-step coassembly process has recently been developed to fabricate hybrid nanocoatings containing a high concentration of wellaligned nanosheets by taking advantage of flow-induced orientation[33].By tailoring the interfacial interaction between the polymer chains and inorganic nanosheets through such a simple coating technique,a highly-orientated structure can be achieved and the formed nanocoatings exhibit high mechanical [33],barrier [33,51],and flame retardant properties[33,52–54].In order to investigate the fundamental mechanism of the coassembly process and explore extended applications in optical and flexible electronics [55],optical and gas barrier properties are key aspects to improve.LP,a synthetic hectorite with similar physicochemical properties of natural clays such as excellent chemical inertness and high adsorption[56],is an ideal candidate for the extended applications of the nanocoatings.LP has a 2:1 phyllosilicate structure with an empirical formula of Si8[Mg5.5Li0.4H4.0O24.0]0.7-[Na0.7]0.7+[57].It has a disk-like platelet shape with a diameter in the range of ca.20–35 nm and a thickness ca.1 nm[58,59].Unlike many natural clay,LP is virtually colorless in an aqueous suspension.As such,LP and its composites have found a wide range of applications[10,60–62],but particularly in optical devices[38,63],and nanocomposites with photochemical behavior[64]which require a high transparency.
The major objective of this project is to study the role of LP on the structure and performance of the fabricated PVA/LP nanocoatings via the facile one-step coassembly technique.An extremely high concentration(up to 70 wt%)of LP nanosheets was successfully aligned in the hybrid nanocoating,which led to exceptional performance.
2.Experimental
Polyvinyl alcohol (PVA) (Mowiol® 8–88,MW:~67,000,86.7–88.7 mol.%hydrolysis)was acquired from Kuraray America Inc.Laponite-RD(BYK Additives Inc.),glutaraldehyde(GA)(50%aqueous solution,Sigma Aldrich),and HCl (37%,Sigma Aldrich) were used as received.Polyethylene terephthalate (PET) films with a thickness of 24 μm were obtained from Toray Plastics(America),Inc.
A 10.0 wt % PVA aqueous solution was prepared by dissolving PVA pellets in water at 90°C,which was used as a stock solution.LP was uniformly dispersed in water with the assistance of stirring for 1 h.A predetermined amount of PVA stock solution was added to the LP dispersion during stirring to achieve a uniform aqueous dispersion.While the mass concentration of LP in the mixture of LP and PVA was varied from 10 to 70 wt %,the total weight concentration of LP and PVA in the aqueous dispersion was maintained at 1.5 wt %.The mixture was stirred for 30 min and ultrasonicated briefly to ensure uniformity.A small amount of crosslinking agent glutaraldehyde (GA) was added to the mixture.The mole ratio of GA to the number of–OH groups on PVA chains was 1:20.HCl was used as the catalyst for crosslinking reaction,with 1:5 of the mole ratio of HCl to GA.Coatings without crosslinking were also prepared as controls.
PET films with dimensions of ca.15 cm × 20 cm were dip-coated using a PVA/LP dispersion (containing GA and HCl) four times,and rotated 180°after every step of coating to smooth out the thickness gradient.The coated films were then hung vertically and dried in an oven at 60°C.The final coated samples were labeled as PET-PVA/LP-x-C/N,in whichxrepresents the mass percentage of LP to the total weight of LP and PVA,C refers to crosslinking,and N represents non-crosslinked samples prepared as a control.
The layered structure of the nanocoatings was characterized by X-ray diffraction (XRD,Bruker D5,30 kV and 40 mA) with a graphite monochromator with Cu Kαradiation(λ=0.15406 nm).The UV–Vis spectra of the coated PET films were collected using a UV–visible spectrophotometer (CARY 100,Varian).The Fourier transform infrared spectroscopy(FTIR) spectra of freestanding nanocoating films were recorded on a Nicolet spectrophotometer(Magna IR-560).Mechanical property testing was also conducted on freestanding nanocoating films using a dynamic mechanical analyzer (DMA Q800,TA Instruments).The regular freestanding nanocoating films are too thin for accurate mechanical testing.Thus,some thick films were intentionally prepared for mechanical testing by increasing the coating process to eight cycles.The freestanding nanocoating films were delaminated from the substrates using a Scotch®tape,similar to the method used to peel graphene from graphite[65].The test specimens were first dried in an oven at 105°C for 5 h and then were equilibrated under 22°C and 30% RH for 24 h before mechanical testing.
The renown42 of the Prince and his adventure had gone before him, and the Emperor sat on his throne awaiting the arrival of the Prince and his companions
To image the cross-section of the nanocoatings,the coated PET films were embedded into epoxy,which was microtomed into thin slices with a thickness of 80–100 nm on a Reichert-Jung Ultracut E ultra-microtome.The thin sections were deposited onto 400-mesh copper grids for imaging under a transmission electron microscopy (JEOL 2010 FasTEM,Tokyo,Japan)with an accelerating voltage of 200 kV.
The thickness of the nanocoatings was measured using a surface profilometer(Veeco Dektak 150).The oxygen transmission rates(OTRs)were tested on a MOCON OX-TRAN 1/50 OTR tester at 23°C and 0% RH following ASTM D3985.
3.Results and discussion
The experimental procedures for fabricating PVA/LP nanocoatings are illustrated in Fig.1.In the uniform aqueous dispersion containing PVA and LP,PVA chains are expected to attach onto the surface of LP nanosheets by hydrogen bonding[56]as depicted in Fig.1.The dispersion was coated onto PET films through a simple dip-coating process,and the films were subsequently hung vertically so that the LP nanosheets can be forced to align by forming a thin layer of liquid (Fig.1).During the drying process,the nanocoatings were crosslinked,which helps improve various physicochemical properties,especially resistance to moisture and mechanical properties.Two types of crosslinking are expected to occur:(1) crosslinking between PVA chains,(2) co-crosslinking between PVA chains and LP nanosheets.
Fig.2 shows the FTIR spectra of LP,PVA,PVA-C,PVA/LP-50-N,and PVA/LP-50-C.The broad band in the range of ca.3200-3500 cm-1is from the stretching of O–H from the intermolecular and intramolecular hydrogen bonds[66].The peak at 1095 cm-1is associated with the stretching of C–OH of the hydroxyl groups in PVA[67].The vibrational band at ca.2940 cm-1is because of the stretching of C–H from the alkyl groups of PVA.The peak at ca.2860 cm-1in PVA-C and PVA/LP-50-C are attributed to C–H stretching of the aldehyde groups from GA[68].The band located at 1735 cm-1is linked to the stretching of C––O from the acetate group remaining from PVA due to the incomplete hydrolysis[66,68].After crosslinking,a considerable reduction of the intensity of–OH peaks (3200-3500 cm-1) and C–OH peaks (1095 cm-1) of PVA-C and PVA/LP-50-C was observed.In comparison to the non-crosslinked PVA,the increased intensity of the peak at 1377 cm-1(C–O–C)in the spectra of PVA-C and PVA/LP-50-C supports that the reaction between the hydroxyl groups of PVA and the aldehydes(shown in Fig.1) occurred[68,69].The new peak observed at 1120 cm-1in PVA/LP-50-C is attributed to the vibration of Si–O–C,suggesting the crosslinking between the hydroxyl groups on LP and GA [46].After crosslinking,the LP nanosheets are covalently bonded with PVA matrix to form a fully integrated system.The nanocoatings are expected to exhibit enhanced mechanical properties,which will be discussed below.
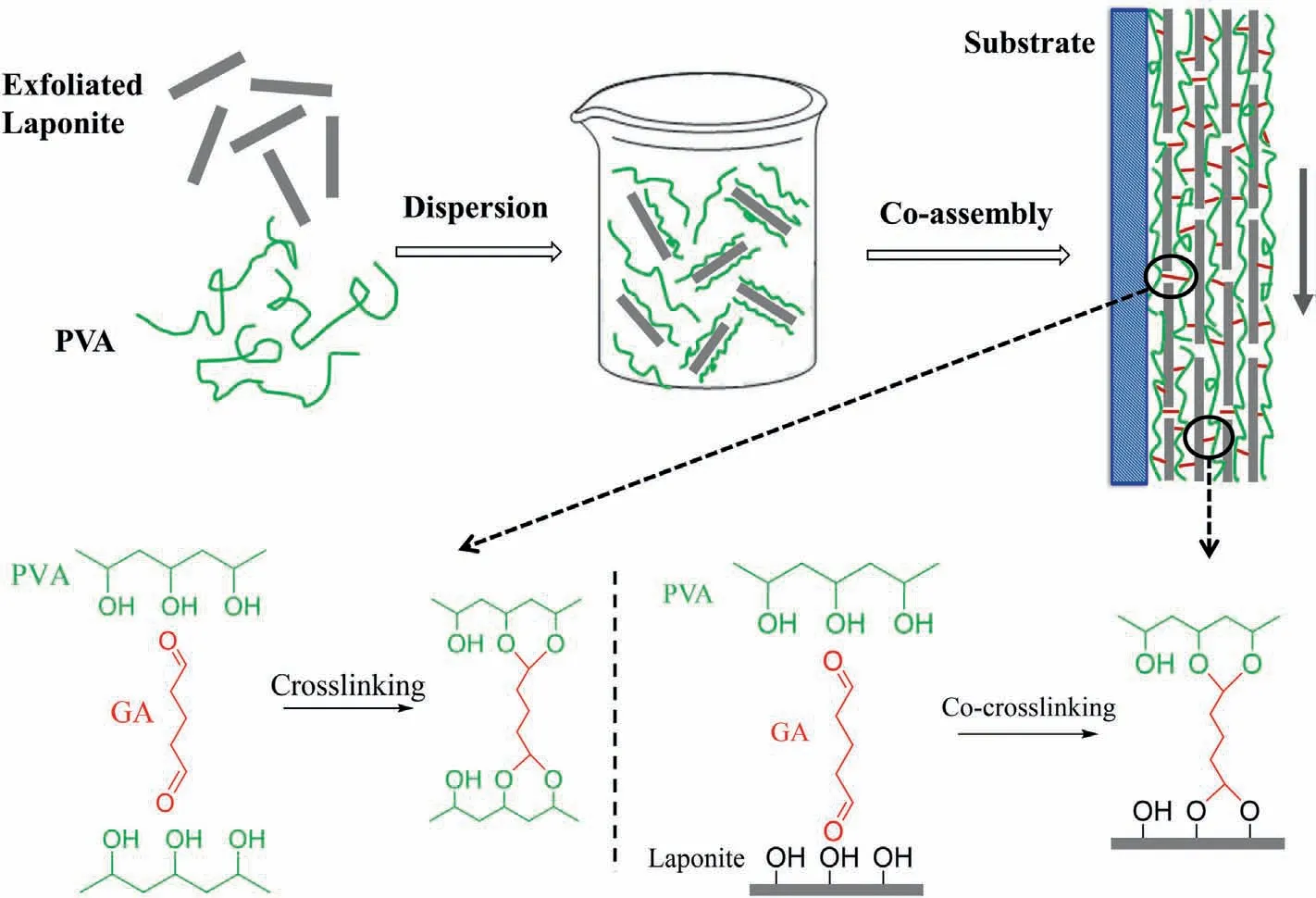
Fig.1.Schematic of the fabrication of PVA/LP nanocoatings with a highly ordered structure and corresponding crosslinking reactions.
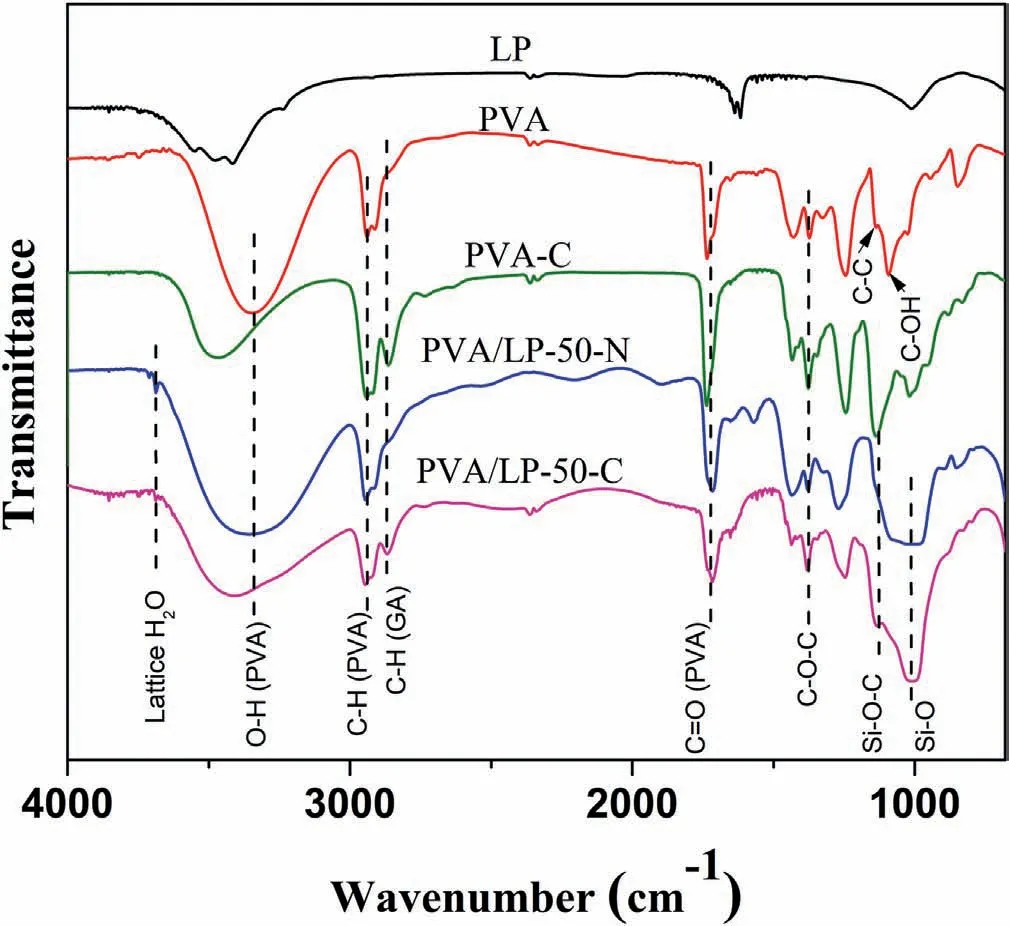
Fig.2.FTIR spectra of LP,PVA,PVA-C,PVA/LP-50-N,and PVA/LP-50-C.
In addition to crosslinking,another critical characteristic determining the overall physicochemical properties of the resultant nanocoating is the level of orientation of the LP nanosheets,which was characterized by XRD and TEM.Fig.3A shows the XRD patterns of the nanocoatings containing various concentrations of LP nanosheets.The diffraction peak at ca.8.5°(10.4 Å)corresponds to the layered structure of the pristine LP[58].Sample PET-PVA/LP-10-C barely exhibited a diffraction peak,indicating a random dispersion of LP nanosheets in the PVA matrix.With more LP nanosheets incorporated in the nanocoating,basal diffraction peaks were observed in the 2θ angle range of 2.3°–4.5°,indicating the formation of a layered structure during coassembly.With an increasing concentration of LP nanosheets,their interlayer distance decreased from ca.37.7 to 19.8 Å(Fig.3B),which is due to the reduced amount of PVA in the galleries of LP nanosheets.The above results indicate that when the concentration of LP nanosheets was too low,it was unfavorable to align the nanosheets,which is probably because there is sufficient space in between the nanosheets and thus they can rotate freely during the coating process.With more LP nanosheets(up to 50 wt%)existing in the dispersion,they became more and more confined with each other and have to stay highly oriented to accommodate neighboring nanosheets,leading to a higher level of orientation.When the concentration of LP nanosheets was further increased to 70 wt %,the interlayer distance keeps shrinking but the diffraction peak became broader,indicating that the nanosheets were less oriented in comparison to those in sample PVA/LP-50-C.This is probably due to the enhanced viscosity at such a high concentration of LP nanosheets,which prevents the nanosheets from rotating freely before the coating was dried and crosslinked.Overall,the XRD result revealed a very clear morphology trend.
Fig.4 shows the TEM images of the cross-section of the nanocoatings.At a low concentration of 10 wt % (Fig.4A),the LP nanosheets were randomly dispersed in the PVA matrix.This agrees well with its XRD pattern in Fig.3A,which does not show any diffraction peak.After increasing the concentration to 30 wt%,the LP nanosheets were loosely packed forming a very rough layered structure (Fig.4B),again corresponding well with the broad hump in the XRD pattern shown in Fig.3A.Further increasing the LP concentration to 50 wt%,sample PVA/LP-50-C exhibited an orientated layered structure with LP nanosheets laying roughly parallel to the substrate surface and their interlayer distances are close to each other(Fig.4C and D).Fig.4F is the gray scale analysis from a representative region in Fig.4D,which shows that the interlayer distance of sample PVA/LP-50-C is ca.2.45±0.30 nm,which is consistent with the XRD result.When the concentration of LP nanosheets reached 70 wt %,a more densely packed structure formed (Fig.4E).Again,this result matches well with its XRD pattern.Overall,the TEM images show the direct evidence of the alignment of LP nanosheets in the nanocoatings,and the overall structural change agrees very well with the XRD characterization results.
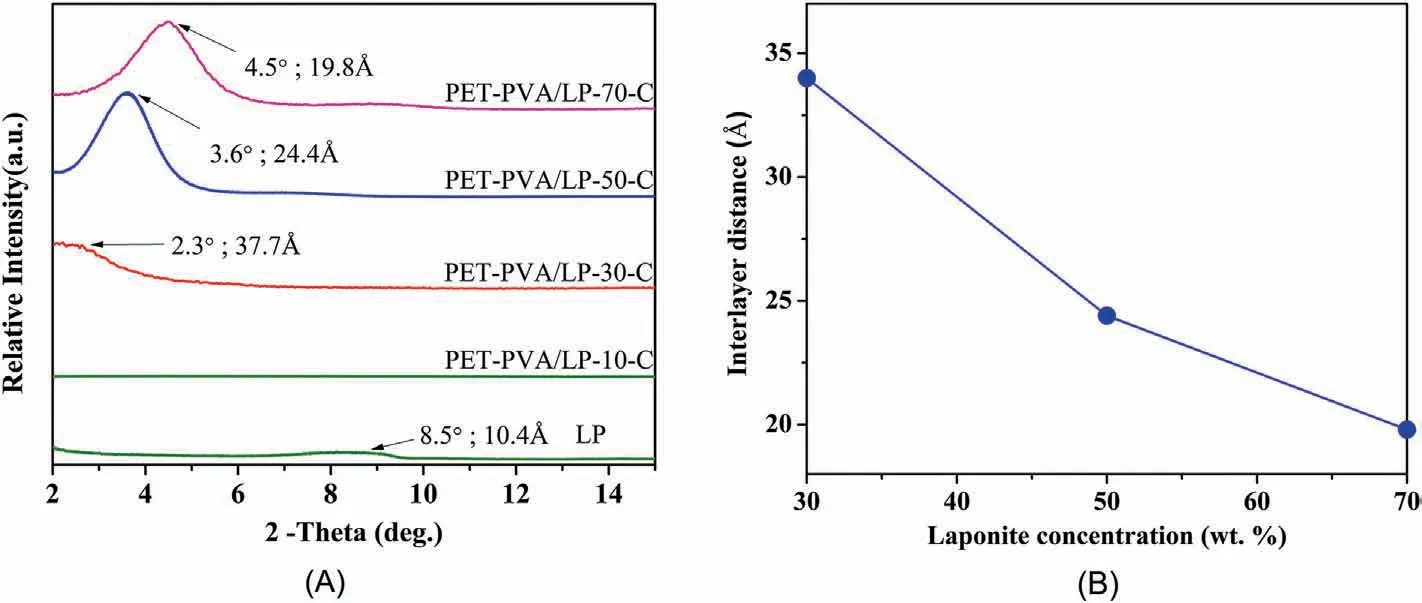
Fig.3.(A)XRD patterns of the coated PET films with various concentrations of LP nanosheets;(B)interlayer distance of the orientated LP nanosheets in nanocoatings as a function of LP concentration.
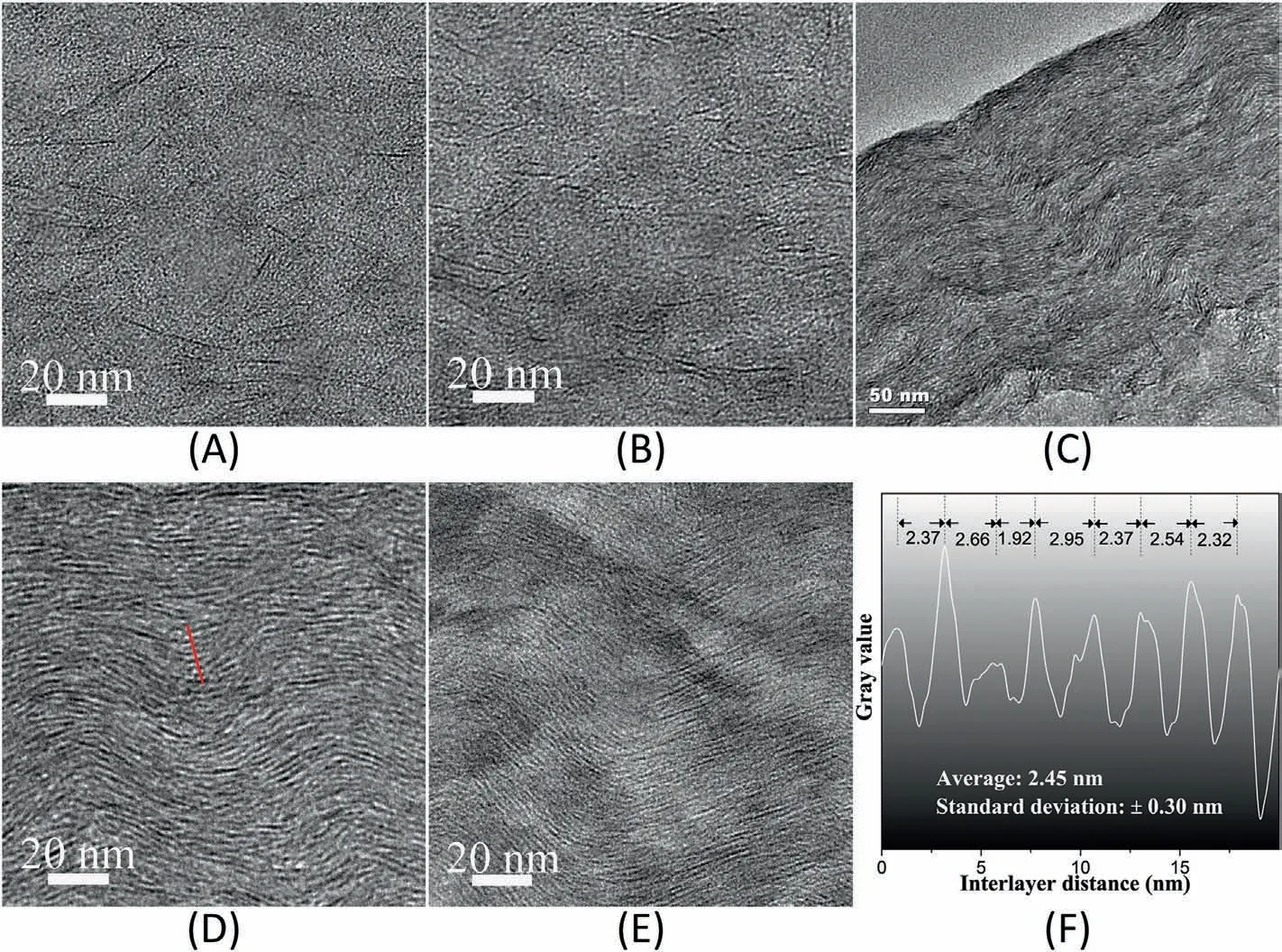
Fig.4.TEM images of the cross-section of the nanocoatings:(A)PVA/LP-10-C;(B)PVA/LP-30-C;(C)PVA/LP-50-C (low magnification);(D)PVA/LP-50-C;(E)PVA/LP-70-C;(F) gray scale analysis of the interlayer distance along the red line marked in (D).
The transparency of the coated films was characterized by UV–vis spectroscopy,as shown in Fig.5.All of the coated PET films exhibited higher transmittance in the visible region compared to the un-coated PET film (Fig.5).In general,a coating layer will negatively affect the transpareny of the coated substrate,especially for a highly transparent subtrate like PET.Inorganic nanosheets will particluarly impact optical clarity[70].The enahnced transparency of the coated PET films indicates that the PVA/LP nanocoating itself is highly transparent and could serves as an anti-reflection coating layer on PET.It is known that when light passes through two substances with different refractive indices,reflection occurs at the interface of the two components.If one additional thin film is deposited on the substrate,two interfaces (air-film and film-substrate) are created,which causes two reflections.A proper control of the film thickness and refractive indices of the film and the substrate could possibly bring about the destructive interference of the two reflected lights with a specific wavelength.Such a coating is called anti-reflection coating.For normal incidence,the optimum refractive index(n1)of the anti-reflection coating is the geometric mean of those of the materials on either siden1=,which can be deduced from the Fresnel equation to achieve the complete cancellation of the reflected lights[71],wheren0andn2are the refractive indices of air and the substrate,respectively.The calculated optimum refractive index of a nanocoating layer that can best improve transparncy for a PET(n2=1.580)substrate is 1.257[72].The refractive indexes of PVA and LP are 1.48 [73] and 1.54 [74],respectively,which are not close to 1.257 but are betweenn0andn2.Therefore the coating still could decrease the intensity of the reflected lights when the optical thickness of the coating is roughly an odd number of specific quarter wavelengths[75].When the concentration of LP was increased,the overall refractive index of the coating layer will shift further away from the optimum,so the anti-reflection properties of the coating layer,as well as the transmittance of the entire film will decrease slightly.In addition,a uniform dispersion of LP nanosheets is also very critical,which eliminates scattering from aggregations[76].In brief,the inherent high transparency of the PVA/LP nanocoating,the uniform dispersion of LP nanosheets,and the proper refractive index of the PVA/LP nanocoating led to the enhanced transparency of the coated PET films.
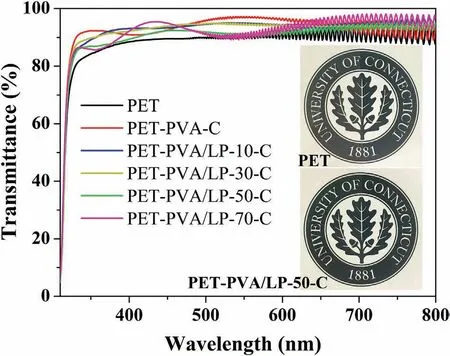
Fig.5.UV–vis spectra of the coated PET films.The two insets show the digital images of an uncoated PET film and a coated PET film placed on the left side of a UCONN logo,demonstrating the high transparency of the coated PET film.
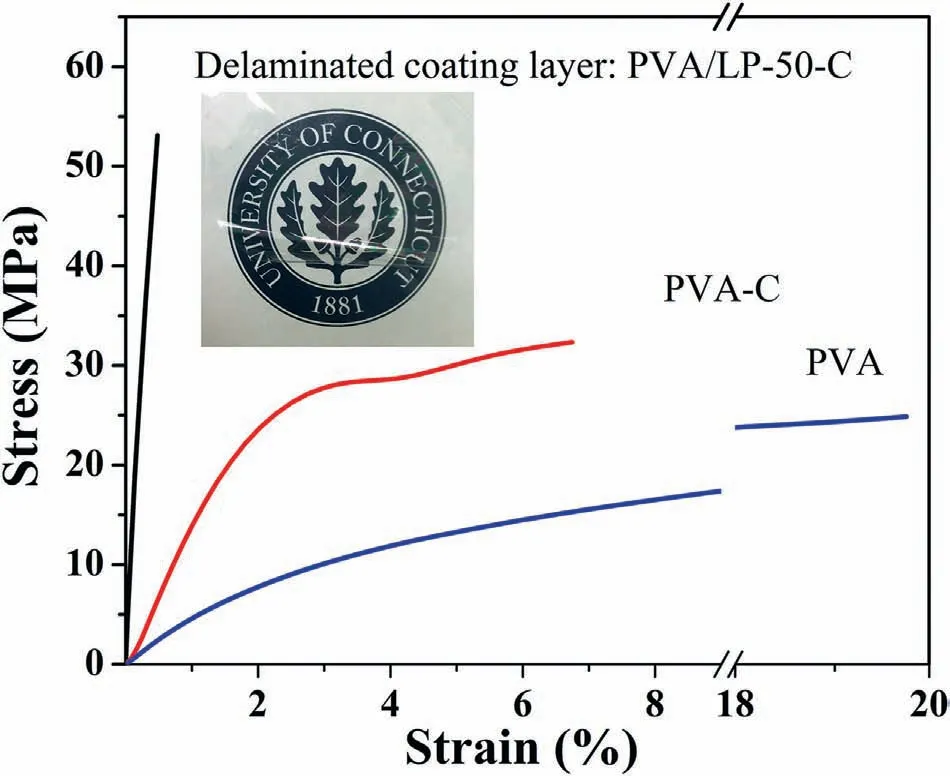
Fig.6.Stress-strain curves of the free-standing nanocoatings.The inset shows a digital picture of a free-standing nanocoating PVA/LP-50-C;note that the sample was intentionally folded for better visibility.
The above systematic characterizations proved that the nanocoatings containing a high loading of well-oriented LP nanosheets can be fabricated through this facile one-step coassembly process,which is expected to lead to outstanding mechanical and barrier properties.
Table 1 shows the tensile test results while the representative stressstrain curves are shown in Fig.6.The nanocoatings after crosslinking exhibited a significant increase in both tensile strength and modulus as compared to the control PVA sample.The highly oriented and uniformly distributed LP nanosheets help effectively reinforce the nanocoatings along the film direction.Meanwhile,the covalent crosslinking and the hydrogen bonding help effectively integrate LP nanosheets into the PVA matrix,which is critical for load transfer and thus further improving the reinforcing effect.As a result,ca.114%improvement in tensile strength and 2180% improvement in Young's modulus was achieved in sample PVA/LP-50-C in comparison to the neat PVA.Nanocoating PVA/LP-70-C was too brittle to be delaminated from the substrate free of defects,and thus we were not able to collect a sufficiently large specimen for tensile testing.The inset in Fig.6 shows the appearance of the free-standing PVA/LP-50-C nanocoating exhibiting ultra-high transparency.

Table 1 Mechanical data of the freestanding nanocoatings.
Due to the well-oriented layered structure and a high LP nanosheet concentration,the nanocoatings also exhibited an outstanding barrier performance,as shown in Table 2.It is well known that volume fraction,level of orientation,and aspect ratio of nanosheets are the main influencing factors of the permeability of the formed nanocomposites[77].Although LP nanosheets have a relatively low aspect ratio of ca.20–35[58],the PVA/LP nanocoatings still exhibited an excellent oxygen barrier performance thanks to the high concentration of well-orientated LP nanosheets.The un-coated PET film exhibited an oxygen transmission rate(OTR)of 64.0 ml/(m2·day).After being coated with a thin layer of PVA-C,the OTR of the coated PET was reduced to 14.8 ml/(m2·day).With an incorporation of LP nanosheets,the OTRs of coated PET films were significantly reduced even further.
Fig.7A shows the OTR data of the coated PET films.At a low LP concentration of 10.0 wt%(5.3 vol%),the OTR of sample PET-PVA/LP-10-C was reduced to 5.2 ml/(m2·day).With an increasing LP concentration up to 50 wt %,the OTR of the coated PET films was further reduced to 0.6 ml/(m2·day),representing an approximately 100-fold reduction in comparison to the un-coated PET films.However,when the LP nanosheet concentration was further increased from 50.0 to 70.0 wt%,the OTR of sample PET-PVA/LP-70-C was slightly increased to 1.1 ml/(m2·day) compared to that of PET-PVA/LP-50-C.According to the barrier models[77],the barrier properties are mainly affected by the volume fraction and aspect ratio of inorganic nanosheets,as well as the level of orientation of the nanosheets.As discussed above and shown in Fig.3,the LP nanosheets exhibited an increasing level of orientation when their concentration was increased from 10.0 to 50.0 wt%,but their level of orientation was then lowered when the LP nanosheets loading was further increased to 70.0 wt%,probably due to an increased system viscosity as discussed above.As such,the OTR data agree well with the overall morphology of the nanosheets in the nanocoatings.In addition,it was observed that the crosslinked nanocoating exhibited a higher oxygen barrier compared to the non-crosslinked counterpart(Fig.7A),which is expected,because after crosslinking,the PVA crystallites region decreased leading to a more compact packing structure and less free volume in the amorphous regions[78].wheredp,φp,andPpare the thickness,volume fraction,and permeability of the polymer substrate,anddg,φg,andPgare the corresponding values of the nanocoating layer.As shown in Table 2 and Fig.7B,the nanocoatings exhibit an excellent oxygen barrier property because of the high concentration of the well-oriented LP nanosheets in the nanocoating.Such highly aligned nanosheets act as impermeable blocking units,significantly increasing the tortuosity of the penetration pathways,thus leading to significantly lowered permeability.
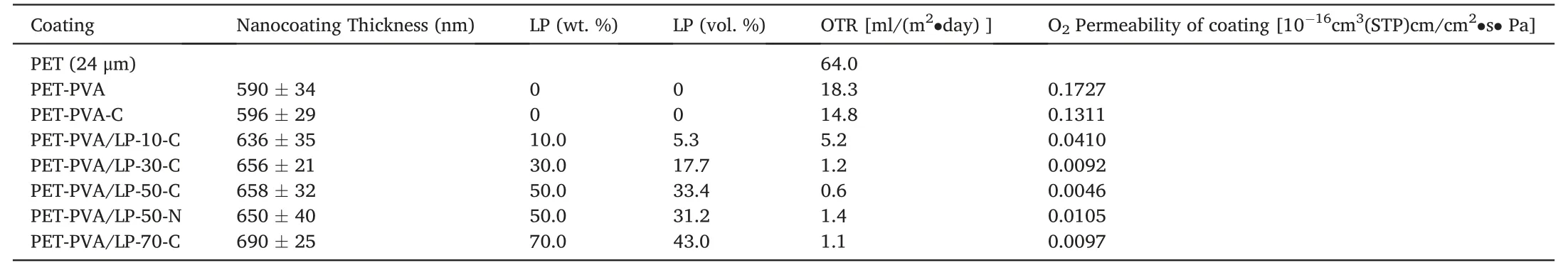
Table 2 Barrier properties of the un-coated and coated PET films.
4.Conclusion
Superior transparent PVA/LP nanocoatings were fabricated by using a facile flow-induced coassembly process.The XRD and TEM characterizations proved the formation of highly packed and well-orientated LP nanosheets in the nanocoatings.Such a thin layer of PVA/LP nanocoating on both sides of a PET substrate helped significantly improve the oxygen barrier property while maintaining the high transparency.Thesenanocoating layers also exhibited superior mechanical properties.Such outstanding properties of the nanocoatings are mainly because of the formation of a high concentration of well-ordered LP nanosheets and the integration between the polymer matrix and the LP nanosheets via chemical crosslinking.
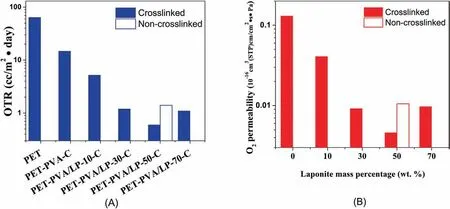
Fig.7.(A) OTR and (B) oxygen permeability of the coated PET films as a function of LP concentration in the nanocoatings.

Note that the OTR data of the coated PET films do not take the thickness into consideration and also include the barrier of the PET substrate.Thus,the O2permeability data based on unit thickness of the nanocoatings excluding the PET substrate were calculated to better evaluate the barrier properties of the nanocoatings by using the ideal laminated theory as shown in equation(1)[79].The permeabilityPof a nanocoating is described as.
Declaration of competing interest
None.
Acknowledgements
L.S.thanks the support by the National Science Foundation (CMMI-1562907).We acknowledge the PVA samples provided by Kuraray and thank Dr.Montgomery Shaw for his valuable discussions.J.L.acknowledges the GE Graduate Fellowship for Innovation and the FEI Fellowship.
杂志排行
Namo Materials Science的其它文章
- Preface of “Trends in Nanomaterials and Nanocomposites:Fundamentals,Modelling and Applications”
--Festschrift in honor of Prof Yiu-Wing Mai's 75th birthday - A comparative study of 85 hyperelastic constitutive models for both unfilled rubber and highly filled rubber nanocomposite material
- On mechanical properties of nanocomposite hydrogels:Searching for superior properties
- VN nanoparticle-assembled hollow microspheres/N-doped carbon nanofibers:An anode material for superior potassium storage
- Molecular dynamics study on mechanical behaviors of Ti/Ni nanolaminate with a pre-existing void
- Core-shell structured silk Fibroin/PVDF piezoelectric nanofibers for energy harvesting and self-powered sensing
