A novel energetic potassium salt of 2,3,5,6-tetranitro-4H,9H-dipyrazolo [1,5-a:5',1'-d][1,3,5]triazinane:Synthesis,crystal structure and performance①
2022-07-11HUOHuanZHANGJunlinBIFuqiangLIXiangzhiLUANJieyuWANGBozhou
HUO Huan,ZHANG Junlin,BI Fuqiang,2,LI Xiangzhi,LUAN Jieyu,WANG Bozhou,2
(1.Xi'an Modern Chemistry Research Institute,Xi'an 710065,China;2.State Key Laboratory of Fluorine & Nitrogen Chemicals,Xi'an 710065,China)
Abstract:A novel energetic potassium salt 2,3,5,6-tetranitro-4H,9H-dipyrazolo [1,5-a:5',1'-d][1,3,5] triazinane (KTNDPT) was synthesized using sodium 4-amino-3,5-dinitropyrazolate as raw material, and structurally characterized by elemental analysis, IR spectra, 1H NMR, 13C NMR. The single crystal of KTNDPT was obtained and analyzed by single-crystal X-ray diffraction. The thermal behavior of KTNDPT was studied by DSC-TG method. Solid phase formation heat and density were calculated by Gaussian 09 program and Trouton′s rule, and the physicochemical detonation properties of AFTF were predicted by means of EXPLO5 detonation software. The results show that the crystal of KTNDPT·H2O belongs to a monoclinic system and space group P21/n with cell parameters: a=8.330(15), b=9.869(17), c=16.61(3)Å, β=102.04(3)°, V=1335(4) Å3, Z=4, Dc=1.966 g/cm3, F(000)=792, μ=0.480 mm-1, S=1.040, the final R= 0.1541 and wR(I> 2σ(I))=0.3779. DSC-TG analysis shows that the decomposition peak temperature of KTNDPT is 241.6 ℃.The heat of formation is 331.6 kJ/mol, detonation velocity is 8647 m·s-1 and detonation pressure is 32.0 GPa. As an energetic ion potassium salt, KTNDPT with excellent detonation performance is expected to use in high energy propellants.
Key words:potassium salt of 2,3,5,6-tetranitro-4H,9H-dipyrazolo[1,5-a:5',1'-d][1,3,5]triazinane;energetic ion salt;detonation performance;crystal structure
0 Introduction
At present, the design and synthesis of new explosives mainly focus on high energy, lower sensitivities and good thermal stability. 1,3,5-triazinane-based energetic compound is one kind of interesting high detonation performance energetic materials, such as a well-known energetic materials 1,3,5-trinitro-1,3,5-triazinane(RDX). In recent years, the energetic materials based on 4H,9H-dipyrazolo[1,5-a:5',1'-d][1,3,5] triazinane with a tricyclic framework in one molecule and the same plane become a prominent family of energetic compounds with high densities and low sensitivities. For example, 2,3,5,6-tetranitro-4H,9H-dipyrazolo[1,5-a:5',1'-d][1,3,5] triazinane(TNDPT) and its ammonium salt(ATNDPT) belong to a kind of the potential high-performance insensitive explosive with some desirable traits, including high detonation velocity, low impact sensitivity, and low friction sensitivity. However, it is noted that ATNDPT has the lower thermal stability(=220.0 ℃) and lower density(1.82 g/cm). In order to improve its properties, potassium ion is introduced to the molecule of 2,3,5,6-tetranitro-4H,9H-dipyrazolo[1,5-a:5',1'-d][1,3,5] triazinane to obtain new energetic ionic salt. Especially, the potassium salt as a new energetic flame inhibitor would be applied for the propellant and gun propellant. At the same time, it is necessary that its crystal structure is thoroughly studied to explain the main reason improving energetic property.
Using sodium 4-amino-3,5-dinitropyrazolate as the raw material, a novel energetic potassium salt of 2,3,5,6-tetranitro-4H,9H-dipyrazolo[1,5-a:5',1'-d][1,3,5] triazinane(KTNDPT) is designed and synthesized via cyclization, acidification and neutralization reaction for the first time. Its single crystal is firstly obtained and analyzed by X-ray single-crystal diffraction analysis, and its thermal behaviors are investigated using DSC-TG under the rate of 10 K/min. Furthermore, the physicochemical and energetic properties are also studied by experimental method and calculated method to supply the basic parameters for its application in the propellant and gun propellant.
1 Experimental
1.1 Materials and instruments
GainH NMR andC NMR in DMSO-on a Bruker AV500 NMR spectrometer. Infrared spectra from KBr pellets on a Nicolet NEXUS870 Infrared spectrometer in the range of 4000~400 cm.Perform elemental analyses(C, H and N) on a VARI-El-3 elementary analysis instrument. Differential scanning calorimetry(DSC) are studied on a Q200 apparatus(TA, USA) at heating rate of 10 K/min under dry oxygen-free nitrogen atmosphere with a flowing rate of 50 ml/min. The TG-DTG experiment is performed with a SDT-Q600 apparatus(TA, USA) operating at heating rate of 10 K/min in a flow of dry oxygen-free nitrogen at 100 ml/min.
Sodium 4-amino-3,5-dinitropyrazolate is prepared according to the published procedures. Other chemicals are obtained from commercial sources and used without further purification.
1.2 Synthesis
The title compound KTNDPT is synthesized by the method in Fig.1.
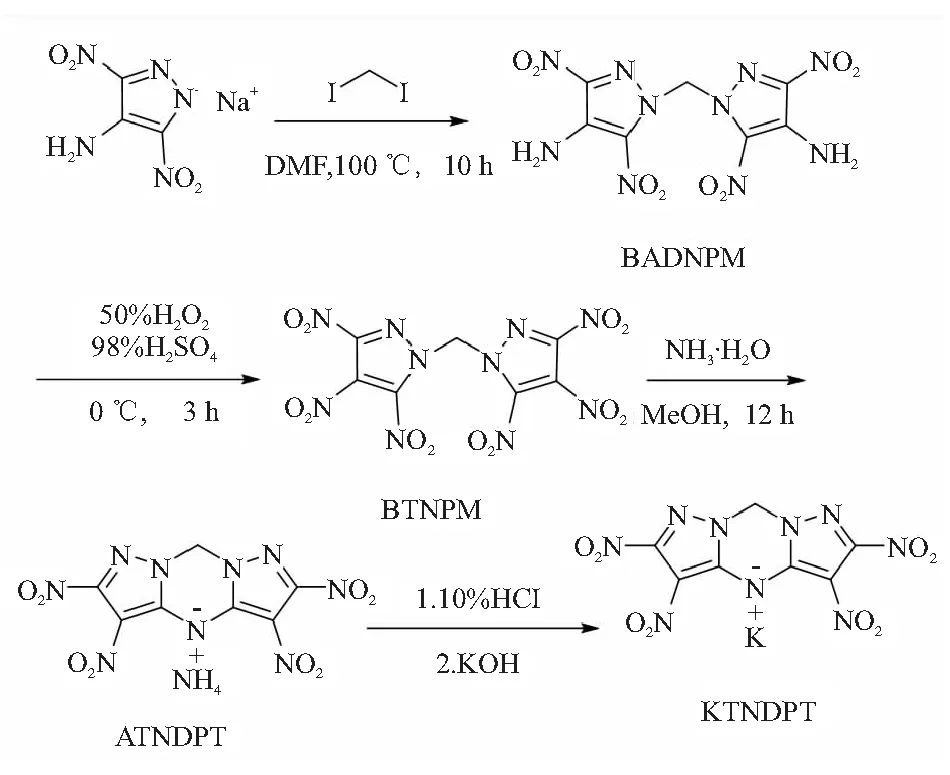
Fig.1 Synthesis of the title compound(KTNDPT)
1.2.1 Preparation of BADNPM
In a 100 ml round-bottom flask equipped with a stir bar, sodium 4-amino-3,5-dinitropyrazolate(10.0 g, 51.3 mmol) and 30 ml of DMF are added sequentially. Then, diiodomethane(6.25 g, 23.3 mmol) is added.The mixture is warmed to 100 ℃, and stirred for 10 h at this temperature, and then dropped to room temperature. The reaction mixture is poured into 200 ml water, and 1.0 g sodium thiosulfate is added, then stirring for 10 min until the suspension turn bright yellow.Then the solid is collected by Büchner filtration and air dried to afford 7.2 g yellow solid BADNPM with a yield of 86.6%.
H NMR(DMSO-, 500 MHz),: 8.897(s, 1H, NH),7.285(s, 4H, NH), 7.166(s, 2H, CH).C NMR(DMSO-, 125 MHz),: 142.982, 131.703, 130.854, 67.115. IR(KBr),(cm): 3484, 3461, 3368, 3350, 2082, 3029, 1644, 1579, 1511, 1480, 1447, 1387, 1353, 1305, 1277, 1234, 1217, 1149, 1102, 1063, 1004, 886, 828, 786, 804, 759, 744, 661, 605, 480. EA (CHNO,%), calcd: C, 23.47; H, 1.69; N, 39.10. Found: C, 23.42; H, 1.74; N, 39.39.
1.2.2 Preparation of BTNPM
In a 100 ml round-bottom flask immersed in an ice bath, HSO(98%, 10 ml) is added, followed by dropwise addition of HO(50%,15 ml). In the mixture, the solution of BADNPM(2.0 g, 5.6 mmol) in HSO(98%, 10 ml) is added dropwise slowly at 0~5 ℃. After the addition is complete, the reaction mixture is stirred for 3 h at this temperature, further stirred for 10 h at room temperature. Then the reaction mixture is poured into ice water(200 ml), the obtained precipitate is filtered off, washed with ice water, and air-dried to obtain 1.9g BTNPM with a yield of 81.7%.
H NMR(DMSO-, 500 MHz),: 7.897(s, 2H, CH).C NMR(DMSO-, 125 MHz),: 144.187, 138.705, 123.794, 67.283. IR(KBr),(cm): 3075, 3008, 2890, 1586, 1566, 1544, 1472, 1364, 1335, 1302, 1264, 1206, 1158, 1086, 1000, 909, 845, 807, 776, 743, 674, 655, 507, 494. EA (CHNO,%), calcd: C, 20.11; H, 0.48; N, 33.50. Found: C, 20.12; H, 0.64; N, 33.59.
1.2.3 Preparation of ATNDPT
Bis(3,4,5-trinitro-1H-pyrazol-1-yl)methane(0.6 g, 1.43 mmol) is dissolved in a solution of methanol(5 ml) and NH·HO (25%~28%, 3.0 g, 49 mol). The mixture is subsequently stirred at room temperature for 10 h. Then the orange precipitate is filtered off and dried in air to afford 0.4 g solid with a yield of 80%.
H NMR (DMSO-, 500 MHz),: 6.197(s, 2H, CH2), 4.459(br, 4H, NH).C NMR(DMSO-, 125 MHz),:147.520, 145.115, 110.502, 63.303. IR(KBr),(cm): 3631, 3548, 3154, 3039, 1562, 1496, 1421, 1360, 1334, 1308, 1246,1221, 1109, 1078, 954, 942, 845, 826, 811, 759, 727, 697. EA(CHNO,%), calcd.: C,23.47; H, 1.69; N, 39.11.Found(%): C,23.62; H,1.77; N, 39.40.
1.2.4 Synthesis of KTNDPT
To a 25 ml round-bottom flask immersed in an ice bath is added 10 ml of 10% aqueous solution of hydrochloric acid. After the solution is chilled to 0 ℃,ATNDPT(0.4 g, 1.1 mmol) is added slowly while the reaction temperature is maintained at 0~5 ℃ during the addition. The mixture is subsequently stirred at same temperature for 30 min, the solid is filtered off and washed with water, then dissolved in ethanol(10 ml) and treated with KOH(0.064 g, 1.1 mmol) in ethanol(5 ml) at room temperature. The precipitate is filtered off and dried in air to afford 0.3 g orange solid with a yield of 72%.
H NMR(DMSO-, 500 MHz) ,: 6.184(s, 2H, CH).C NMR(DMSO-, 125 MHz),:149.871, 145.635, 111.130, 63.271. IR(KBr),(cm): 3640, 3554, 3301, 3254, 3040, 1619, 1561, 1497, 1420, 1362, 1333, 1307, 1247, 1220, 1170, 1108, 1079, 955, 941, 847, 827, 813, 759, 727, 697, 628, 612. EA(CHNOK·HO,%), calcd:C,21.16; H, 1.01; N, 31.73. Found(%):C, 21.42; H, 1.01; N, 31.84.
2.3 Crystal structure determination
The single crystal for KTNDPT·HO is cultivated by slow evaporation from methanol at room temperature in order to give satisfactory crystals for-ray determination. The crystal with approximate dimensions of 0.35 mm× 0.27 mm×0.14 mm is mounted on the top of a glass fiber in a random orientation. The unit cell determination and data collection are performed with a Mo-radiation (= 0.71073 Å) on a Bruker Smart APEX-CCD diffractometer equipped with a-scan mode in the range of 2.41°≤≤25.10° at 296(2) K. A total of 6285 reflections are collected with 2369 unique ones (= 0.2263), of which 2139 observed reflections with>2() are used in the succeeding refinements. Diffraction data are collected for 384 unique reflections with -9≤≤9, -11≤≤6 and -19≤≤17. Absorption correction is not used. The structure is solved by direct methods using SHELXS program of the SHELXL-97 package and refined with SHELXL package. The final refinement is performed by full-matrix least-squares method with anisotropic thermal parameters on F2 for the non-hydrogen atoms. Crystal data and refinement results are summarized in Table 1. Crystallographic data for the structural analysis have been deposited in the Cambridge Crystallographic Data Center, CCDC No.1952606.
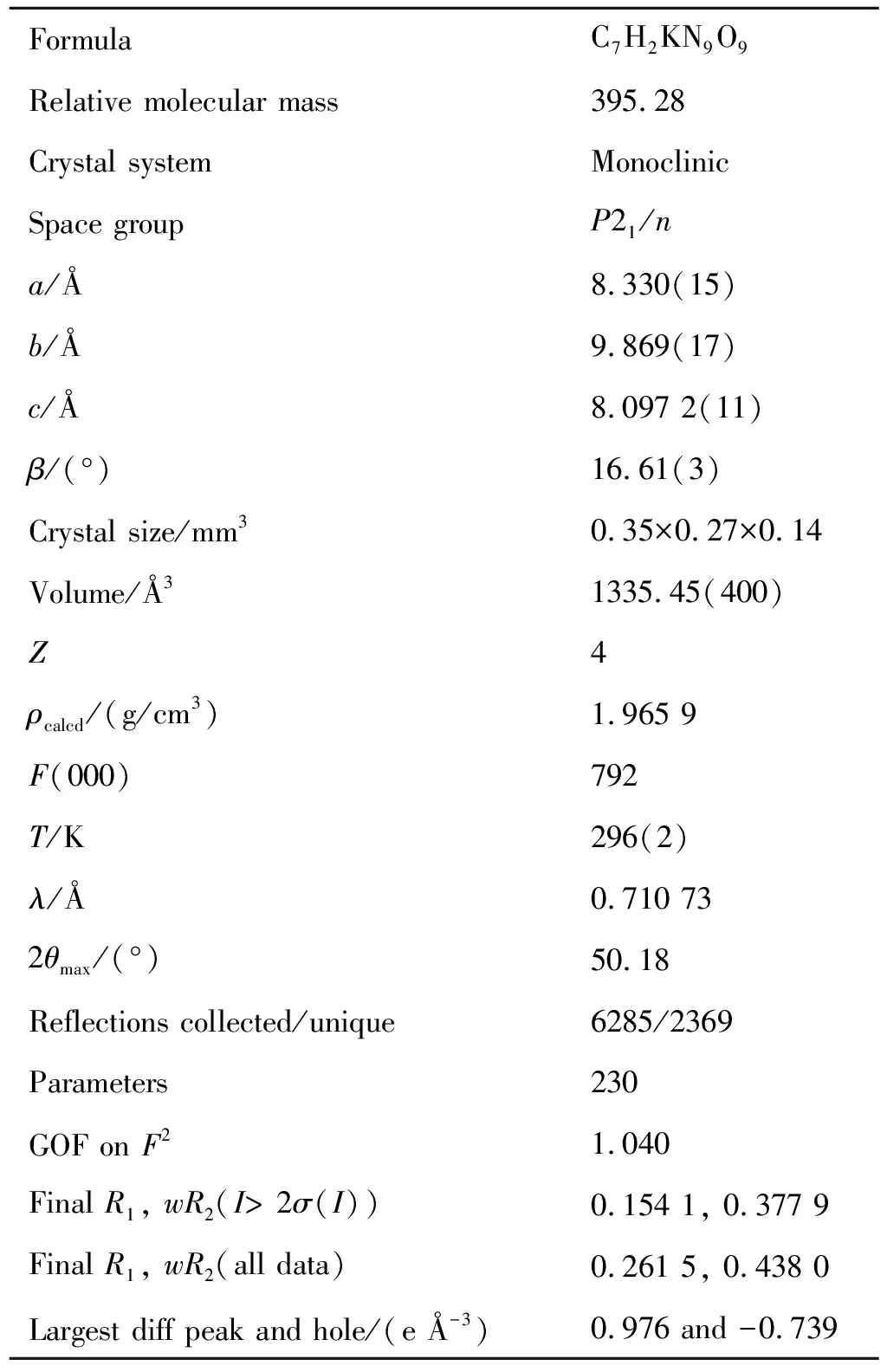
Table 1 Crystal data and structure refinement details
3 Results and discussion
3.1 Crystal structure
The crystal of KTNDPT·HO belongs to monoclinic with space group2, and containing four molecular moieties in the unit cell with the higher density(1.996 g/cm). The molecular structure of KTNDPT·HO is shown in Fig.2, and intermolecular hydrogen bonding interactions in the crystal structure are shown in Fig.3.
Selected bond lengths, bond angles and dihedral angles are listed in Table 2. Hydrogen bond lengths and bond angles are given in Table 3.
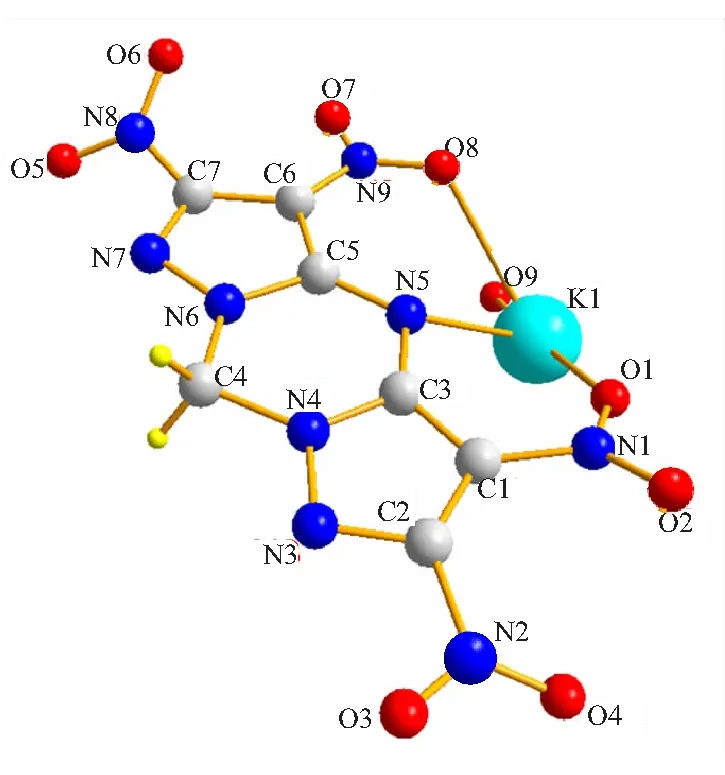
Fig.2 Molecular structure of KTNDPT·H2O

Fig.3 Molecular packing of the unit cell in thecrystalline state of KTNDPT·H2O
In the molecular structure of KTNDPT·HO, there is one central Kion, one TNDPTanion and one water solvent molecule, and this water molecule is coordinated to the potassium center. Each Kion in the unit cell is connected with TNDPTanion through four K—O coordination bonds(K(1)—O(1)(2.650(13) Å), K(1)—O(9)(2.744(13) Å), K(1)—O(8)(2.880(13) Å), K(1)—O(5)#1(2.893(13) Å) and a K—N coordination bond(K(1)—N(5)(2.961(11) Å)). At the same time, each TNDPTanion interacts with four adjacent Kions through the same coordination interactions. Herein, we also observe some weak K—O interactions(K(1)—O(7)#2(3.056(12) Å), K(1)—O(8)#2 (3.058(12) Å), K(1)—O(5)#3(3.086(12) Å)) and K(1)—N(7)#1 interaction(3.188(12) Å) in the crystal packing of KTNDPT. All finally expand into a three-dimensional net structure through coordination bonds, electrostatic forces(Fig.3).
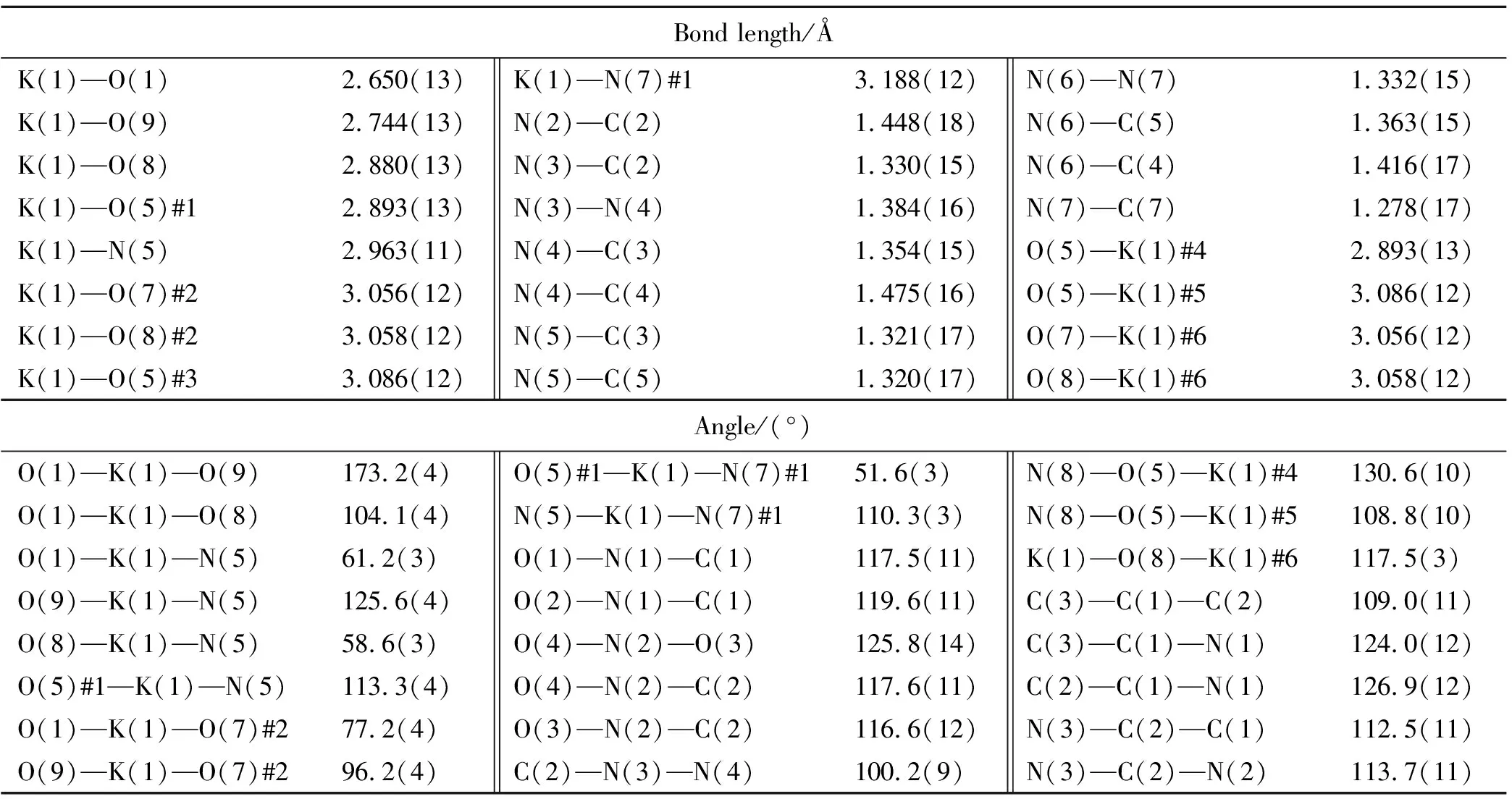
Table 2 Selected bond length and bond angles of the title compound

Table 3 Selected torsion angles(°)
As seen in Table 1, according to X-ray diffraction structural determination, the bond lengths of the pyrazole and triazinane rings in the molecular structure of KTNDPT·HO lie between the lengths of formal C—N and N—N single and double bonds(C—N: 1.47, 1.22 Å; N—N: 1.48, 1.20 Å), and each ring is almost planar. It is interesting to find that the N—C bond length(N(5)—C(5): 1.320(17) Å, N(5)—C(3): 1.321(17) Å) are slightly shorter than other N—C bond length(N(4)—C(4): 1.475(16) Å, N(4)—C(3): 1.354(15) Å, N(6)—C(5): 1.363(15) Å, N(6)—C(4): 1.416(17) Å) in the triazinane ring. As expected, the molecule of dipyrazolo[1,5-a:5',1'-d][1,3,5]triazinane shows a complete planar, since the torision angles of C(2)—N(3)—N(4)—C(4), C(5)—N(5)—C(3)—C(1), C(4)—N(6)—N(7)—C(7), C(4)—N(4)—C(3)—C(1), N(3)—N(4)—C(3)— N(5) and N(7)—N(6)— C(4)—N(4) are -179.3(11)°, -177.1(14)°, 177.9(12)°, 178.6(12)°, -178.0(12)° and 179.4(11)°, respectively. The results show that the title molecular has good thermal stability because of the-stacking and existing of a conjugated system in the molecular structure.
3.2 Thermal behavior
Thermostability of an energetic compound is especially important for processing and storage. In order to investigate its thermostability, typical DSC and TG-DTG experiments are employed on KTNDPT in the range of 50~450 ℃, and the curves (Fig.4 and Fig.5) exhibit good thermal stability as evidence. The DSC curves of KTNDPT in Fig.5 exhibit one thermal decomposition peak temperature of 241.6 ℃ with the heating rate of 10 K/min, which is without previous melting. The TG-DTG curves of KTNDPT are shown in Fig.5. For KTNDPT, there are two decomposition processes with the DTG peaks. The first exothermic process, ranging from 214.3 ℃ to 243.8 ℃, occurr in an intensive way with the peak temperature located at 230.83 ℃ and a total mass loss of 32.6%. The following exothermic process is from 243.8 ℃ to 399.0 ℃ and accompanied with 25.33% mass loss. It is noteworthy that there is still 35.65% residue observed when further heated to 487.1 ℃, indicating an incomplete decomposition behavior of the material.

Fig.4 DSC curve of KTNDPT

Fig.5 TG-DTG curves of KTNDPT
3.3 Physicochemical and energetic properties
Quantum computation studies are carried out with the Gaussian 09(Revision A. 01) suite of programs for analysis of the physicochemical and energetic properties of KTNDPT. Optimized structures are characterized to be true local energy minima on the potential-energy surface without imaginary frequencies and the gas phase heats of formation are calculated with the atomization method based on the Gaussian 09 program package at the CBS-4M level of theory. The enthalpies of the gas-phase species are estimated according to the atomization energy method. Gas phase heat of formation is transformed to solid phase heat of formation by Trouton′s rule. Based on the data of density and heat of formation, the detonation velocity and detonation pressure for KTNDPT is calculated by EXPLO5 6.04. All the data are summarized and compared with those of TNDPT, ATNDPT and RDX in Table 4.
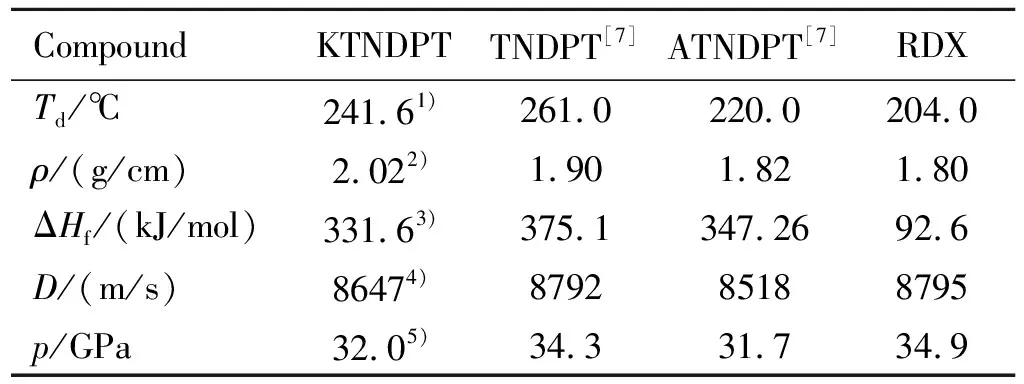
Table 4 Physicochemical and energetic properties of
Interestingly, KTNDPT exhibits slightly higher thermal stability than ATNDPT and slightly lower thermal stability than TNDPT. In the same time, the compound KTNDPT also demonstrated excellent detonation properties which is similar to TNDPT(=8792 m/s,=34.3 GPa,=1.90 g/cm), ATNDPT(=8518 m/s,=31.7 GPa,=1.82 g/cm) and RDX (=8763 m/s,=35.1 GPa,=1.81 g/cm).
4 Conclusions
A new energetic potassium salt of 2,3,5,6-tetranitro-4H,9H-dipyrazolo[1,5-a:5',1'-d][1,3,5] triazinane (KTNDPT) is synthesized and characterized thoroughly. KTNDPT·HO crystallize in the monoclinic system with space group2and a density of 1.996 g/cm. Its thermal properties are investigated by DSC and TG with decomposition peak temperature of 241.6 ℃. Additionally, for KTNDPT, the physical and energetic properties are studied by calculation and experiment. The calculated detonation velocities and pressures of KTNDPT(=8647 m/s,= 32.0 GPa) is comparable with those of ATNDPT and RDX. The results show KTNDPT has good thermal stability and some characteristics of explosive, which might be used as a potential energetic material or as an ingredient for priming composition.
