Green Synthesis of Nitrogen-to-Ammonia Fixation: Past,Present, and Future
2022-07-04JianyunZhengLiJiangYanhongLyuSanPingJiangandShuangyinWang
Jianyun Zheng* , Li Jiang, Yanhong Lyu, San Ping Jiang, and Shuangyin Wang
1. Introduction
Food and energy security and sustainability are the two most grand challenges facing humankind today across the world.Ammonia(NH3)is one of the most critical ingredients in the food supplier chain as NH3is the essential fertilizer for the agricultural and food production sector.[1,2]Since the discovery of Haber–Bosch(HB)process in 1909,the important process has produced a large proportion of global NH3production over 100 years.[3]The world production of NH3by HB process is over $60 billion annually, and nearly, 80% of the produced NH3is used as the fertilizer in agriculture(see Figure 1).The practical NH3production via HB process enables the global population to nearly quadruple since the rapid implementation of the process in the early 20th century. In the energy field, NH3is currently acknowledged as a promising hydrogen energy carrier because of high volume energy density (13.6 GJ m-3) and easy transportation characteristics (boiling temperature of -33.5 °C).[4]However, to drive the rupture of N≡N and hydrogenation reaction, the HB process involves in high temperature (400–500 °C) and pressure (10–20 MPa) reaction conditions, which accounts for around 1.5% of total global carbon dioxide(CO2) emissions and consumes about 2% of the world’s annual energy supply.[5]Therefore,pursuing an alternative, green, and environmentally efficient process for nitrogen (N2)-to-NH3fixation with renewable energy is very significant for sustainable NH3production.[6–8]
In view of compatibility with renewable energy source, low product cost and potential scalable production, photocatalytic, electrochemical, photoelectrochemical (PEC), and plasma-driven approaches are recognized as the promising and competitive next-generation NH3synthesis technologies.[9–13]These approaches not only carry out the N2-to-NH3fixation under mild conditions like room temperature and atmospheric pressure but also can be powered by renewable energy source such as sun and wind.[14–16]Generally, the photocatalytic process is directly driven by sunlight to propel the activation and hydrogenation of N2. This type of devices is most simple and low-cost, but shows the low chemical utilization of solar energy.Green electrolytic reaction units for N2reduction reaction(NRR) are powered by solar cells and wind turbines, which usually necessitate use of two encapsulation and support structures. The integrated modularity is the most mature and benefit with high technology readiness,but its cost is also highest.In comparison with photocatalytic and electrochemical method,PEC device is an economically viable solution by combining the catalyst and the solar absorbers into a fully integrated system, which has the considerable chemical utilization of solar energy and acceptable cost. The plasma-based processes can generate highly reactive species to activate N2and facilitate NH3synthesis under atmospheric pressure. Although this approach can obtain high NH3production rate,low selectivity,high energy consumption,and expensive devices limit the application of plasma-driven NRR. Furthermore,in addition to the direct fixation of N2to NH3, an indirect conversion route including oxidation of N2to nitrate and reduction in nitrate to NH3has been implemented by the use of the above approaches.[17–19]
Indeed,the great prospect has inspired a flurry of research activity to increase the NH3production rate and conversion efficiency of the approaches. Important milestones in the research and development of this emerging field are highlighted in Figure 2.[20–25]The research activities in the green conversion of N2to NH3can be constructively divided into three major groups: 1) the selectivity and adjustment of various catalysts;[26–29]2) the type of electrolyte/solvent system;[22]and 3) the investigation of reaction conditions.[25,30]Recently, much effort and progress have been made in green NH3synthesis using photocatalytic and (photo-)electrochemical approaches, and meanwhile,some questions that the detected NH3is derived from the extraneous contamination rather than N2have arisen among some researchers in this field (Figure 1).[31]Herein, we briefly discuss the past advances and recent critical activities in the area of sustainable N2fixation and subsequently provide a perspective for rational and healthy development of this area.
2. Selectivity and Adjustment of Catalysts
Catalysts are the core component of both photocatalytic and (photo-)-electrochemical N2-to-NH3fixation and are absolutely vital for the N2absorption,hydrogenation reaction,and NH3desorption dynamic processes to influence the performance of NRR.[32,33]To date, a series of catalysts have been designed and prepared via various theoretical and experimental routes to carry out sustainable NH3production.Currently,the study of catalysts can primarily concentrate on the types of materials and improvement strategies, including noble metal-based materials,non-noble metal-based materials, nonmetal-based materials, and defect engineering. Ruthenium (Ru),[28]gold (Au),[34]and palladium(Pd)[35]are usually explored in photocatalytic and (photo-)electrochemical NRR under mild conditions (see Figure 3a). For example,Han et al. have reported that a catalyst with diatomic Pd-Cu sites dispersed on N-doped carbon show high activity and selectivity with an NH3formation rate of ~69.2 µg∙h-1∙mg-1and a faradic efficiency of~24.8%.[35]Non-noble metal-based materials such as Bi, Ti, and Cu have been currently explored as efficient catalysts for photocatalytic and(photo-)electrochemical NRR.An Bi4O5I2catalyst with oxygen vacancy and hydroxyl functional group, which can mimic “π back-donation”behavior by the presence of sufficient vacant orbitals, has been used to enhancing NRR activity in neutral media.[36]This catalyst reaches a splendid faradic efficiency of 32.4%superior to most of the other NRR catalysts in mild conditions.[36]Furthermore,nonmetal-based materials can not only offer good mechanical flexibility and electrical conductivity,but also more importantly,have sufficient catalytic active centers by the introduction of defects.[37]To date,some nonmetal-based materials including conducting polymers and organic carbon-based materials have been explored as catalysts for green NRR.[38]In addition to the use of defect engineering,other enhanced routes such as Li+incorporation,[10]aerophilic-hydrophilic heterostructure,[23]and interface engineering[39]have been investigated for green conversion of N2to NH3under mild conditions. Besides, many of theoretical calculations have also showed that these materials can be major active centers to enhance the N2adsorption, decrease the reaction energy barrier and permit the stabilization of hydrogenated N2species.
3. Type of Electrolyte/Solvent System

Jianyun Zheng received his Ph.D.degree in Physical Chemistry from Shanghai Institute of Ceramics,Chinese Academy of Sciences in 2015.From September 2015 to September 2019,he successively worked in Lanzhou Institute of Chemical Physics as an assistant research fellow and Hunan University and Curtin University as a united postdoctoral researcher.Currently,he is an associate professor in the College of Chemistry and Chemical Engineering in Hunan University.His main interests focus on the preparation of semiconductor materials,design,and assembly of photoelectrodes and photoelectrochemical devices,and their performance in photo(electro-)catalysis.

Li Jiang is currently a graduate student in Hunan University,under the supervision of Prof.Jianyun Zheng and Prof.Shuangyin Wang. Her current research interest is photoelectrochemical nitrogen reduction reaction.

Yanhong Lyu received her Pd.D. degree in Physical Chemistry from Shanghai Institute of Ceramics, Chinese Academy of Sciences in 2015.She currently works in Hunan First Normal University as a researcher. Her researches mainly focus on the (photo-)-electrochemistry, nanoscale analysis, and surface engineering of the materials for water splitting and nitrogen reduction.

San Ping Jiang is a John Curtin Distinguished Professor at the Western Australian School of Mines: Minerals,Energy and Chemical Engineering and Deputy Director of Fuels and Energy Technology Institute, Curtin University,Australia. Dr Jiang obtained his PhD from The City University,London in 1988. Before 2010,Dr. Jiang worked at Nanyang Technological University in Singapore. His research interests encompass fuel cells, water electrolysis, supercapacitors,carbon dioxide reduction, single-atom catalysts, and nanostructured functional materials.
As important as the catalyst, the electrolyte/solvent system is responsible for sufficient reaction elements or compounds at the solid/liquid interface, efficient conductivity in the overall reaction process, and appropriate pH environment toward targeted production, contributing to outstanding catalytic performance. As mentioned in the section of Catalysts, aqueous electrolytes have drawn attentions of numerous researchers to frequently explore and investigate in green NRR process because of environmental friendliness and rich reserves of water resource. However, a tremendous challenge for the use of aqueous electrolyte is low N2solubility and immediate availability of H+leading to poor NRR selectivity. Thus, an effective way to enhance the NRR performance is changing the electrolyte media, especially ionic liquid.Ionic liquid is a typical non-aqueous electrolyte, which only contains trace of water to offer the proton source and effectively suppresses the H2evolution. Meanwhile, certain ionic liquid can provide a high N2solubility under ambient conditions, as much as 20 times higher than aqueous electrolyte. For instance, MacFarlane group has reported ionic liquids with high N2solubility as electrolytes to obtain a high conversion efficiency of 60% for electrocatalytic NRR on a Fe-based catalyst(Figure 3b).[22]A series of other ionic liquids have been also tested for NRR at room temperature and enhanced the reaction selectivity toward NH3production. The NH3yield rates for NRR are quite low in ionic liquids although high conversion efficiency is achieved.In addition,the ionic liquids are non-green and expensive, not in accordance with the green synthesis requirements.

Shuangyin Wang received his Ph.D. in 2010 from Nanyang Technological University, Singapore. He was a postdoctoral fellow working with Prof. L.Dai (2010–11) and Prof. A.Manthiram (2011–12). He was a Marie Curie Fellow at the University of Manchester with Prof. K. Novoselov (2012–13).He is currently a Professor of the Key Laboratory for Graphene Materials and Devices and College of Chemistry and Chemical Engineering, Hunan University. His research interests are in novel catalysts, defects in various crystals and their application in electrocatalysis.
4. Investigation of Reaction Conditions
To further overcome the obstacles of yield rate and conversion efficiency,certain studies have started to control the reaction conditions to change the thermodynamic of NRR.According to Le Chatelier’s principle, the pressurized reaction environment can facilitate the balance toward the NH3production for NRR as a volume-reduced reaction and inhibit the hydrogen evolution owing to a reaction of an increasing volume.[30]In addition, the N2solubility in the electrolytes is directly proportional to the reaction pressure, which can affect the supply and diffusion of N2source. Encouragingly,the recent outstanding research work has revealed that the increased reaction pressure can be beneficial for improving NRR performance,achieving a record-high NH3yield rate of~74.2 µg∙h-1∙cm-2and a faradaic efficiency of ~20.4%, which exhibit 7.3-and 4.9-fold enhancements than those produced at ambient pressure(Figure 3c).[25]Actually, compared with the improvement of catalysts and electrolytes,the investigation and development of system pressure could be more promising to break the current limitations of NH3yield rate for NRR.
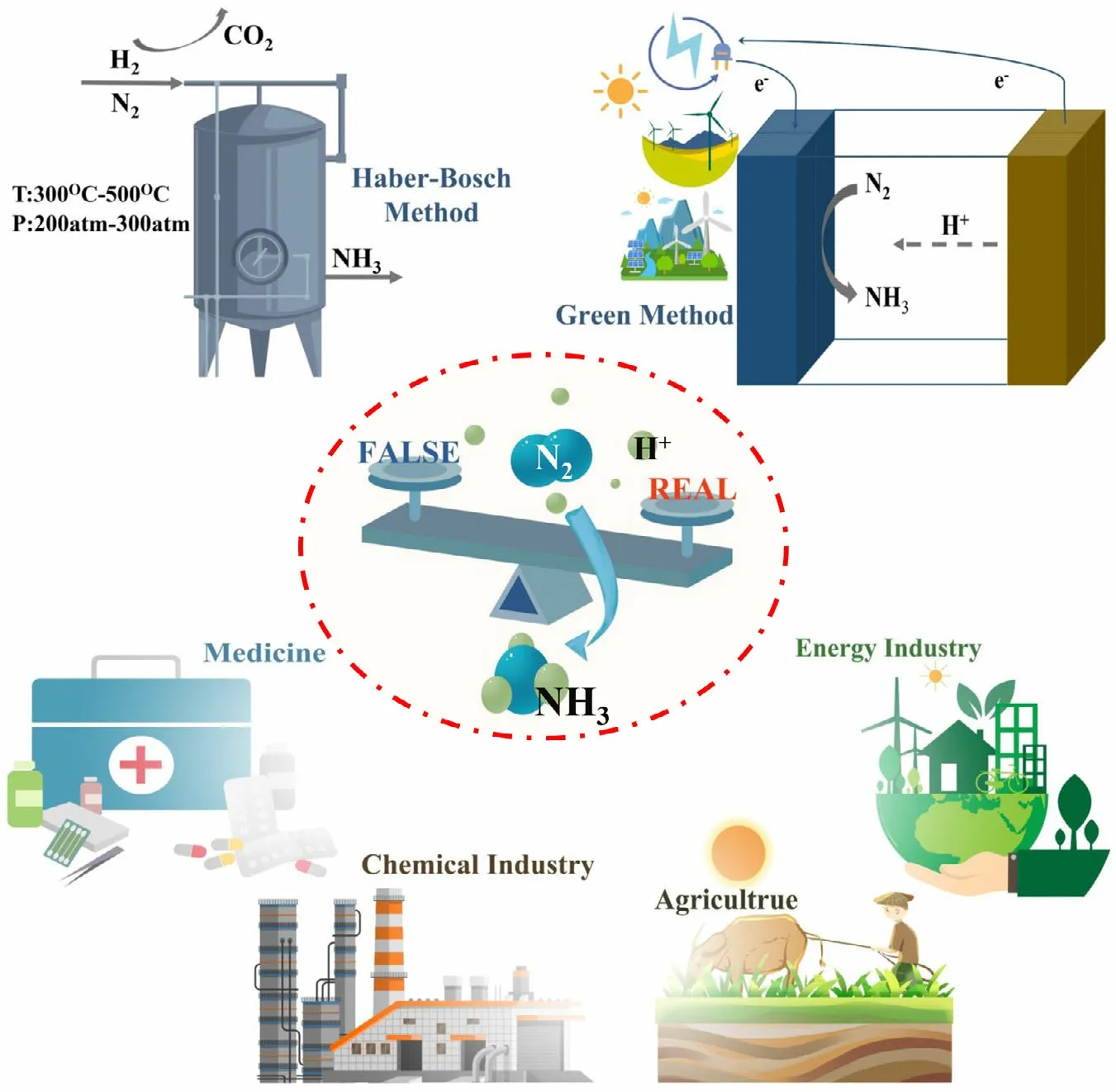
Figure 1. Schematic diagrams for N2-to-NH3 fixation, including its synthesis methods, current dilemma, and application domain.
5. Current Dilemma
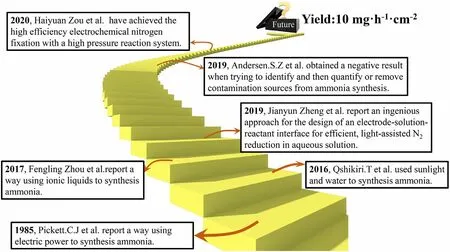
Figure 2. Timeline of the key developments in the field of green N2-to-NH3 fixation.
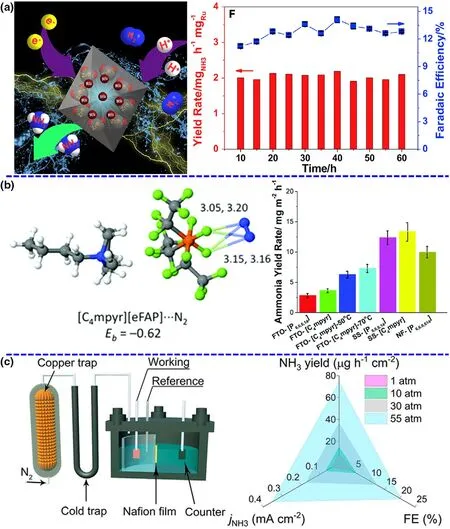
Figure 3. Short overview of green NRR from three major groups. a) Schematic diagram and NH3 yield rate of electrocatalytic NRR by Ru single. Reproduced from ref. 28 with permission from Elsevier B.V. (Copyright 2019).b) N2 binding energy and NRR performance in the ionic liquids. Adapted from ref. 23 with permission from The Royal Society of Chemistry (Copyright 2017). c) Schematic of the pressurized NRR setup and NH3 yield rate at the different N2 pressures. Panels were reproduced with permission from ref. 25, National Academy of Sciences (Copyright 2020).
The research field of green NRR still faces various problems in current stage although some achievements have suggested the potential values of this method. The major problem has been discussed as to whether the green N2-to-NH3fixation could be a practical and feasible or fictional and false way. Significant doubt and uncertainty in the NRR research are mainly derived from the various and potential contamination sources.[40]According to the source of contamination, the contamination can be grouped as extra-systematic and intra-systematic contamination.The NH3and labile nitrogen-containing compounds (e.g., NOx)from ambient environment such as the air and rubber gloves are referred to as the extra-systematiccontamination,which can be easily excluded by a closed system and rigorous operation.The intrasystematic contamination present in the catalysts, electrolytes, and feed gas has a significant influence on the true NRR performance.However,such intra-systematic contamination can be identified and eliminated by a series of control experiments and reaction units. Based on the rough calculation, all these contaminations can provide dozens or even hundreds of microgram of NH3production, showing a similar order of magnitude compared with the reported results of green NRR.[41]So far, the NH3yield rate by green NRR process ranges in the value from 1 to 70 µg∙h-1∙cm-2,which is too low to satisfy the practical production and suppress the interference of contamination. Under the current situation, it is extremely necessary for the researchers to adopt a more cautious attitude to treat all the results of green NRR or contamination. After all, enhancing the accuracy and reliability of the published literatures can be conducive to develop the green synthesis of N2-to-NH3fixation. In addition, the disturbance of contamination only exists in certain conditions and objects and is not of universality. It is confirmed that there is no discernible amount of NH3production detected in many NRR tests by different research groups and reaction methods.In fact,the involved literature reports on the false positives of NRR can be aimed at underlining and eliminating the effect of special contamination. Finally, all of the reported nitrogen-containing contamination can be effectively cleared away via an appropriate reaction unit, a rational experimental process,and a series of useful control experiments to achieve a“genuine” NRR. To improve the current situation of NRR, many of the research groups have proposed several rigorous and complicated protocols on basis of their own experimental routes.[24]Nevertheless, these protocols are too complicated to apply in all of the laboratories,especially for cash-strapped research group, and a complicated experimental process usually involves more experimental steps resulting in the more possibilities to introduce the contamination. In this perspective,we will also describe a facile protocol with a simple reaction unit available for reference purposes in the next section. In a word, the main reason for the difficulty in the NRR or contamination issues is the extremely low NH3yield rate by green methods. Therefore, the top priority of green NRR in future is to increase the yield rate by leaps and bounds.

Figure 4. Schematic diagrams for increasing the NH3 yield rate, which contributes new opportunities for developing green NRR.
6. Future Challenge, Strategy, and Prospect
The major challenges we face in pursuit of practical application of green NRR are the very low NH3production rate with the disturbance of contamination. The low NH3production by green NRR under mild conditions can be attributed to the inherent limitations of reaction process like the rupture of N≡N and low N2solubility.The disturbance of contamination is regarded as the technical puzzles of green NRR in its infancy, which has often happened in creating and exploiting a new reaction or method. When the NH3yield rate still maintains the super low level, the results of NRR not only are difficult to be characterized by the existing NH3detection methods but also are easily affected by the contamination to display the “false positive.” On the other hand, the low NH3production from so-called NRR makes no sense to be reported when the contamination involves in the experiments.
With respect to the breakthrough of NH3yield rate, there are several points in the NRR system should be considered in priority (see Figure 4). There is no doubt about the significance of the catalysts for NRR, but the routine improvements of the catalysts hardly break through the bottleneck of green NRR. The composite catalysts with different active sites to adsorb, activate, and hydrogenate N2and desorb NH3in series will be the inevitable development direction of NRR, but how to precisely synthesize and characterize the catalysts and testify the tandem and coupling mechanism can be the key points.In comparison with the catalysts, we can pay more attention to the selection and control of methods, electrolytes, and reaction conditions.Current technology for green NRR basically uses a single method,such as photocatalysis or electrocatalysis, which shows a poor NRR efficiency. In fact, utilizing the complementarity between methods, a few efforts on coupling the different methods can effectively enhance the performance of NRR, possibly involving the field enhancement effect and multi-step reaction chains (e.g., electrochemistry-photoelectrochemistry tandem device). On the other hand, developing novel and characteristic electrolytes has drawn the attentions of the researchers. We think that the future electrolytes may be neither single aqueous electrolytes nor ionic liquid electrolytes and can be a kind of mixed electrolytes with multiple phase to integrate the required functions (e.g., the mixture of metal-organic framework (or covalent organic frameworks) to adsorb and dissociate N2and aqueous electrolyte to provide protons). Finally, the reaction conditions like temperature and pressure should be changed to promote the N2-to-NH3fixation in the infancy of green NRR field. As everyone knows, high temperature and pressure can facilitate the NRR toward NH3production. Thus, to dramatically improve the NRR performance, rationally increasing the reaction temperature (e.g., 100 °C) or pressure (e.g.,1 MPa) can be a most fast and effective route, which establishes indepth understanding of green NRR and smart integration of comprehensive theories.
For the protocol, we firstly emphasize that NRR is carried out in a clean and isolated room at least. The researchers in the NRR process should be careful and patient to well treat all the experimental details. The laboratory rookie for NRR should be accompanied by the master to guide the normative operation. It will be best to usually use a buddy system for NRR, which can enhance the accuracy of the results and reduce the occurrence of false positive. In addition, some facile and advanced experimental conditions and setups can be employed to exclude the disturbance of nitrogen-containing contamination. A series of cells to assemble a gas-tight reaction unit are used to carry out NRR, where each cell can play the different functions including the purification of N2gas, the NRR, the reabsorption of NH3production, and the effect of liquid seal. The rational design of control experiments is vitally important, which can confirm the N source of NH3from N2and check the composition of the catalysts, reaction reagents, and reaction gas to exclude the effect of contamination.
Exploitation of a sustainable process N2-to-NH3fixation for both agriculture and energy industry can give rise to a massive global impact in the food and energy security and supply field in this century,as done at 100 years ago by Haber–Bosch process. In the face of great social and economic profit, we should keep confidence and strive to fundamental understanding and innovation in the NRR process for NH3production. We enthusiastically recommend the standard operational practices and new reaction modes to pursue such important task. We believe that all positive attempts and comments can make a good contribution to developing the green NRR.
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China(51402100,21573066,21825201,22075075,21805080,and U19A2017),the Provincial Natural Science Foundation of Hunan (2016JJ1006, 2020JJ5044, and 2016TP1009),and Australian Research Council(DP180100568 and DP180100731)for financial support of this research.
Conflict of Interest
The authors declare no conflict of interest.
Keywords
current dilemma, enhanced performances, future challenges, green synthesis,nitrogen-to-ammonia fixation
Received: March 4, 2021
Revised: March 22, 2021
Published online: March 23, 2021
[1] T. N. Ye, S. W. Park, Y. Lu, J. Li, M. Sasase, M. Kitano, T. Tada, H. Hosono, Nature 2020, 583, 391.
[2] I. ˘Cori′c, B. Q. Mercado, E. Bill, D. J. Vinyard, P. L. Holland, Nature 2015,526, 96.
[3] W. Guo, K. Zhang, Z. Liang, R. Zou, Q. Xu, Chem. Soc. Rev. 2019, 48,5658.
[4] L. Hui, Y. Xue, H. Yu, Y. Liu, Y. Fang, C. Xing, B. Huang, Y. Li, J. Am.Chem. Soc. 2019, 141, 10677.
[5] A. J. Mart′ın, T. Shinagawa, J. P′erez-Ram′ırez, Chem 2019, 5, 263.
[6] L. Wang, M. Xia, H. Wang, K. Huang, C. Qian, C. T. Maravelias, G. A.Ozin, Joule 2018, 2, 1055.
[7] R. F. Service, Science 2018, 361, 120.
[8] D. Bao, Q. Zhang, F. L. Meng, H. X. Zhong, M. M. Shi, Y. Zhang, J. M.Yan, Q. Jiang, X. B. Zhang, Adv. Mater. 2017, 29, 1604799.
[9] H. Li, J. Shang, Z. Ai, L. Zhang, J. Am. Chem. Soc. 2015, 137, 6393.
[10] G. F. Chen, X. Cao, S. Wu, X. Zeng, L. X. Ding, M. Zhu, H. Wang, J. Am.Chem. Soc. 2017, 139, 9771.
[11] L. Shi, Y. Yin, S. Wang, H. Sun, ACS Catal. 2020, 10, 6870.
[12] S. Zhang, Y. Zhao, R. Shi, C. Zhou, G. I. N. Waterhouse, Z. Wang, Y.Weng, T. Zhang, Angew. Chem. Int. Edit. 2020, 60, 2554.
[13] R. Hawtof, S. Ghosh, E. Guarr, C. Xu, R. M. Sankaran, J. N. Renner, Sci.Adv. 2019, 5, eaat5778.
[14] X. Cui, C. Tang, Q. Zhang, Adv. Energy. Mater. 2016, 8, 1800369.
[15] Y. Zhao, L. Zheng, R. Shi, S. Zhang, X. Bian, F. Wu, X. Cao, G. I. N.Waterhouse, T. Zhang, Adv. Energy. Mater. 2020, 10, 2002199.
[16] L. Li, C. Tang, B. Xia, H. Jin, Y. Zheng, S. Z. Qiao, ACS Catal. 2019, 9, 2902.
[17] L. Hollevoet, F. Jardali, Y. Gorbanev, J. Creel, A. Bogaerts, J. A. Martens,Angew. Chem. Int. Edit. 2020, 59, 23825.
[18] G. F. Chen, Y. Yuan, H. Jiang, S. Y. Ren, L. X. Ding, L. Ma, T. Wu, J. Lu,H. Wang, Nat. Energy 2020, 5, 605.
[19] J. Wang, L. Ling, Z. Deng, W. X. Zhang, Sci. Bull. 2020, 65, 926.
[20] C. J. Pickett, J. Talarmin, Nature 1985, 317, 652.
[21] T. Oshikiri, K. Ueno, H. Misawa, Angew. Chem. Int. Edit. 2016, 55, 3942.
[22] F. Zhou, L. M. Azofra, M. Ali, M. Kar, A. N. Simonov, C. McDonnell-Worth, C. Sun, X. Zhang, D. R. MacFarlane, Energy Environ. Sci. 2017,10, 2516.
[23] J. Zheng, Y. Lyu, M. Qiao, R. Wang, Y. Zhou, H. Li, C. Chen, Y. Li, H.Zhou, S. P. Jiang, S. Wang, Chem 2019, 5, 1.
[24] S. Z. Andersen, V. Colic, S. Yang, J. A. Schwalbe, A. C. Nielander, J. M.McEnaney, K. Enemark-Rasmussen, J. G. Baker, A. R. Singh, B. A. Rohr,M. J. Statt, S. J. Blair, S. Mezzavilla, J. Kibsgaard, P. C. K. Vesborg, M.Cargnello, S. F. Bent, T. F. Jaramillo, I. E. L. Stephens, J. K. Norskov, I.Chorkendorff, Nature 2019, 570, 504.
[25] H. Zou, W. Rong, S. Wei, Y. Ji, L. Duan, Proc Natl Acad Sci USA 2020,117, 29462.
[26] J. Liu, M. S. Kelley, W. Wu, A. Banerjee, A. P. Douvalis, J. Wu, Y. Zhang,G. C. Schatz, M. G. Kanatzidis, Proc Natl Acad Sci USA 2016, 113, 5530.
[27] C. Liu, K. K. Sakimoto, B. C. Col′on, P. A. Silver, D. G. Nocera, Proc Natl Acad Sci USA 2017, 114, 6450.
[28] H. Tao, C. Choi, L. X. Ding, Z. Jiang, Z. Han, M. Jia, Q. Fan, Y. Gao, H.Wang, A. W. Robertson, S. Hong, Y. Jung, S. Liu, Z. Sun, Chem 2019, 5,204.
[29] F. Wang, L. Mao, H. Xie, J. Mao, Small Struct. 2021, 2, 2000075.
[30] H. Cheng, P. Cui, F. Wang, L. X. Ding, H. Wang, Angew. Chem. Int. Edit.2019, 58, 15541.
[31] L. Li, C. Tang, D. Yao, Y. Zheng, S. Z. Qiao, ACS Energy Lett. 2019, 9,2111.
[32] L. Li, Y. Wang, S. Vanka, X. Mu, Z. Mi, C. J. Li, Angew. Chem. Int. Edit.2017, 56, 8701.
[33] P. Li, Z. Jin, Z. Fang, G. Yu, Angew. Chem. Int. Edit. 2020, 59, 22610.
[34] J. Zheng, Y. Lyu, M. Qiao, J. P. Veder, R. D. Marco, J. Bradley, R. Wang,Y. Li, A. Huang, S. P. Jiang, S. Wang, Angew. Chem. Int. Edit. 2019, 58,18604.
[35] L. Han, Z. Ren, P. Ou, H. Cheng, N. Rui, L. Lin, X. Liu, L. Zhuo, J. Song, J.Sun, J. Luo, H. L. Xin, Angew. Chem. Int. Edit. 2020, 60, 345.
[36] C. Lv, L. Zhong, Y. Yao, D. Liu, Y. Kong, X. Jin, Z. Fang, W. Xu, C. Yan,K. N. Dinh, M. Shao, L. Song, G. Chen, S. Li, Q. Yan, G. Yu, Chem 2020,6, 2690.
[37] J. Zheng, Y. Lyu, B. Wu, S. Wang, EnergyChem 2020, 2, 100039.
[38] D. Zhu, L. Zhang, R. E. Ruther, R. J. Hamers, Nat. Mater. 2013, 12, 836.
[39] C. Tang, Y. Zheng, M. Jaroniec, S. Z. Qiao, Angew. Chem. Int. Edit. 2021.https://doi.org/10.1002/anie.202101522
[40] C. Tang, S.-Z. Qiao, Chem. Soc. Rev. 2019, 48, 3166.
[41] J. Choi, H.-L. Du, C. K. Nguyen, B. H. R. Suryanto, A. N. Simonov, D. R.MacFarlane, ACS Energy Lett. 2020, 5, 2095.
杂志排行
Energy & Environmental Materials的其它文章
- Progress of Pb-Sn Mixed Perovskites for Photovoltaics:A Review
- Development Strategies in Transition Metal Borides for Electrochemical Water Splitting
- Polymer-/Ceramic-based Dielectric Composites for Energy Storage and Conversion
- Controllable Construction of Bifunctional CoxP@N,P-Doped Carbon Electrocatalysts for Rechargeable Zinc–Air Batteries
- Unveiling the Underlying Mechanism of Transition Metal Atoms Anchored Square Tetracyanoquinodimethane Monolayers as Electrocatalysts for N2 Fixation
- Rational Design of High-Performance Bilayer Solar Evaporator by Using Waste Polyester-Derived Porous Carbon-Coated Wood
