Microstructures and mechanical properties of as-cast Mg-Sm-Zn-Zr alloys with varying Gd contents
2022-07-13KiGunDisukeEgusEijiAbeJinghuiZhngXinQiuQingYngJinMeng
Ki Gun, Disuke Egus, Eiji Abe,d, Jinghui Zhng, Xin Qiu, Qing Yng,∗∗,Jin Meng
a Department of Materials Science & Engineering, The University of Tokyo, Tokyo 113-8656, Japan
b Key Laboratory of Superlight Material and Surface Technology, Ministry of Education, College of Material Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, PR China
c State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, PR China
d Research Center for Structural Materials, National Institute for Materials Science, Tsukuba 305-0047, Japan
Abstract
Keywords: Magnesium alloys; Microstructure characterization; Mechanical properties; Transmission electron microscopy (TEM).
1.Introduction
As the lightest metal structural materials, magnesium (Mg)alloys with high specific strength show great prospects in aerospace and automobile industries.However, the practical applications of traditional Mg alloys are severely restricted by their low strength.Great efforts have been made to develop high-performance Mg alloys,and rare earth(RE)elements are recognized as the most effective alloying elements that can remarkably improve the mechanical properties of Mg alloys by providing grain boundary strengthening, solution strengthening as well as dispersion strengthening [1–16].
Recently, one of the cheap RE elements, samarium (Sm)with the largest maximum solid solubility (5.8 wt.%) in Mg among the light RE elements has attracted much attention[17–34].For instance, the mechanical properties of commercial ZK60 alloys can be significantly improved at ambient and elevated temperatures by a small amount of Sm addition[18].The precipitation sequence of Mg-Sm binary alloys were widely investigated by atomic-resolution scanning transmission electron microscopy (STEM) [19–21].Moreover, quaternary Mg-Sm-Zn-Zr alloys were frequently reported, whereZr [16,17]and Zn [4,5]can significantly refine grains and promote aging precipitation in Mg alloys, respectively.The as-cast Mg-Sm-Zn-Zr alloy has been proved to have excellent creep resistance [23].The effects of Sm content on the microstructures and tensile properties of Mg-Sm-Zn-Zr alloys were systematically investigated, and the results reveal that the Sm/Zn ratio plays a critical role in determining the crystal structure of intermetallic compounds [24].Among a variety of Mg-Sm-Zn-Zr alloys, the as-extruded Mg-3.33Sm-0.55Zn-0.46Zr alloy exhibited the optimized mechanical properties,its tensile yield strength was measured to be 363 MPa at room temperature, and that was further improved to 416 MPa after aging treatment at 200 °C for 24 h [24–25].It should be noted that, compared with Mg-Gd and Mg-Y alloy systems,the aging strengthening effect of Mg-Sm-Zn-Zr alloy is insufficient due to the relatively small maximum solid solubility of Sm in Mg matrix.
In order to further improve the aging strengthening effect of Mg-Sm-based alloys, a second RE element, especially heavy RE with large solid solubility in Mg, is generally added.Luk’yanova et al.[34]pointed out that Sm addition can distinctly accelerate the aging hardening and improve the mechanical properties of Mg-RE based alloys.It is well known that the maximum solid solubility of Gd in Mg matrix is 23.3 wt.%, which is one of the most appropriate candidates for the development of high-performance Mg alloys.Hence a series of Mg-Sm-Gd based alloys have been developed [35–39].Numerous prismatic precipitates were observed in the peak-aged Mg-4Sm-6Gd-0.4Zr alloy, which significantly enhanced alloy’s hardness and yield stress at room temperature[35].The predominant intermetallic compounds of the corresponding as-cast alloy were identified as face centered cubic(fcc) Mg6.2(Sm0.56Gd0.44) phase (a= 2.2879 nm) [36].Apart from prismatic precipitates, a number of precipitates formed on the basal plane of matrix were revealed in aged Mg-Sm-Gd-Zn-Zr alloys due to Zn addition [37,38].In this case,both the basal and non-basal dislocation slips are effectively hindered during plastic deformation, which can consequently improve the mechanical properties of Mg alloys at ambient and elevated temperatures [38].The dominant intermetallic compounds of the as-cast Mg-3Sm-0.5Gd-0.3/0.6Zn-0.5Zr alloys were roughly inferred to be Mg41RE5phase according to X-ray diffraction and chemical composition analysis results [37].Afterwards, based on the combination of electron diffraction pattern and chemical composition analysis,the eutectic phase was identified asfcc(Mg, Zn)3RE phase(a= 0.727 nm) in the as-cast Mg-2.6Sm-1.3Gd-0.6Zn-0.5Zr alloy [39].It is obvious that the reported crystal structures of the dominant phase in Mg-Sm-Gd-Zn-Zr alloys are controversial.Additionally, it’s reported that RE/Zn ratio plays a decisive role in formation of intermetallic phases in various Mg-RE based alloys [24,40].The dominant intermetallic phase was Mg3Sm phase with abundant Zn enrichment (~15 at%)in Mg-Sm-Zn-Zr alloys with Sm content less than 5 wt.%,which was completely replaced by Mg41Sm5phase with much lower Zn enrichment (~2 at%) as Sm content increased to 6.5 wt.% [24].However, the influence of Gd content onthe intermetallic phase in Mg-Sm-Gd-Zn-Zr system is still unknown.
It is worth noting that the microstructure and intermetallic compounds play an important role in deciding the mechanical properties and heat resistance of Mg alloys[41,42].Therefore,clarifying the crystal structures and chemical compositions of dominant intermetallic compounds is beneficial to the development of novel high-performance Mg alloys.In the present work,the microstructures and mechanical properties of as-cast Mg-Sm-Zn-Zr alloys with varying Gd additions, particularly the crystal structures of intermetallic compounds, were systematically investigated.
2.Experimental procedures
The alloys with nominal compositions of Mg-3Sm-xGd-0.5Zn-0.5Zr (x= 0, 3, 5 and 7) in wt.% were prepared with Mg-20 wt.% Sm, Mg-25 wt.% Gd and Mg-30 wt.% Zr master alloys, pure Mg and Zn.The raw materials were melted at about 760 °C in a steel crucible under the protection of 1 vol% SF6and 99 vol% CO2mixed atmosphere.Then the melt was allowed to stand at about 740 °C for half an hour after being fully stirred.Finally, the melt was poured into a preheated steel mold with a dimension ofd90 mm×800 mm at about 715 °C.After solution treated at 520 °C for 8 h,the samples were treated by isothermal aging at 225 °C.Cubic specimens with a side-length of 8 mm were machined from the ingots for the subsequent microstructure observations.The tensile specimens with gage dimensions of 15 mm length, 3 mm thickness and 4 mm width were machined by electrical discharge machine.The tensile test was performed on Instron 5869 testing machine with an initial strain rate of 1.0 × 10-3s-1at ambient temperature.The corresponding values of tensile properties are the average of five effective tests.The Vickers hardness was measured using a micro-Vickers hardness tester under a load of 98 N for 15 s.
Inductively coupled plasma atomic emission spectroscopy(ICP-AES) was used to test actual chemical compositions of the studied alloys, and the corresponding result was listed in Table 1.Microstructural features of these alloys were characterized by Olympus-GX71 optical microscopy (OM), Bruker D8 FOCUS X-ray diffractometer (XRD) with Cu-Kαradiation (λ= 0.15406 nm), Hitachi S-4800 scanning electron microscopy (SEM) equipped with energy-dispersive X-ray spectroscopy (EDS), and FEI Tecnai G2F20 transmission electron microscopy (TEM) equipped with EDS operating at 200 kV.After mechanical grinding with different grades of SiC emerypapers, the specimens used for OM and SEM observations were etched in an aqueous mixture of picric acid, acetic acid and ethanol.Thin foils with a diameter of 3 mm for TEM observations were milled using a Gatan 691 precision ion polishing system (PIPS) equipped with liquid nitrogen cooling system.

Table 1Actual chemical compositions of the presented alloys.

Fig.1.OM images of as-cast Mg-Sm-xGd-Zn-Zr alloys.(a) SG30 alloy, (b) SG33 alloy, (c) SG35 alloy and (d) SG37 alloy.
3.Results and discussion
3.1.Microstructure evolution


Fig.2.XRD patterns of as-cast Mg-Sm-xGd-Zn-Zr alloys.
Fig.1a–d present the low-magnification OM micrographs of as-cast Mg-3Sm-xGd-0.5Zn-0.5Zr (x= 0, 3, 5 and 7) alloys (denoted by SG30, SG33, SG35 and SG37).It can be seen that all the alloys have no obvious casting defects and are composed of equiaxed Mg grains and dark regions at grain boundaries.The latter can be clearly identified as intermetallic compounds according to the inset in Fig.1d.By means of Nano Measure 1.2 software, the average grain size of SG30, SG33, SG35 and SG37 alloys is measured to be 49.6 ± 5.7, 47.7 ± 5.1, 42.6 ± 3.6 and 33.3 ± 4.2 μm, respectively.The result shows that Gd addition can effectively refine the grains of Mg-Sm-Zn-Zr alloys, especially when the content of Gd is high.Similar grain refinement effect of ascast alloys are frequently reported, which can be explained by constitutional supercooling induced by accumulation of solute atoms in front of the liquid-solid interface during solidification [43,44].The grain refinement effect can be described by the growth-restriction parameterQ[43,44],which is expressed as in whichmis the liquidus slope,kis the solute partition coefficient, andC0is the solute concentration in the alloy melt.It can be seen directly that solute concentration plays a key role in grain refinement.Regarding the present alloys,with increasing Gd content, constitutional supercooling in the liquid region ahead of an advancing Mg solid front was enhanced by the increased solute concentration during solidification, which can prominently promote nucleation in liquid region and refine grains.XRD was performed to preliminarily distinguish the intermetallic compounds of the present alloys,as shown in Fig.2.Apart from the primary diffraction peaks corresponding to Mg matrix, some additional ones with relatively weakintensity from intermetallic compounds are displayed in XRD patterns.In SG30 and SG33 alloys, all the additional peaks can be indexed by Mg3RE phase withfcccrystal structure,although they slightly shift towards large 2-theta side comparing to standard peaks.This result is similar to the reported(Mg, Zn)3RE phase in the as-cast Mg-2.6Sm-1.3Gd-0.6Zn-0.5Zr alloy [39].It should be noted that the peak intensity in SG33 alloy is stronger than that in SG30alloy, indicating that Gd addition increases the amount of intermetallic compounds.In comparison with SG33 alloy, several new extra faint diffraction peaks that represent Mg5RE phase are observed in SG35 alloy, which are obviously enhanced in SG37 alloy.It implies that the volume fraction of Mg5RE phase increases with increasing Gd content in this alloy system.The results show that the content of Gd can affect not only the type of intermetallic compounds but also their quantity.

Fig.3.Backscattered SEM images along with the SEM-EDS results of as-cast (a) SG30 alloy, (b) SG33 alloy, (c) SG35 alloy and (d) SG37 alloy.
Fig.3a–d show the backscattered SEM images of the ascast alloys with various Gd additions.It is distinct that a mass of intermetallic compounds with blocky and reticular morphologies widely distributed along grain boundaries.Meanwhile, some particulate phases are sporadically observed in grain interiors and boundaries.Further inspection of these images reveals that all the intermetallic phases exhibit bright contrast in SG30 and SG33 alloys, whereas in SG35 and SG37 alloys,in addition to the bright phase,some gray phases are also apparently observed.Combined with the XRD result, these bright and gray phases are likely to be identified as Mg3RE and Mg5RE phases, respectively.It is to be noted that the volume fraction of intermetallic phases increases obviously with increasing Gd content, which can properly support the XRD result.SEM-EDS was conducted to investigate the influence of Gd content on the composition of intermetallic compounds in these four alloys.The specific compositions of typical intermetallic compounds marked with purple crosses in Fig.3 are attached in the corresponding images.The chemical compositions of the bright phases in SG30,SG33,SG35 and SG37 alloys are(A)Mg70.1Sm15.6Zn14.2Zr0.1,(B)Mg76.2Sm7.2Gd8.7Zn7.9Zr0,(C)Mg74.5Sm7.5Gd9.8Zn8.1Zr0.1and (D) Mg77.8Sm7.3Gd8.1Zn6.8Zr0, respectively.It is evident that Sm element in bright phases is partially substituted by Gd after adding Gd.Simultaneously, Gd addition leads to the decrease of Zn segregation in bright phases.As for the gray phases in SG35 and SG37 alloys,their chemical compositions are measured to be (C’) Mg83.7Sm6.7Gd4.5Zn5.1Zr0and (D’)Mg86.1Sm4.6Gd5.5Zn3.7Zr0.1, respectively.Apparently, the contents of Sm, Gd and Zn in gray phases are much lower than those in bright phases, which can well explain their relatively low contrast in the backscattered images.By the way, the Zr content is very low in all intermetallic compounds, implying that Gd addition has little effect on the distribution of Zr.
3.2.TEM characterizations of intermetallic compounds
In order to thoroughly investigate the crystal structures of the various phases, the present alloys were meticulously characterized by TEM.Fig.4a–c present the typical brightfield TEM (BF-TEM) images of reticular, rod-like and granular phases in SG30 alloy, respectively.The correspondingselected area electron diffraction (SAED) patterns shown in Fig.4d–f reveal that all three types of intermetallic compounds arefccMg3Sm phase with lattice parameter ofa=0.7127±0.006 nm.It is important to notice that the lattice parameter is considerably smaller than that (a= 0.7346 nm)reported in binary Mg-Sm alloy [45], supporting the XRD result.Considering the specific composition of M3Sm phase shown in Fig.3a, the reduced lattice parameter can be ascribed to the remarkable segregation of Zn.Because the atomic radius of Zn (0.137 nm) is much smaller than that of Mg (0.160 nm) and Sm (0.180 nm).

Fig.4.(a–c) BF-TEM images and (d–f) the corresponding SAED patterns of as-cast SG30 alloy.
The typical BF-TEM images recorded from SG33 alloy,the corresponding SAED patterns, and high-resolution TEM(HR-TEM) images along with fast Fourier transform (FFT)patterns are shown in Fig.5.The massive reticular and blocky phases (Fig.5a and b) were identified asfccMg3RE phase with lattice parameter ofa= 0.7196±0.008 nm according to the analysis of relevant SAED patterns (Fig.5d and e).This can be further confirmed by the analysis results of FFT patterns(Fig.5j and k)generated from HR-TEM images(Fig.5g and h).Similar to SG30 alloy, the experimental lattice parameter of Mg3RE phase in SG33 alloy is definitely smaller than that reported in binary Mg-Sm alloy (a= 0.7346 nm)[45]and Mg-Gd alloy (a= 0.7324 nm) [46].In addition,very few blocky intermetallic compounds with comparatively small dimension are rarely observed in lots of TEM observations for SG33 alloy, the typical one is shown in Fig.5c.It can be demonstrated to befccMg5RE phase by analyzing the corresponding SAED pattern (Fig.5f), HR-TEM image(Fig.5i) and FFT pattern (Fig.5l).The experimental lattice parameter of Mg5RE phase in the present alloy is revealed asa= 2.225 nm, which is obviously smaller than that reported in binary Mg-Sm alloy (a= 2.246 nm) [45]and Mg-Gd alloy (a= 2.234 nm) [47].However, the diffraction peaks from Mg5RE phase cannot be distinctly observed in the XRD pattern (Fig.2) of SG33 alloy, which may be because its volume fraction was too small to be detected.Furthermore, a few of tiny plate-like intermetallic compounds with longitudinal axis roughly parallel to grain boundary are observed in SG33 alloy, as shown in Fig.6a.The SAED pattern presented in Fig.6b can be readily recognized by two sets of lattice parameters corresponding to Mg matrix and Mg3RE phase.The HR-TEM image taken from the interface between the intermetallic phase and the left matrix, and its FFT pattern are shown in Fig.6c and d.The regions indicated by yellow and pink dotted frames in Fig.6c are, respectively confirmed as Mg and Mg3RE phase on the basis of their separate FFT patterns displayed in Fig.6e and f.Their orientation relationship is (220) Mg3RE// (0001) Mg and [1–1–1]Mg3RE//[11–20]Mg, suggesting the tiny plate-like phase was nucleated and grown up at the grain boundary during the cooling process after solidification.The lattice parameter of the platelike Mg3RE phase is measured to bea= 0.7187 nm, which is basically equivalent with that of the reticular and blocky ones in Fig.5.
The TEM observation results of SG35 alloy are presented in Fig.7.The coarse phase with irregular shape presented in Fig.7a is identified as Mg3RE phase (fcc, a= 0.7169 nm)according to the relevant SAED pattern(Fig.7d),and the FFT pattern (Fig.7j) produced from the HR-TEM image (Fig.7g).In a similar way, the SAED patterns (Fig.7e and f) and the HR-TEM images (Fig.7k and l) together with the FFT patterns (Fig.7h and i) reveal that both the reticular phase(Fig.7b) and the blocky one (Fig.7c) arefccMg5RE phase with the lattice parameter ofa=2.220±0.001 nm.Compared with the standard lattice parameters of Mg3RE and Mg5RE phases in binary Mg-Sm/Gd alloys [45–47], the experimental values in SG35 alloy are obviously small, but comparable to those of SG33 alloy, supporting the presence of Zn segregation shown in Fig.3c.

Fig.5.(a–c) BF-TEM images, (d–f) SAED patterns, (g–i) HR-TEM images and (j–l) the corresponding FFT patterns of as-cast SG33 alloy.
With respect to SG37 alloy, the representative high-angle annular dark-field scanning transmission electron microscopy(HAADF-STEM)image presented in Fig.8a exhibits differentZ(atomic number) contrast, indicating the chemical compo-sition difference between the intermetallic compounds.The dark phase labeled with A and the bright one labeled withBare reliably identified as Mg5RE phase (fcc, a= 2.216 nm)and Mg3RE (fcc, a= 0.7222 nm) phase with the assistance of the corresponding SAED patterns insets.Fig.8b shows the EDS mappings corresponding to the region highlighted by blue dashed rectangle in Fig.8a, illustrating that segregated Zn in the bright Mg3RE phase is obviously richer than that in the gray Mg5RE phase.The significant segregations of both Sm and Gd are revealed, but it is difficult to distinguish the detailed difference in Sm/Gd segregations between these two phases based on the EDS mappings.So the alloying elements of the two phases were quantitatively analyzed, as shown in Fig.8c.It is quite apparent that all alloying elements except negligible Zr are more abundant in Mg3RE phase (marked withBin Fig.8c) than in Mg5RE phase (marked withAin Fig.8c).The TEM-EDS results are basically consistent with those of SEM-EDS.Moreover, Mg3RE and Mg5RE in the Gd-modified alloys can be approximately expressed as Mg3(Sm, Gd, Zn) and Mg5(Sm, Gd, Zn) according to the specific compositions listed in Figs.3 and8.This result can provide a good interpretation to the reduced lattice parameters of the intermetallic phases since the atomic radius of Zn (0.137 nm) is smaller than that of Sm (0.180 nm) and Gd (0.180 nm).It should be noted that the fraction of Zn enriched in Mg3RE phase is 6–8 at%for the Gd-modified alloys,while the proportion of enriched Zn is approximately 15 at%in Mg3Sm phase for SG30 alloy without Gd addition.It can be concluded that the segregation behavior of Zn in Mg3Sm phase is inhibited to some extent by Gd addition.The corresponding mechanism can be attributed to electronegativity difference between Sm/Gd and Zn elements.The electronegativity values of Sm, Gd and Zn are 1.17, 1.20 and 1.65,respectively.It is evident that the electronegativity difference of Zn-Sm (0.48) is larger than Zn-Gd (0.45), indicating Zn-Sm combination is more preferable.In addition, as shown in Fig.8a, several tiny particles highlighted by the white circle are observed in grain interior.The BF-TEM image of an oval particle is shown in Fig.8d, whose chemical composition can be determined as Mg29.63Sm1.65Gd2.19Zn38.14Zr28.39based on the EDS profile shown in Fig.8e.The representative HR-TEM image of the particle and the corresponding FFT pattern are shown in Fig.8f and g, respectively.Fig.8h and i, present the individual FFT patterns, respectively generated from the regions marked by yellow and pink dashed rectangles in Fig.8f.According to the comprehensive analysis of these FFT patterns, the oval particle can be approximately indexed by primitive tetragonal Zn2Zr3phase [48]that frequently reported in Zn/Zr-containing Mg alloys after solution treatment [33,49].In this work, based on the experimental chemical composition, the oval particle can be termed as MgZnZr phase with a trace of Sm/Gd segregation.Its experimental lattice parameters(a=0.7449 nm andc=0.6855 nm)are slightly smaller than the standard values (a= 0.7633 nm andc= 0.6965 nm) of Zn2Zr3phase, which can be attributed to the composition deviation between them.It is worth notingthat the orientation relationship between the MgZnZr phase and matrix is revealed as (310)MgZnZr//(0001)Mg and [1-32]MgZnZr//[11–20]Mg, which is different from that reported between Zn2Zr3phase and Mg matrix [33,49].Analogous to Fig.5 for SG33 alloy, a certain number of undersized particles along grain boundary are observed in the HAADF-STEM image (Fig.9a) for SG37 alloy.The SAED pattern obtained from the particle indicated by the yellow arrow in Fig.9a is given in Fig.9b, revealing the coexistence of Mg3RE (fcc,a= 0.7185 nm) phase and Mg matrix.The HR-TEM image (Fig.9c) and the relevant FFT pattern (Fig.9d) demonstrate that the Mg3RE phase and Mg matrix are coherent,and their orientation relationship is (220)Mg3RE//(0001)Mg and [1–1–1]Mg3RE//[11–20]Mg.This can be well supported by the separate FFT patterns (Fig.9e and f) corresponding to the regions marked by pink and yellow dashed squares in Fig.9c.The experimental results of SG37 alloy are basically coincident with those of SG33 alloy, suggesting that the precipitates along grain boundaries are generally Mg3RE phase regardless of Gd content.

Fig.6.(a) BF-TEM image, (b) SAED pattern, (c) HR-TEM image along with (d) FFT pattern of as-cast SG33 alloy, and (e,f) FFT patterns corresponding to the regions marked by yellow and pink dashed rectangles in (c), (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).

Fig.7.(a–c) BF-TEM images, (d–f) SAED patterns, (g–i) HR-TEM images and (j–l) the corresponding FFT patterns of as-cast SG35 alloy.
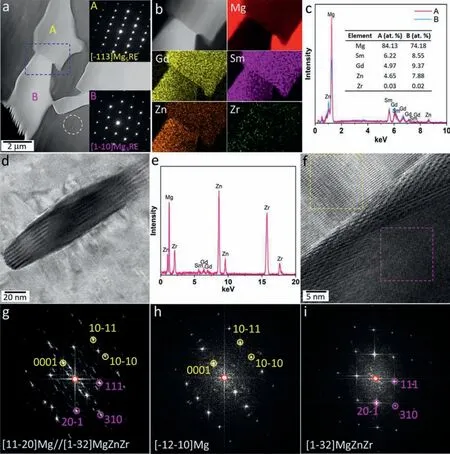
Fig.8.(a) HAADF-STEM image along with SAED patterns of as-cast SG37 alloy, (b) EDS mappings corresponding to the blue dashed rectangle region in(a), and (c) EDS spectrums for A and B regions in (a).(d) BF-TEM image, (e) EDS spectrum, (f) HR-TEM image and (g) the corresponding FFT pattern,and (h,i) FFT patterns generated from the regions marked by yellow and pink dashed rectangles in (f), (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.3.Mechanical properties
The tensile properties of as-cast SG30, SG33, SG35 and SG37 alloys at ambient temperature are shown in Fig.10.It is evident that both the true ultimate tensile strength (UTS)and true yield strength (YS) are significantly improved by increasing Gd content, whereas the elongation (EL) is gradually decreased.SG37 alloy exhibits optimal UTS(~241 MPa)and YS (~197 MPa), which are improved by ~49 MPa and~78 MPa, respectively, compared with SG30 alloy without Gd addition.
It is well known that the enhanced mechanical properties of as-cast Mg alloys can be ascribed to strengthening effect form grain refinement, intermetallic compounds, precipitation and solid solution.The grain refinement can remarkably improve the YS in Mg alloys, according to the Hall-Petch relation[50]:

wherekanddrepresent the proportional constant and the average grain size, respectively.As shown in Fig.11, theaverage grain size decreases monotonically with the increase of Gd content.Accordingly the grain refinement strengthening effect in the present alloys is gradually enhanced with the increase of Gd content.The dominant solute atoms in the matrix, Sm (0.180 nm) and Gd (0.180 nm), whose atomic radius is larger than Mg (0.160 nm), can effectively inhibit dislocation slip during plastic deformation.The solid solution strengthening can be described as [50]:
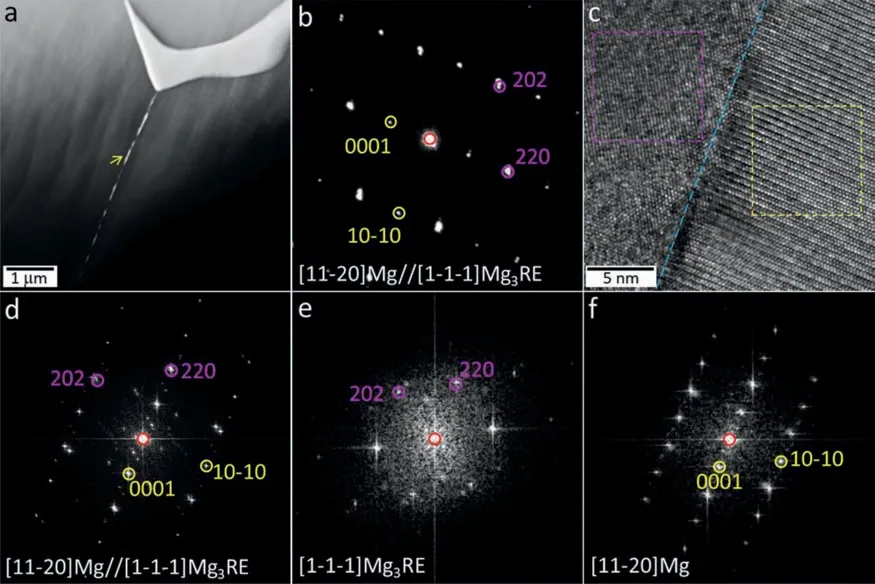
Fig.9.(a) HAADF-STEM image, (b) SAED pattern, (c) HR-TEM image and (d) the corresponding FFT pattern of as-cast SG37 alloy, and (e,f) FFT patterns generated from the regions marked by pink and yellow dashed rectangles in (c), (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).

Fig.10.The tensile properties of as-cast Mg-Sm-xGd-Zn-Zr alloys at ambient temperature.


Fig.11.The average grain size and the volume fraction of intermetallic compounds in as-cast Mg-Sm-xGd-Zn-Zr alloys.
whereCiis a constant for elementi, Xiis the atomic fraction of solutei.The maximum solid solubility of Gd in Mg matrix is 23.3 wt.% (4.48 at%), which is significantly larger than that of Sm, 5.8 wt.% (0.99 at%).Even at 200 °C, the solid solubility of Gd (3.8 wt.% or 0.61 at%) in Mg matrix is still greater than that of Sm (0.4 wt.% or 0.06 at%) [51].Therefore, the addition of Gd can significantly improve the solid solution strengthening effect of the alloy.As shown in Fig.8, some plate precipitates on basal plane are occasionally observed, which can provide effective barriers to gliding dislocations during the plastic deformation.Thus these nanoscale precipitates with large aspect ratio, although their volume fraction is not very large, can enhance the YS to some content through precipitation strengthening according to theOrowan equation [52]:

Fig.12.OM images of solution-treated Mg-Sm-xGd-Zn-Zr alloys.(a) SG30 alloy, (b) SG33 alloy, (c) SG35 alloy and (d) SG37 alloy.

whereMis Taylor factor,Gis the shear modulus of Mg,bis the Burgers vector,νis Poisson ratio (ν= 0.35),λis the interparticle spacing,r0is the core radius of the dislocation taken equal tob, anddpis the mean diameter of the precipitates.

Fig.13.Age-hardening curves of solution-treated Mg-Sm-xGd-Zn-Zr alloys aged at 225 °C.
In general, the RE-containing intermetallic compound,which is harder than the matrix, distributed at grain boundary can effectively inhibit grain boundary movement during deformation.It was reported that the strengthening effect of intermetallic compounds is largely dependent upon their crystal structure [41, 42].Chia et al.[41]demonstrated thatfccMg3RE phase provides more influential strengthening effect than body-centered tetragonal Mg12RE in Mg-RE binary alloys.Analogously, Lv et al.[42]revealed thatα-Al11La3phase (body-centered orthorhombic structure,a= 0.443 nm,b= 1.314 nm, andc= 1.013 nm) exhibits better strengthening effect thanη-Al3La phase (monoclinic structure,a= 0.4437 nm,b= 0.4508 nm,c= 0.9772 nm,andβ= 103.5 °) in a die-cast Mg-Al-La alloy.In practice,however, both the Mg3RE and Mg5RE phases in the present alloys havefccstructure.Hence,it can be inferred that the two types offccintermetallic compounds with the same content can provide equivalent strengthening effect at room temperature from the perspective of crystal structure.Significantly,the major difference between the two phases is the chemical composition, which may lead to the difference in thermal stability.The melting temperatures of Mg3Sm and Mg5Sm in Mg-Sm binary alloy are 700 °C and 565 °C, and those of Mg3Gd and Mg5Gd in Mg-Gd binary alloy are 706 °C and 642 °C, respectively [51].It can be seen that the melting temperature of Mg3RE phase is generally higher than thatof Mg5RE phase, suggesting Mg3RE phase may provide better strengthening effect at elevated temperature.In addition to crystal structure, the strengthening effect of intermetallic compounds at room temperature can be influenced by their volume fraction, morphology and even continuity.More importantly,it was demonstrated that the volume fraction of Mg-RE intermetallic compounds exerts a stronger influence on the YS than their morphology and continuity [41].We estimated the volume fraction of intermetallic compounds in each alloy by analyzing at least ten backscattered SEM images with the magnification of 500x.As shown in Fig.11, the volume fraction of intermetallic compounds increases monotonically with the increase of Gd content, i.e.3.1%, 5.4%, 8.2% and 11.7%for SG30, SG33, SG35 and SG37 alloys, respectively.Thus,it can be deduced that the volume fraction of intermetallic compounds plays a crucial role in the enhancement of YS.On the other hand, the large volume fraction of the intermetallic compound would inevitably lead to the increase of incoherent interface between the Mg-RE phase and the matrix, which further results in a greater tendency to produce voids leading to ductile failure during plastic deformation.Besides, some massive intermetallic compounds sporadically distributed along grain boundary in the alloy with high content of Gd can easily induce the stress concentration during plastic deformation, which can serve as initiation site of micro-crack.From the above discussion, it is evident that Gd addition can significantly enhance the strength of the as-cast Mg-Sm-Zn-Zr alloys at room temperature, mainly attributed to the strengthening effect of grain refinement and Mg-RE intermetallic compounds.

Fig.14.(a, b) BF-TEM images and (c, d) the corresponding SAED patterns of the typical peak-aged samples.(a, c) SG30 alloy, (b, d) SG37 alloy.
3.4.Age-hardening behavior
The representative OM images of the solution-treated samples are presented in Fig.12.It is obvious that for all kindsof alloys, the average grain size is slightly increased after solution treatment and the majority of intermetallic compounds are dissolved into the matrix.As shown in Fig 12c and d,only a small quantity of particles remain at grain boundaries in SG35 and SG37 alloys, which can be attributed to the relatively large volume fraction of intermetallic compounds in the corresponding as-cast alloys shown Figs.3 and 11.Additionally, some precipitates are distinctly observed in the interior of grains of these four samples,which have been identified as Zn2Zr3, Zn2Zr, and MgZn2phases in previous works[33,49].
Fig.13 presents the age-hardening curves of the solutiontreated Mg-Sm-xGd-Zn-Zr alloys aged at 225 °C.SG30 alloy without addition of Gd exhibits a relatively weak agehardening effect,the maximum hardness(73.3 HV)is reached after aging treatment for 8 h,which is slightly improved compared with the solution-treated sample (54.5 HV).It is worth noting that the age-hardening effect is significantly enhanced and the peak-aging time is obviously shortened in the Gdmodified alloys.The peak hardness increases with the increasing content of Gd, and the optimal hardness (145 HV) is obtained in the SG37 alloy aged for 2 h, which is improved by approximately 60 HV compared with the solid-solution state.This demonstrates that the combined addition of two RE elements belonging to different subgroups can provide surprising strengthening effect in Mg alloys.Similar results were also reported in previous works [35,51,53–55].For instance,Mg-Gd-Sm-Zr alloys exhibited remarkable age-hardening effect,and the peak hardness of Mg-10Gd-2Sm-0.4Zr alloy was nearly 120 HV [35].It should be noted that the peak hardness of the present SG37 alloy is apparently higher than that of Mg-10Gd-2Sm-Zr alloy, although the latter contains more RE elements.This is mainly attributed to the fact that Zn addition can effectively promote the age-hardening response[4,5].
It is generally known that the size, density and type of precipitates play a critical role in the age-hardening behavior of Mg alloys.SG30 alloy without Gd addition and the typical Gd-modified SG37 alloy are selected for TEM characterization.Fig.14a presents the BF-TEM image of the peak-aged SG30 sample, in which a number of prismatic precipitates are clearly observed.This can be further demonstrated by the weak reflection spots distributed between (0000)Mgand {10–10}Mgspots in the corresponding SAED pattern presented in Fig.14c.In addition, a small number of basal precipitates marked with red arrows are sparsely distributed in the peakaged SG30 alloy although there is no distinct extra reflection along {0002}Mgin the SAED pattern.As shown in Fig.14b,both prismatic and basal precipitates are observed in the peakaged SG37 alloy.Similar to SG30 alloy, the volume fraction of basal precipitates in SG37 alloy is relatively low.But the remarkable thing is that both the volume fraction and the density of prismatic precipitates in SG37 alloy are dramatically higher than those in SG30 alloy,which can be supported by the much more distinct reflection spots distributed at 1/4,1/2 and 3/4 {10–10}Mgreflection spots in the corresponding SAED pattern presented in Fig.14d.According to the SAED pattern analysis, the predominant precipitates in SG30 and SG37 alloys are identified asβ” phase (D019 structure,a= 0.64 nm,c= 0.52 nm) andβ’phase (orthorhombic structure,a= 0.64 nm,b= 2.27 nm,c= 0.52 nm) [56], respectively.Therefore, it can be concluded that the addition of Gd can effectively increase the volume fraction and density of prismatic nano-scale precipitates in Mg-Sm-Zn-Zr alloy, thus improving the age-hardening effect prominently.
4.Conclusion
The microstructures and mechanical properties of as-cast Mg-3Sm-xGd-0.5Zn-0.5Zr (x= 0, 3, 5 and 7) alloys have been systematically investigated.The main conclusions can be drawn as follows:
(1) The intermetallic compounds with multiple morphologies are identified as Mg3Sm phase in Mg-Sm-Zn-Zr alloy.Mg5RE phase is induced by Gd addition, and its volume fraction gradually increases with increasing Gd content.
(2) The lattice parameters of both Mg3RE and Mg5RE phases are reduced by Zn segregation, and the concentration of segregated Zn in Mg3RE phase is clearly higher than that in Mg5RE phase.The addition of Gd can significantly reduce the percent of Zn segregation in Mg3RE phase.
(3) Mg3RE precipitates are generally formed along grain boundaries regardless of Gd content.Its orientation relationship with Mg matrix is (220) Mg3RE// (0001)Mg and [1-1-1]Mg3RE// [11–20]Mg.A novel primitive tetragonal MgZnZr phase (a= 0.7449 nm andc= 0.6855 nm) with trace Sm/Gd segregation is revealed in Mg matrix, their orientation relationship is(310) MgZnZr// (0001) Mg and [1-32]MgZnZr// [11–20]Mg.
(4) Both the YS and UTS at ambient temperature increase with the increase of Gd content in the present alloys,which is mainly attributed to the decreased average grain size and the increased volume fraction of intermetallic compounds.However, the latter obviously degrades the ductility of the alloy.
(5) The volume fraction and density of prismatic nano-scale precipitates in the present alloys are significantly enhanced by Gd addition, which consequently improve the age-hardening response.
Acknowledgments
This work was supported by JSPS KAKENHI for Scientific Research on Innovative Areas “Materials Science of a Mille-feuille Structure (Grant Numbers JP18H05475,JP18H05479)”, the Open Funds of the State Key Laboratory of Rare Earth Resource Utilization (RERU2020012), and“Nanotechnology Platform” of the MEXT, Japan.K.G.was supported by Grant-in-Aid for JSPS Fellows (JP19F19775).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Critical review of superplastic magnesium alloys with emphasis on tensile elongation behavior and deformation mechanisms
- Advances in hydroxyapatite coatings on biodegradable magnesium and its alloys
- Impressive strides in amelioration of corrosion and wear behaviors of Mg alloys using applied polymer coatings on PEO porous coatings: A review
- Alloying design and microstructural control strategies towards developing Mg alloys with enhanced ductility
- Friction self-piercing riveting (F-SPR) of aluminum alloy to magnesium alloy using a flat die
- The evolution of coarse grains and its effects on weakened basal texture during annealing of a cold-rolled magnesium AZ31B alloy
