Study on the Adsorption of Preservatives by Eyeliner Pen Packaging Materials
2022-07-14LiuLiGongHui
Liu Li,Gong Hui
(JALA (group) Co.Ltd.,China
Abstract In order to study adsorption of preservatives by eyeliner pen package,the chemical compositions of the commercially available Eyeliner package were analyzed by infrared spectrometer.It was found that these package components were PP,PET,modified PP,modified PET,ABS,nylon,PU and so on.The adsorption effects of these components on three preservatives,i.e.phenoxyethanol,Methyl paraben and Propyl paraben have been further studied by high performance liquid chromatography.The results demonstrated that the four chemical materials PET,PP,modified PP and ABS had no adsorption on the above three preservatives.However,the component named of water diversion core which was composed of modified PET,and nylonmade cotton head have been observed of slight adsorption in this study.And components as the function of preventing liquid leakage,which was made of PU,have presented the most serious adsorption on these three preservatives,with the adsorption order as Propyl Paraben > Methyl Paraben > phenoxyethanol.
Key words eyeliner pen packing material;preservative;Infra-red spectrometry;liquid chromatography;adsorption
In the past two years,due to the impact of corona Virus Disease 2019,people must wear masks in public places,and the focus of cosmetics consumers has gradually shifted from lip makeup to eye makeup[1].Eyeliner is an important category of eye makeup,its formula is made up of film forming agent,colorant,preservative as the main raw materials[2].In order to ensure the safety of the product and the service life of the product,eyeliner formula was often added by preservatives,including:alcohols such as phenoxyethanol,paraben esters such as methyl-phydrxoybenzoate,propyl-p-hydroxybenzoate,etc[3,4].
The packaging material of eyeliner is composed of cotton head,diversion core,throttle,storage container and its functional accessories such as sponge of liquid anti-leakage.After our investigation,it was found that the chemical material of the packaging component is generally synthetic plastics,such as polypropylene (PP),acrylonitrile-butadienestyrene copolymer (ABS),polyethylene terephthalate(PET),polyurethane (PU),etc.Due to the different design functions of chemical components,a single plastic often can not meet the required needs.Therefore,other means were normally applied,such as choosing composite materials or chemical modification of the material,representative PP modification,PET modification,etc.
At present,the adsorption research on preservatives is not adequate in the cosmetics industry.The structure of eyeliner packing material is complex,and there is few research works in this field.In this paper,some eyeliner pens were collected to study the compatibility of packaging components and preservatives,and the experimental strategy was designed to investigate this problem.After chemical material classification according to infrared spectroscopy,7 parts of package were chosen and soaked in the formula simulation solutions.After placement for a specific time,analysis method of liquid chromatography was used to monitor the procedure of preservatives’ absorption.All the test results have shown that this method could be referred for the formula engineer to test the finished product as quality-control standard,and also provide ways for the packing engineer to screen the eyeliner pen packing material.
1 Experimental section
1.1 Main instruments and reagents
Total reflection fourier transform infrared(iS5),Thermo Electron,HPLC (Model 1260 with diode array detector),Agilent,Electronic Balance(ME204),Metler-Toledo.Methanol,Ethanol,HPLC grade,CNW;Phenoxylethanol,paraben methyl ester,paraben propyl ester for liquid chromatograph,99%purity,Germany Dr.Ehrenstorfer;paraben methyl and propyl ester,UENO,Japan;phenoxylethanol,DuPont;laboratory water,high pure grade.
1.2 Research method of packing material on preservatives
12 common eyeliner pen packaging was selected from the market respectively.They are 6 straight liquid eyeliner packing material,including steel beads,and 6 cotton core eyeliner packing material.The 6 disassembled components contained brush head,diversion core,throttle,tank,i.e.formulacontacting part,leakage prevention sponge.
The chemical composition of each component was tested using an infrared spectrometer.The acquired IR spectrum was processed and compared with the standard atlas[5]to identify the chemical structure of the packaging components and classify these components.Seven categories of representative material components were summarized for subsequent studies[6,7].
The simulation solutions were prepared according to the amount of preservative added in the common liquid eyeliner formula.For simplification,the simulation solution contained only preservatives and water-alcohol solvents.The solute consisted of 3 preservatives,and the added amount is same as the actual amount of preservative in eyeliner formulation.For the proportion of water and alcohol in the solvent,the proportion of water and organic matter in the main reference formula was usually concerned.This study implemented two kinds of water-alcohol ratio:normal ratio 20:80 considering the existing formula water and organic matter;extreme ratio 50∶50.The seven kinds of packaging components were soaked in the simulation solution as the samples to be tested and placed for a period of time.The preservative content of the simulated solution and the seven samples was tested by the liquid chromatograph in order to study the adsorption of the preservatives by the packaging components.
1.3 Adsorption model and liquid chromatography test conditions
1.3.1.Prepare the simulation solution
Accurately weigh phenoxyanolol,paraben methyl ester and propyl ester in 100 mL volumetric flasks,dissolved with mixed solvent (ethanol:water =50∶50 (V/V)),and shaken,then weighed as formula simulation solution A.Simulation solution B was prepared in the same way and dissolved by ethanol:water =20∶80 (V/V).The composition of the simulation solution was shown in Table 1.

Table 1.Preparation of simulated solution A and B
1.3.2 Liquid chromatography detection
Prepare 20 mL glass reagent bottles with PP cap and PTFE cushion.Put in 0.1 g of each of 7 components,and then add 5 mL of pre-prepared simulation solution,for that the components were completely immersed.Place the reagent bottles at room temperature for 2 days.1 mL of solution was filtered by 0.45 μm hydrophobic PTFE filter membrane,and the filtrate was injected into liquid chromatography.

Liquid chromatography conditions:Athena WPC18 column (4.6 mm,250 mm,5 μm),sampling volume of 5 uL,column temperature of 30 ℃,detection wavelength of 220 nm (phenoxyethanol),254 nm (paraben methyl ester,paraben propyl ester).The mobile phase was made of methanol and 0.1%phosphoric acid water with a flow rate of 1 mL/min.Gradient elution procedures are shown in Table 2.
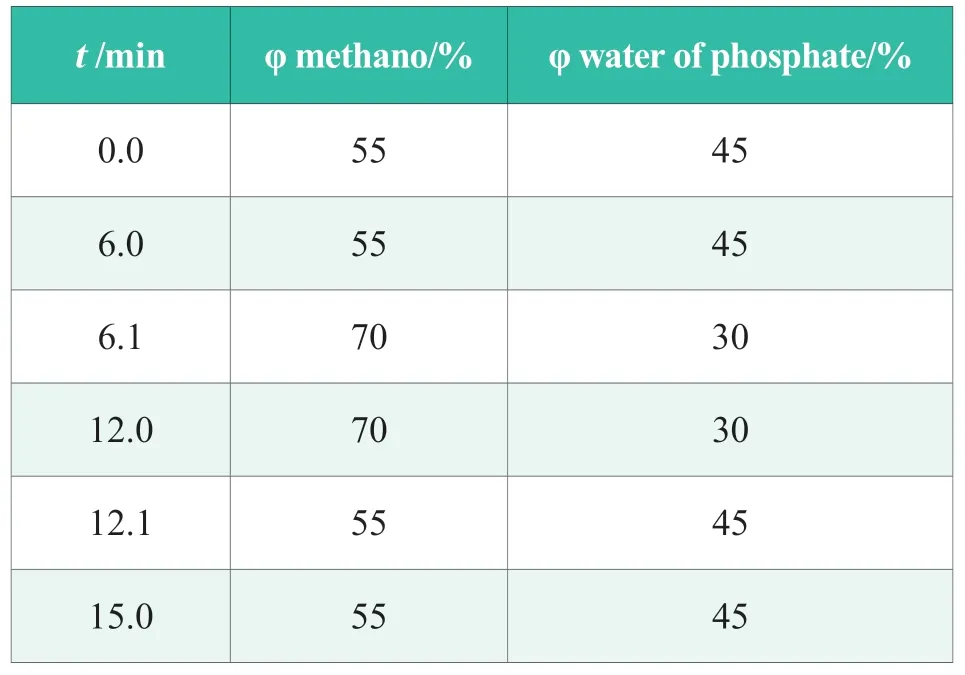
Table 2.Gradient elution procedure
2 Results and Discussion
2.1 Infrared spectrum

The collected 12 eyeliner pens were disassembled.All the 50 packaging components were chemical classified,which could be summarized into 7 groups:PET,PP,PP modification,ABS,Nylon,PET modification and PU,respectively labeled as No.1 ~7.The infrared spectral test results of the 7 materials were shown in Figure 1,and the collected eyeliner pens according to the component chemical materials are summarized in Table 3.Comparing the IR spectra with the standard spectrum database,the results showed that the 50 components could be divided into two groups according to their complexity.One of groups was common plastics such as PP and PET,and the other group was denaturing plastics such as PP modification and PET modification.Unlike PP materials,PP modified components was observed a strong absorption peak at around 1,060 cm-1.In contrast of PET,an extra infrared absorption peak of 1,030 cm-1was found in modified PET,probably related to the C-O-C ether bond functional group.
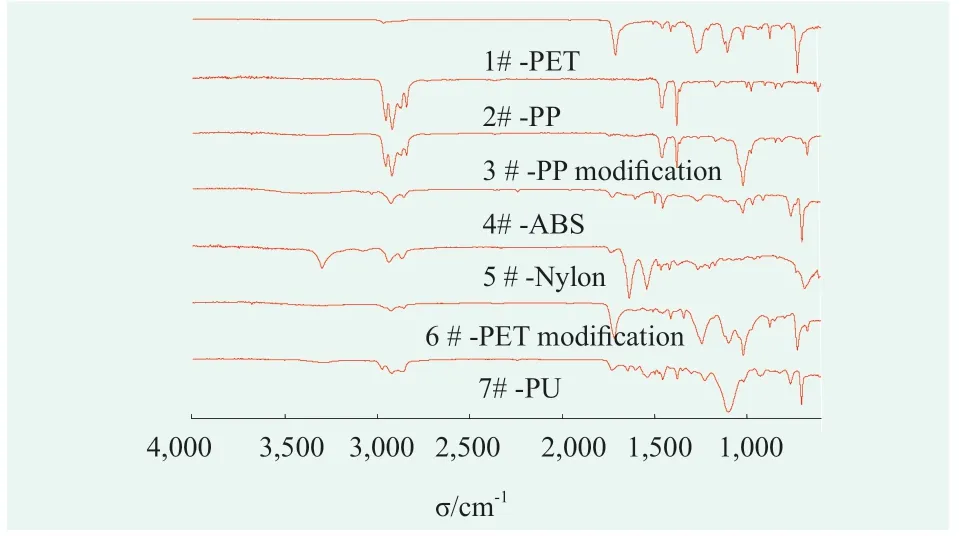
Figure 1.Infrared spectrum of seven representative components of Eyeliner Pen
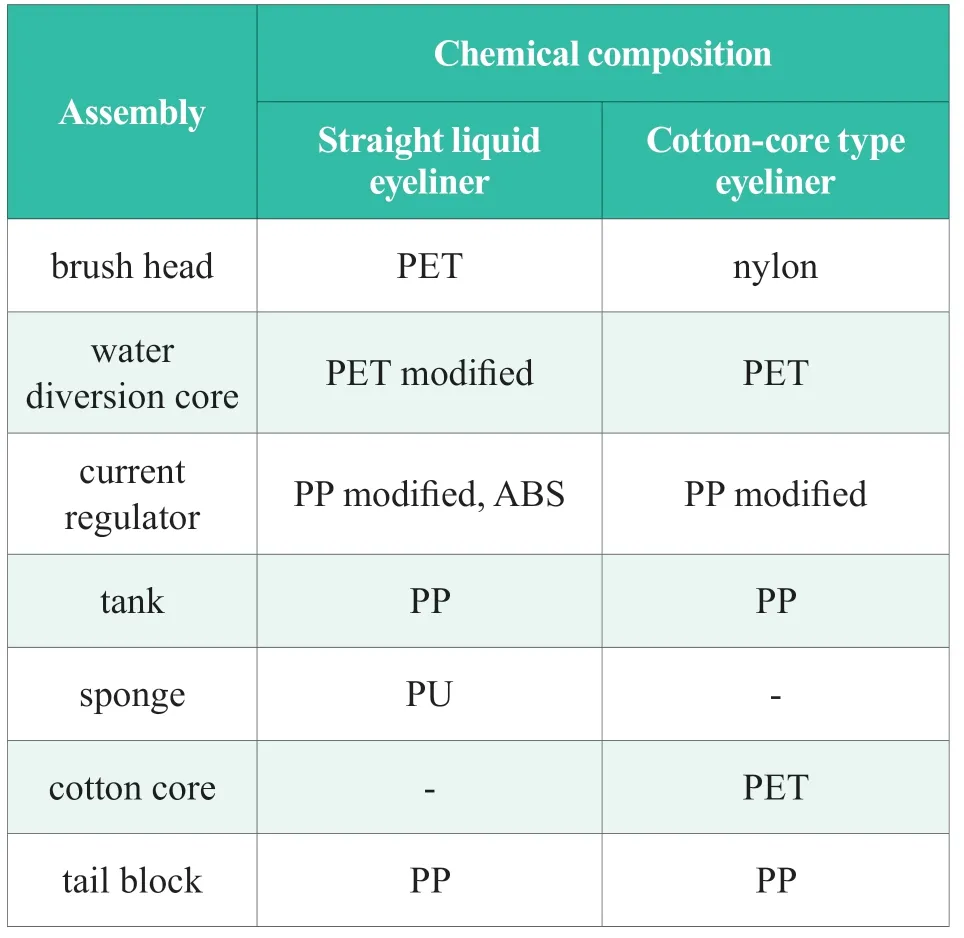
Table 3.Chemical classification of Eyeliner Pen components
2.2 HPLC
Phenoxylethanol,paraben methyl ester,paraben propyl ester,numbered a,b,c.Liquid chromatography was conducted on 16 solutions with external standard method.The relative standard deviation i.e.RSD of 48 groups of data was from 0.8%~1.5%.The arithmetic value of three measurements was taken as the result of preservative contents in solution.The adsorption of the three preservatives of seven materials in simulated solution A and B was shown in Figure 2 and 3.
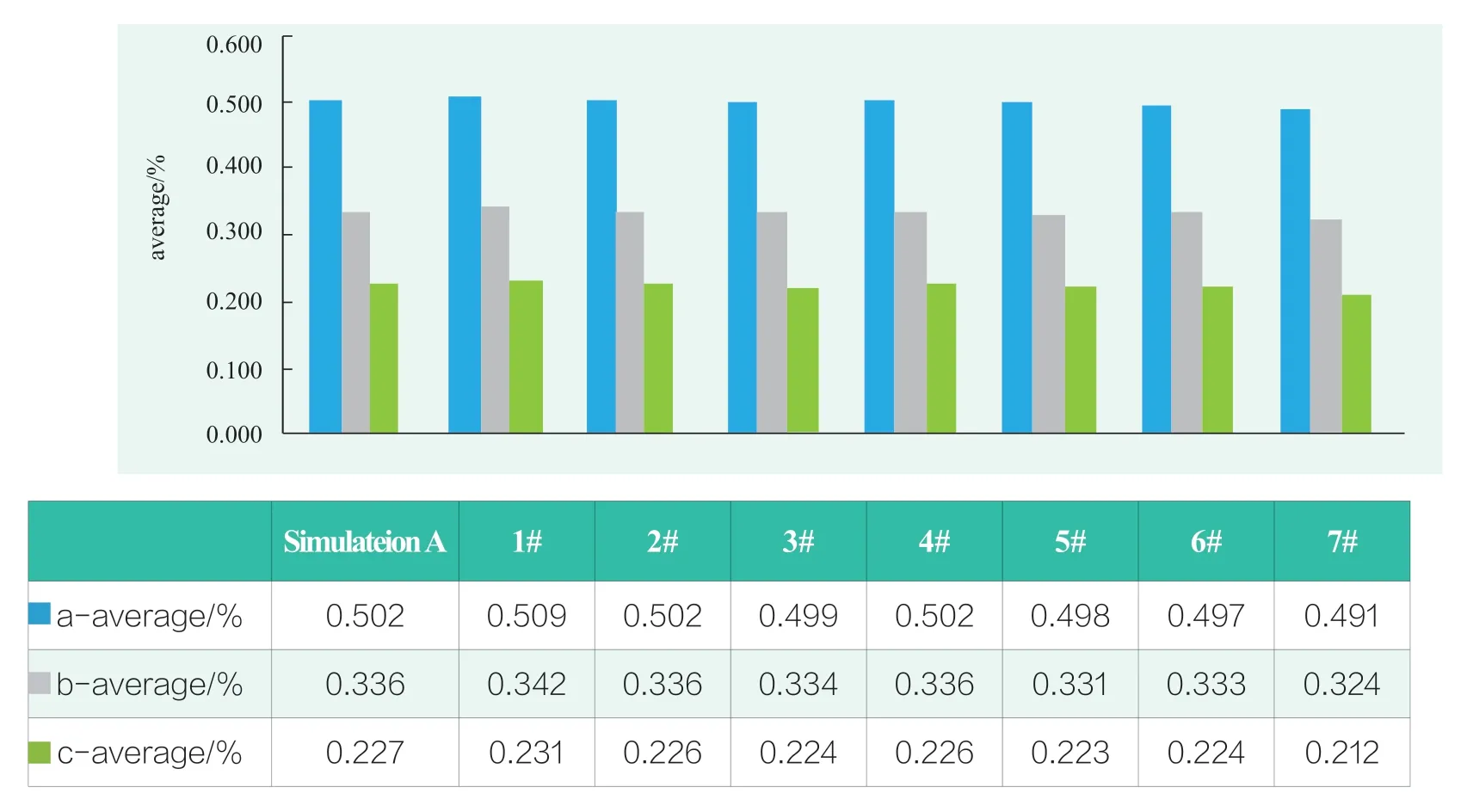
Figure 2.Adsorption comparison of three preservatives in simulated solution A

Figure 3.Adsorption comparison of three preservatives in simulated solution B
Figures 2 and 3 showed that there was a clear difference between the adsorption phenomenon of simulated solution A and B.It could be seen that the proportion of alcohol-water in the simulation solution affected the adsorption of preservatives.For example,the amount of preservative differed significantly for the components 5 # and 6 # after contacting simulation solution A and B.No adsorption was observed in simulation liquid A,however significant adsorption occurred in simulation liquid B.It could be seen that the lower the proportion of organic solvents in the simulation liquid,the more obvious the adsorption effect of the components on preservatives occurred.
After the liquid chromatography study of simulated liquid B,it was found that 1 # ~4 #components did not adsorb the three preservatives,5 # and 6 # only showed mild adsorption,and 7 #samples showed strong adsorption effect.It could be calculated that the preservatives phenoxythanol,paraben methyl ester and paraben propyl ester were adsorbed 17%,46% and 76% by PU materials respectively.The comparison of the controlled simulation sample B and 7 # tested solution shown in Figure 4 presented that the peak area of all three preservatives was significantly reduced.

Figure 4.HPLC results of simulated sample B and 7#component sample
Compared with simulation liquid A,the volume ratio of organic solvent ethanol in simulation liquid B was decreased from 50% to 20%,which was convenient to study the adsorption of packaging material to preservatives.It was speculated that the oil solubility of ethanol is strong,which was a competitive relationship with the adsorption of preservative.When the ethanol content in the solvent was high,the preservative could not be effectively adsorbed by the packaging material,which is reflected in the adsorption research by HPLC with less difference between control samples and tested samples.It showed that the proportion of organic phase in experimental design is an important factor whether adsorption phenomenon can be observed.
3 Conclusion
The experimental results showed that the PET,PP,modified PP,ABS materials had no adsorption phenomenon.However,the PU adsorption problem was proved to be the most serious,followed by Nylon and PET modified materials.The chemical composition and structure of the packaging material components were the reasons for the difference in the adsorption capacity of the preservatives.In terms of molecular structure,PU contained not only aromatic groups but also ester and amide groups,which explained why the three preservatives with similar molecular structure showed strong adsorption effects.After analyzing the molecular structure of the modified PET of water diversion core and Nylon of cotton head,we found that they were far different from the chemical structure of the three preservatives.However,because both belonged to porous materials and had a large specific surface area,this was one of the reasons for their adsorption to preservatives.

