β-PbO2和BDD电极处理印染反渗透浓水性能
2022-06-29刘梓锋郑睿豪周青青王家德史学儒
刘梓锋,郑睿豪,周青青,王家德,史学儒
β-PbO2和BDD电极处理印染反渗透浓水性能
刘梓锋,郑睿豪,周青青,王家德*,史学儒
(浙江工业大学环境学院,浙江 杭州 310014)
系统比较了β-PbO2/Ti-Ti和BDD/Si-Ti两种电极体系处理实际印染行业反渗透浓水(ROC)的性能, 考察了同步去除化学需氧量(COD)和总氮(TN)的动力学,以及对废水可生化性的改善情况.结果表明,BDD/Si-Ti电极体系的析氧电位(2.45V)和析氯电位(1.90V),以及阳极氧化电位和阴极还原电位的绝对值均高于β-PbO2/Ti-Ti电极体系;两种电极体系对COD以及TN的去除符合拟一级动力学,其中BDD/Si-Ti电极体系对COD去除的表观速率常数和能量利用效率均优于β-PbO2/Ti-Ti电极体系;而β-PbO2/Ti-Ti电极体系对TN去除的表观速率常数和能量利用效率更优.β-PbO2/Ti-Ti电极体系在5mA/cm2的电流密度下电解15min,可使反渗透浓水BOD/COD从0.18升至0.42 (提高1.33倍),而BDD/Si-Ti电极体系仅提升0.78倍.两者相比, BDD/Si-Ti电极体系适用于矿化污染物,β-PbO2/Ti-Ti电极体系适用于改善废水可生化性.
反渗透浓水;电化学;印染废水;动力学
反渗透是工业水处理领域清洁高效的水处理技术.印染行业,反渗透浓水(ROC)盐度高、色度高,截留污染物成分复杂, 可生化性差[1],纳管(或直排)前需要作进一步处理.针对这类反渗透浓水,单一生物处理效果差,吸附处理存在吸附剂次生污染问题.相比臭氧氧化[2]、光催化[3]、芬顿反应[4]等高级氧化,使用电氧化方法处理反渗透浓水,通过电极表面的电子迁移以及活性物种的化学反应,实现污染物转化或完全矿化,反应条件温和,设备操作简单,且无额外添加反应药剂,处理过程清洁绿色[5],为这类废水处理提供了一条新途径[6-7].目前,电氧化技术已经成功应用于农药、纺织以及垃圾渗滤液等行业废水的深度处理[8-9].反渗透浓水高含盐,为电化学体系提供了支持电解质,且无需添加额外电解质,降低了槽电压,节约能耗;同时,Cl-和SO42-电氧化生成的活性氯和过硫酸根[10],会与污染物发生氧化分解反应[11],提高了反应效率.
废水电氧化处理常用的阳极材料有二氧化铅(PbO2)电极和掺硼金刚石(BDD)电极,它们在电解过程中具有高析氧电位和低析氧产率[12],氧化性能强.β-PbO2/Ti电极成本低,表面活性层呈开放的多孔结构,活性表面积大,电子迁移率高,稳定性强[13-15]. BDD/Si电极电化学和理化性能优,电化学势窗宽、背景电流小[16-17],可实现高电流效率和有机物完全矿化[18-19].电极材料会直接影响电氧化体系的性能[20],目前,国内外针对两种电极体系的对比多倾向于特定的物质降解[21-24],而非应用于特定行业的实际废水,因此,本文通过系统地比较β-PbO2/Ti-Ti和BDD/Si-Ti电极体系处理实际印染行业反渗透浓水的性能,考察同步去除化学需氧量(COD)、总氮(TN)的动力学、能耗以及对废水可生化性的改善情况,为废水的电化学处理技术实际工程应用提供支持.
1 材料与方法
1.1 原水水质
反渗透浓水取自浙江绍兴柯桥实际印染厂反渗透末端外排浓水.经分析检测,水质参数如表1所示.

表1 反渗透浓水的基本水质特征
1.2 实验装置
本文所使用的β-PbO2/Ti电极以Ti电极为基底、通过热分解法以锡锑氧化物作为底层、碱性电镀α-PbO2层和酸性电镀β-PbO2作为活性层,共4步骤制成;BDD/Si电极的制备方法主要以硅板作为基底采用化学气相沉积技术(CVD)所制成.

电极性能测试主要在常规的三电极体系中完成(图1a),电解试验在电解槽中进行(图1b).阳极选用β-PbO2/Ti电极和BDD/Si电极,阴极选用网板钛(Ti)电极,分别构成β-PbO2/Ti-Ti电极体系和BDD/ Si-Ti电极体系.
1.3 分析项目及测试方法
使用电化学工作站CHI660(中国)进行电极循环伏安测试, 扫描速率100mV/s,工作电极为β- PbO2/Ti电极(1cm2)、BDD/Si电极(1cm2),对电极为Pt片(1cm2),参比电极为饱和甘汞电极,测试在室温下进行,支持电解质为0.5mol/LNa2SO4;线性伏安测试扫描速率10mV/s,测试条件同上,电解质体系分别为0.5mol/LNa2SO4和0.5mol/L NaCl以及实验所使用的反渗透浓水,测得电极的析氧、析氯电位,以及在实际废水中的阳极氧化电位;电极电化学阻抗谱(EIS)中交流阻抗测试频率范围为104~100Hz,扰动振幅为0.5mV.
化学需氧量(CODCr)使用重铬酸钾法测定(GB 11914-89) ,在预处理中使用硫酸汞屏蔽废水中氯离子的干扰;五日生化需氧量(BOD5)使用Hach BOD TRAK Ⅱ(美国)装置测定;总有机碳(TOC)使用岛津TOC-V(日本)测定;总氮(TN)采用碱性过硫酸钾消解紫外分光光度法,使用Hach DR6000紫外分光光度计(美国)测定;氨氮选用纳氏试剂比色法确定;pH值使用EZDO B6339仪器(中国台湾)测定,TDS、电导率使用EZDO W6277便携式测定笔(中国台湾)测定;Cl-、SO42-、NO3-等阴离子,Na+、Mg2+、NH4+等阳离子浓度使用离子色谱仪器Dionex ICS-2000(美国)测定.
所有实验数据均取自3个独立实验的平均值,并且计算得出的误差<6%.
2 结果与讨论
2.1 电极的基本特征
在扫速为100mV/s时,在图2(a)1.2V处有氧化峰,在0.9V处有还原峰,属于PbO2/PbO的氧化还原对[25-26],是典型的PbO2电极.图2(b)可看出与β- PbO2/Ti相比BDD/Si电极背景电流小,电化学势窗口较大,析氧电位明显高于β-PbO2/Ti电极.
在电化学阻抗谱(EIS)中,判断电子在基体和镀层之间的难易程度主要是依据圆弧半径的相对大小[10].不同电极在0.5mol/L Na2SO4溶液中的电化学交流阻抗图如图2(c)所示, BDD/Si电极的电化学反应界面的电荷转移电阻小,说明相比β-PbO2/Ti电极,BDD/Si电极具有更高的电化学活性.

图2 β-PbO2/Ti 和BDD/Si电极的循环伏安及阻抗图 (vs. SCE)
如图3所示,β-PbO2/Ti电极析氧电位为1.78V,析氯电位为1.61V;BDD/Si电极的析氧电位为2.45V,析氯电位为1.90V;设定电流密度为10mA/cm2时 ,使用实际废水作为溶液 BDD/Si电极电位为2.35V, β-PbO2/Ti电极电位为1.69V.两者相比,BDD/Si电极具有较高的析氧电位、析氯电位及实际废水中的阳极氧化电位[18-27],具有更强的电催化性能.两种电极的阳极氧化电位均介于析氧电位和析氯电位之间,实际废水发生电解反应时,析氧副反应可以得到有效控制,但析氯反应无法避免.
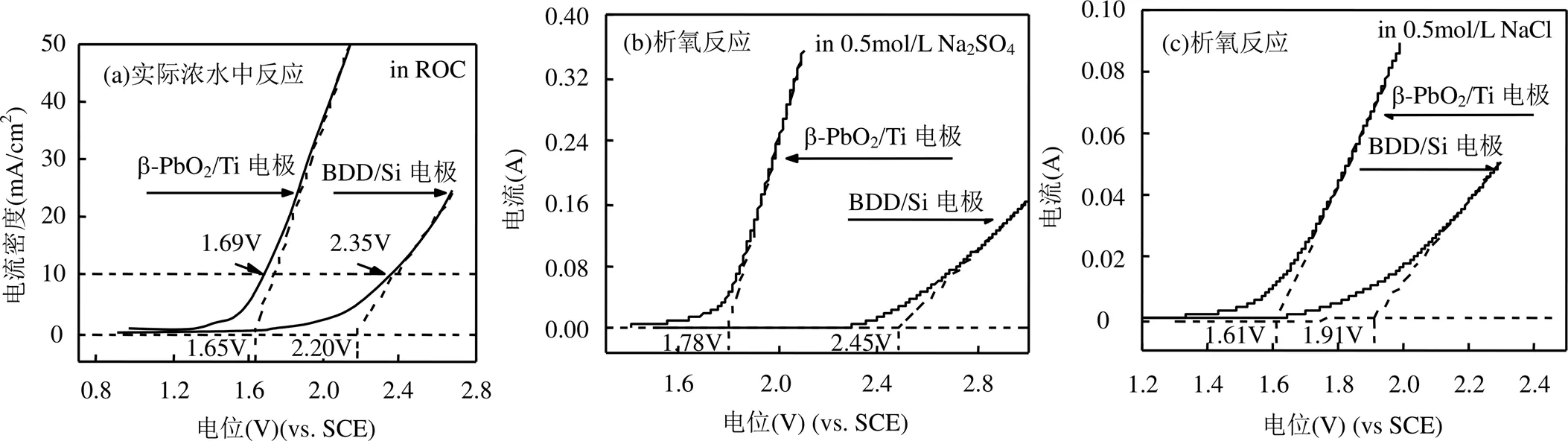
图3 β-PbO2/Ti 和BDD/Si电极的线性伏安扫描图(vs. SCE)
2.2 不同电极体系的电位分布
实际电解体系包括阳极和阴极,体系中电极电位直接影响着污染物分解.实验设计盐桥检测不同电流密度下的两种电解体系阴阳两极电位分布,以此揭示电极反应本质,科学控制副反应.
如图4所示,实际电解体系伏安特性曲线具有与图3极化曲线相一致的形状,以10mA/cm2电流密度下举例,阳极电位2.77V(BDD/Si电极)、1.60V (β-PbO2/Ti电极),Ti阴极电位-2.30V(BDD/Si-Ti电极体系)、-2.03V(β-PbO2/Ti-Ti电极体系),控制相同电流密度,BDD/Si-Ti电极体系的电压绝对值大于β-PbO2/Ti-Ti电极体系,这意味着达到同样的电极电位,BDD/Si-Ti电极体系输入的电流密度小于PbO2电极体系.同时,过高的电极电位容易导致反应过度(如阴极NO3-,NO2-过度还原成NH3,影响TN和NH3-N的去除).
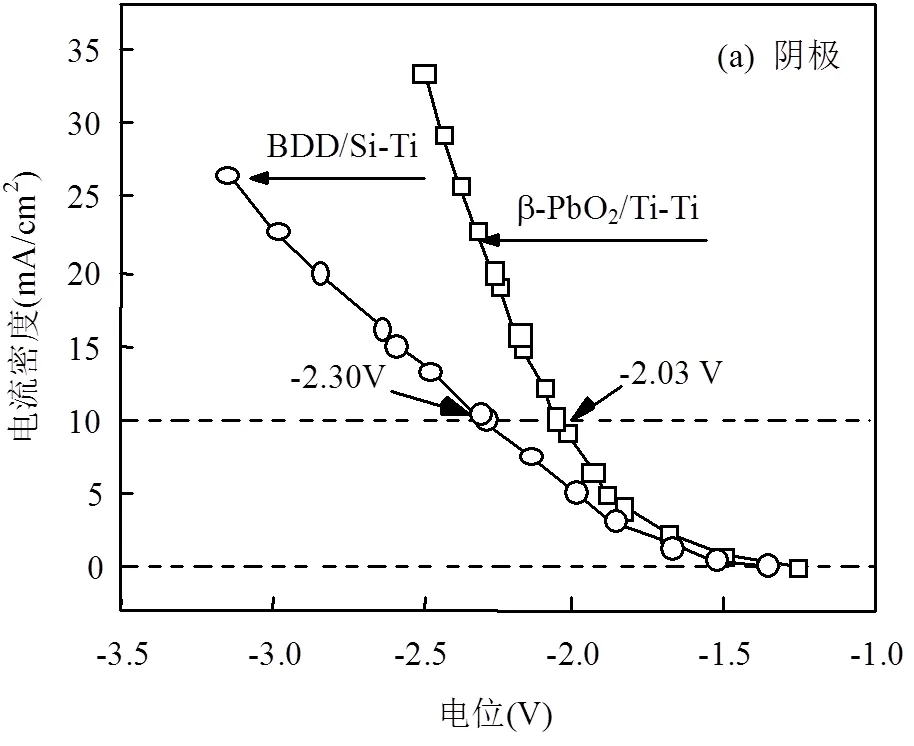
2.3 动力学分析
2.3.1 CODCr去除 低污染物浓度废水电氧化过程符合拟一级动力学[28].

式中:[COD]0/[COD]是t时间(min)相对于初始时间的浓度相对值;app是拟一级表观速率常数.
实际反渗透浓水电解实验结果显示(图5),不同电流密度下的ln[COD]0/[COD]和app成线性关系,电流密度越大,app的值越大,如表2所示,通过对表观速率常数和电流密度之间的关系拟合,可以得到描述两者关系的数学式.

表2 COD去除拟一级表观速率常数
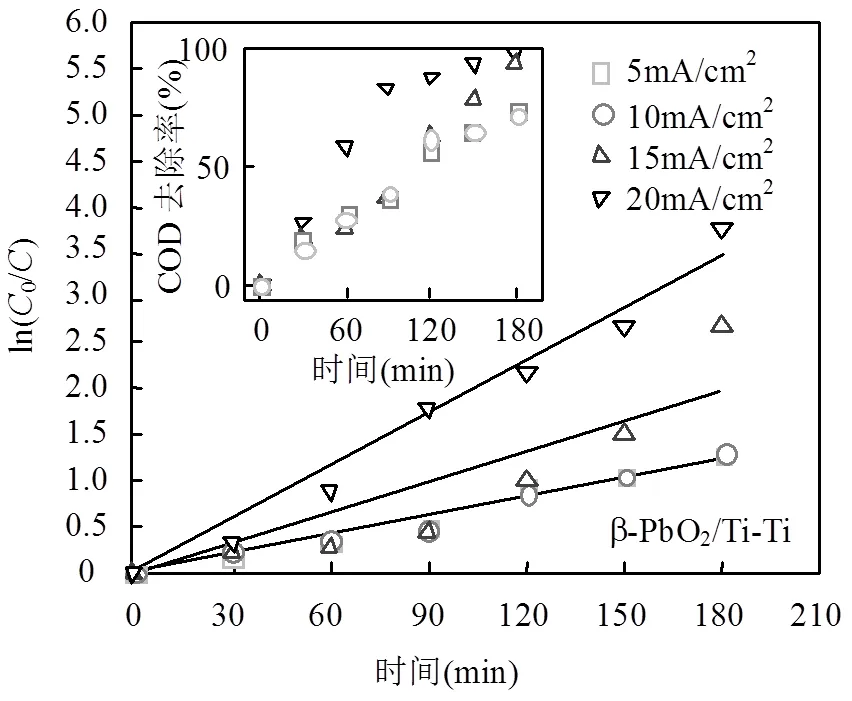
β-PbO2/Ti-Ti电极体系:

BDD/Si-Ti电极体系:

式中:为电流密度,mA/cm2.
两种电极体系均能有效去除污染物,但相同电流密度下(如10mA/cm2),BDD/Si-Ti电极体系对COD去除性能强于β-PbO2/Ti-Ti电极体系.120min所达到去除率分别为68.7%以及57.7%,前者app是后者的1.84倍.两种电极体系下阳极对有机污染物的氧化性能,往往取决于析氧副反应[29](式(4)),由于BDD电极比β-PbO2电极具有更高的析氧电位,析氧反应较难发生,减少了析氧副反应.在实际电解中,相比具有水合性的β-PbO2电极,BDD电极的惰性表面对其产生羟基自由基(·OH)的吸附性能弱[28-30]·OH更容易参与有机物氧化反应(式(5)),同时,由于体系中Cl-存在,通电过程中发生析氯反应产生大量活性氯(式(6))且在溶液中转换成不同形态(式(7)、(8)),不同形态的活性氯参与间接氧化降解有机物质(式9)[31-33],提高了系统对COD的处理能力.
除了上述原因之外,大量研究结果已经证明BDD电极可以产生SO4·-与过硫酸根(式(10)、(11)),SO4·-(2.5~3.1V)具有比·OH(1.8~2.7V)更高的氧化电位,能够进一步提升电极本身的氧化能力[28]. Sun等[30]比较两种电极氧化蒽醌染料茜素红S,BDD电极直接矿化有机物;PbO2电极则先裂解—C=O基团附近的C—C键,形成邻苯二甲酸、小羰基等中间体.Lazhar等[34]考察了两种电极处理甲基橙水溶液,BDD电极处理污染物快速、矿化彻底,而PbO2电极体系倾向于产生中间体再进一步矿化. 相比之下,PbO2电极产生SO4·-仅在TiO2纳米管结构时被观测到[35-36],在这之前鲜有报道.实验所使用的并非TiO2纳米管结构电极,因此相比之下,BDD/ Si-Ti电极体系具有更强的矿化能力.
S(·OH)→S+1/2O2+H++e-(4)
R+S(·OH)→S+CO2+H2O+H++e-(5)
2Cl-→Cl2+2e-(6)
R+ Active Chlorine→CO2+H2O+zCl-(9)
SO42-+S(·OH) →S(SO4·-)+OH-(10)
SO42-+S(SO4·-) →S2O82-+e-(11)
2.3.2 TN去除 印染反渗透浓水TN包括NO3--N、NO2--N和NH3-N.电化学去除TN主要为阳极上NH3-N氧化成N2(式(12)和(13)),NO3--N、NO2--N在阴极还原成N2(式(15)和(16)),并且存在一个适合的电位范围[37].在溶液HOCl浓度远大于NH3的条件下,HOCl与NH3反应生成NO3-以及N2[31-38].高电极电位下,阳极上NH3-N易过氧化生成NO3-- N、NO2--N,阴极上NO3--N、NO2--N还原成NH3-N,这些反应均会影响NH3-N和TN的去除.
2NH3+6OH-→N2+6H2O+6e-(12)
2NH3+3HOCl→N2+3H2O+3H++3Cl-(13)
NH3+4HOCl→NO3-+H2O+6H++4Cl-(14)
NO3-+H2O+2e-→NO2-+2OH-(15)
NO2-+4H2O+6e-→N2+8OH-(16)
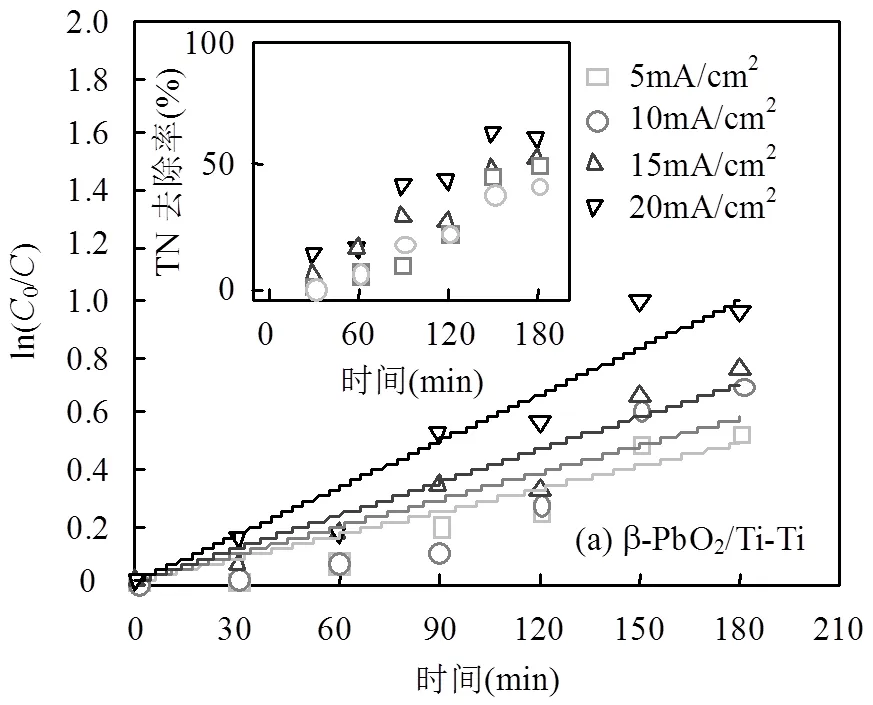
实际反渗透浓水TN电解去除如图6所示,不同电流密度下的ln[0]/[C]和app也成线性关系,电流密度越大,app值越大,同样通过对表观速率常数和电流密度之间的关系拟合,可以得到描述两者关系的数学式.
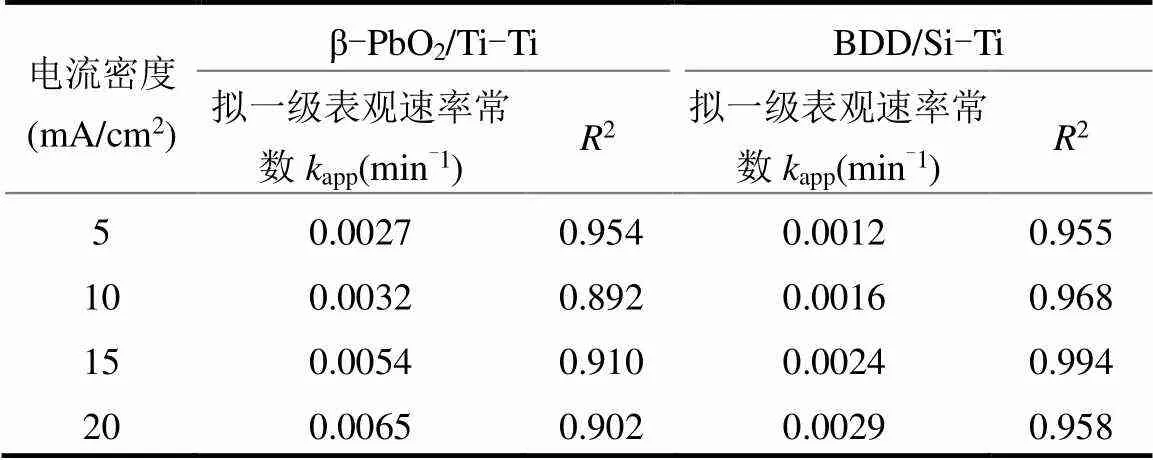
表3 TN去除拟一级表观速率常数
β-PbO2/Ti-Ti电极体系:
app=0.0019e0.0632J(2=0.954)(17)
BDD/Si-Ti电极体系:
app=0.0019e0.0611J(2=0.975)(18)
由上述可知,在相同电流密度下,BDD/Si-Ti电极体系对TN去除性能低于β-PbO2/Ti-Ti电极体系,并且在相同电流密度下,BDD/Si-Ti体系在3h的处理时间内难以达到理想的去除率50%以上(TN< 30mg/L),这与阴极过度还原和阳极过度氧化有关.实际电解时,低阴极电位易发生析氢副反应,大量的·H吸附在电极表面上,促进N-H结合,不利于N-N形成,NO3-还原成NH4+(式(19))[39];高阳极电位使析氯反应剧烈,活性氯将NH4+过氧化生成NO3--N、NO2--N[40],从而导致TN去除效果减弱.BDD/Si-Ti电极体系的阴极电位和阳极电位绝对值均高于β-PbO2/Ti-Ti电极体系,阴极析氢反应强,导致NO3--N、NO2--N过度还原为NH3-N,形成N2的选择性下降.
NO3-+10H++8e-→NH4++3H2O(19)
NH4++4HOCl→NO3-+H2O+6H++4Cl-(20)
2.4 可生化性实验
生物法是废水处理最经济的方法.污染物通过生物代谢活动分解为CO2、H2O或成为生物自身物质,整个处理过程经济安全绿色.相比之下,电化学彻底分解污染物能耗高,相对经济的方法是提高废水可生化性后,再用生物工艺处理.反渗透浓水TDS为12.85~ 13.83g/L,盐度为1%~1.5%,对照Kokabian[41]、Abou-Elela等[42]研究成果,该浓度下不会对生物处理产生大的影响.
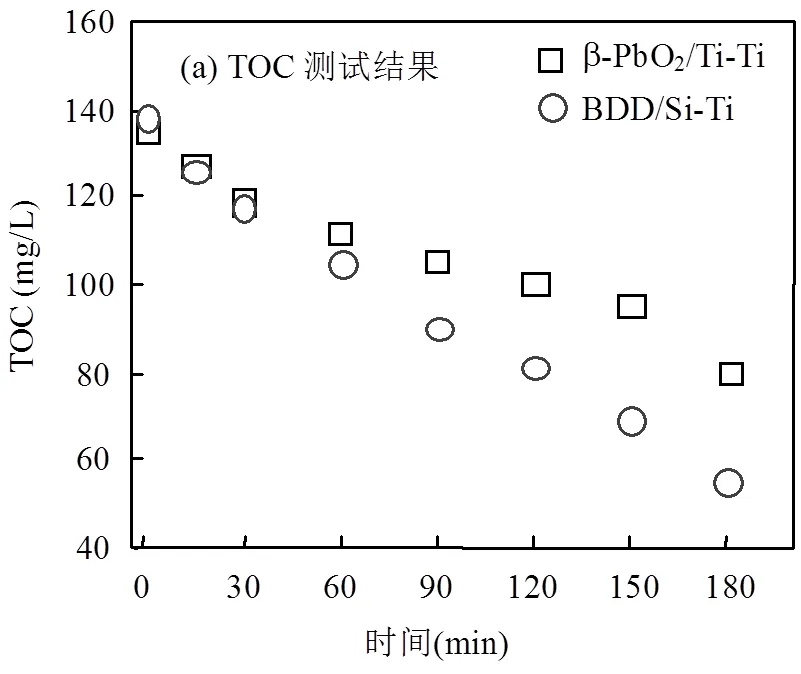
使用BOD/COD(B/C比)比作为生化指标,试验考察了反渗透浓水电分解后的B/C比变化.图7为相同电流密度下,两种电极体系TOC、BOD值以及B/C比随时间的变化情况.根据图7(a)可以看出在电流密度5mA/cm2时,BDD/Si-Ti电极体系在长时间的电解情况下去除TOC效率高于β-PbO2/Ti-Ti电极体系,且β-PbO2/Ti-Ti电极体系在60~150min TOC去除效率有所放缓,而由图7(b)可知,随着电解进行,两个体系BOD值与B/C比均经历了先升后降的过程.电流密度5mA/cm2、电解15min,β-PbO2/Ti-Ti电极体系将反渗透浓水的B/C比从0.18提升至0.42(>0.35),高于BDD/Si-Ti电极体系,结合前面COD、TN去除情况,BDD/Si-Ti电极体系阳极氧化电位高,催化能力强,适合污染物矿化;β-PbO2/Ti-Ti电极体系阳极氧化电位低,更适合将污染物转化为可生化性好的物质,便于后续生物降解.
2.5 能耗评估
大量研究表明,低污染物浓度废水电氧化过程符合拟一级动力学[28-34],COD以及TN的去除符合拟一级动力学,因此本文引入指数能耗eo[43-44],科学评估电化学体系污染物去除的耗能情况以及电化学系统的能量利用效率,计算公式如(21).
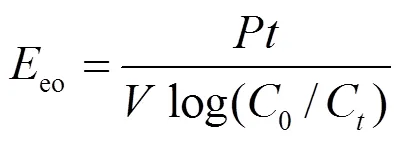
式中:eo是电解降低一个数量级所需要的能量, kW·h/(m3·order);是平均功率,kW;是反应时间,h;是溶液的体积,m3可以根据拟一级动力学简化该表达式.
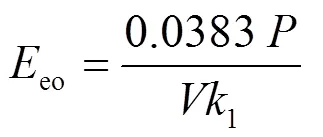
式中:0.0383是转换因子(1/60/0.4343);1是拟一级速率常数,min-1.
对于特定的电极体系,电流密度小(接近极限电流密度),去除单位COD能耗低,能量利用效率高,但反应速度慢,完成相同反应量需要时间长,设备体积大.实际电解过程电流密度大小与能耗有一个平衡点.表4和表5分别列出了不同电流密度下的指数能耗(eo),同一电流密度下,BDD/Si-Ti电极体系对COD去除的能量利用效率高于β-PbO2/Ti-Ti电极体系.分析认为,实验所选取电流密度的阳极氧化电位均高于析氯电位,略低于析氧电位,易发生析氯析氧副反应;相比之下,BDD/Si-Ti体系阳极具有更低的·OH基团的吸附能力[45],有利于COD去除[34-46],能量利用率高.进一步地,两种电极对·OH基团的吸附量受电流密度影响不同, β-PbO2/Ti电极较BDD/Si电极敏感,低电流密度提升阶段电极表面·OH基团吸附量上升迅速,析氧副反应剧烈,能耗增加明显.

表4 不同电流密度下两种电极体系去除COD的Eeo
TN去除主要取决于阳极和阴极的电位控制[47].由表5可知,同一电流密度下,β-PbO2/Ti-Ti体系TN去除效果更好,能量利用效率更高.分析认为,两种体系实际阴极还原电位均低于析氢电位,但β-PbO2/ Ti-Ti电极体系具有相对较低的阴极电位,减少NH4+形成,提高NO3-、NO2-还原为N2的选择性,提高了能量利用效率;而BDD/Si-Ti电极体系过度氧化和过度还原严重,不利于TN的高效去除.
综上所述, COD和TN去除的eo随着电流密度增大而增大,且高电流密度下析氧析氯副反应也更加剧烈,考虑实际情况,电化学处理时间需要控制在2.5h以内,应尽可能选用低电流密度.以《纺织染整工业水污染物排放标准》(GB4287-2012)现有企业间接排放标准(COD<200mg/L、TN<30mg/L)作为参考,β-PbO2/Ti-Ti电极体系建议选用15mA/cm2作为主要处理参数,BDD/Si-Ti电极体系在仅考虑COD达标的情况下应选用10mA/cm2作为主要处理参数.

表5 不同电流密度下两种电极体系去除TN的Eeo
图8为COD和TN达到上述排放标准时,不同电流密度下所需的电解能耗c.c计算见式(23).
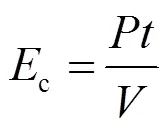
式中:c是处理单位体积废水的能耗,kW·h/m3.
相比之下,β-PbO2/Ti-Ti电极体系处理单位体积废水的能耗高于BDD/Si-Ti电极体系.需要说明的是, β-PbO2/Ti-Ti电极体系能实现COD和TN 两者达标, 而BDD/Si-Ti电极体系仅COD达标,对TN最高去除率仅为35%,出水TN浓度大于30mg/L,难以达到同步去除要求.
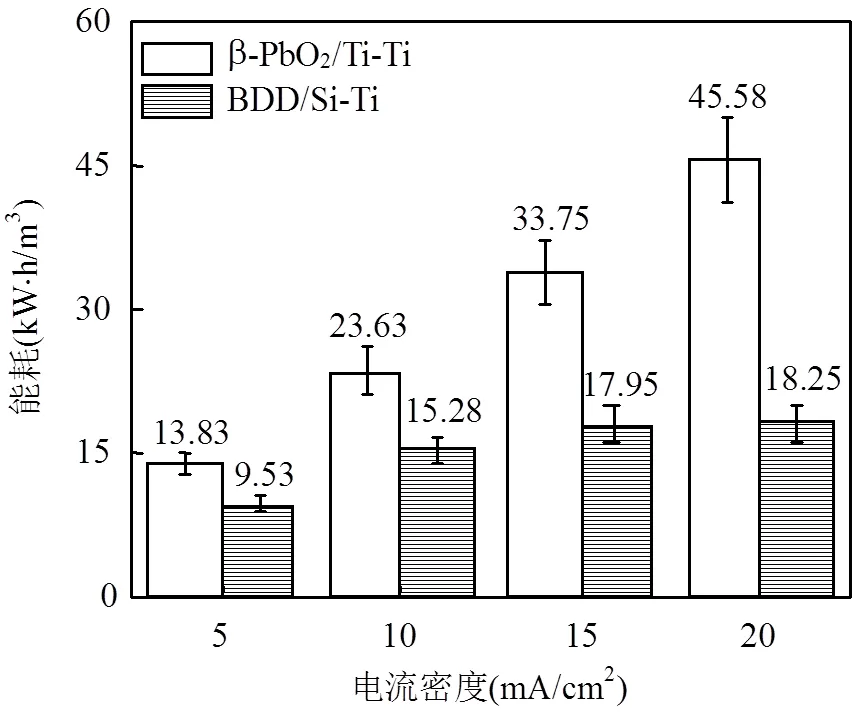
图8 废水处理至达标排放的能耗对比
按上述的排放标准,对比分析单独电化学深度处理和电化学仅作预处理(提升B/C)后续结合生物处理两种工艺所需的总能耗,其中电化学深度处理部分能耗来源于图8;电化学作为预处理提高B/C的生化处理能耗则选取通电处理的时间为15min时不同的电极体系所消耗的能耗(此时β-PbO2/Ti-Ti电极体系预处理废水B/C为0.42,BDD/Si-Ti电极体系预处理废水B/C为0.30),生化处理部分参考Yan He等研究成果[46].结果如表6所示,相比之下,β-PbO2/ Ti-Ti电极体系预处理反渗透浓水,之后采用生物处理至达标,能耗仅为1.74kW·h/m3,远低于直接电解至达标所消耗的能耗,节能显著.
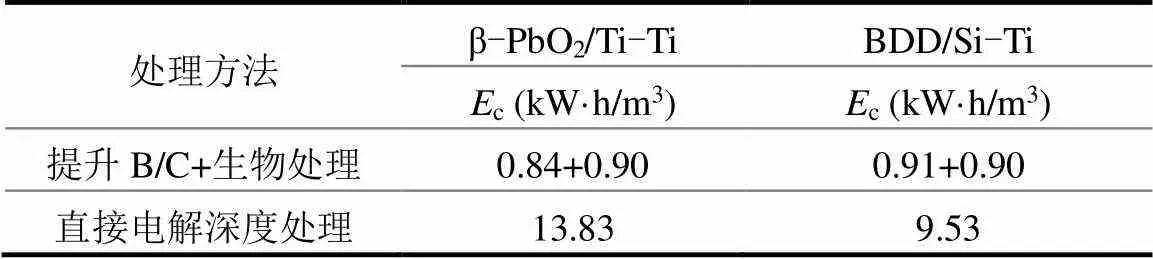
表6 不同处理方式下两种电极体系的能耗Ec
3 结论
3.1 BDD/Si阳极的析氧电位(2.45V)和析氯电位(1.90V)高于β-PbO2/Ti阳极,BDD/Si-Ti电极体系的电极电位绝对值大于β-PbO2/Ti-Ti电极体系;控制相同电流密度,BDD/Si-Ti电极体系的槽电压会高于β-PbO2/Ti-Ti电极体系.
3.2 两种电极体系对COD和TN去除遵循拟一级动力学模型,在电流密度为5~20mA/cm2范围内,根据拟合方程及实验结果,BDD/Si-Ti电极体系COD去除表观速率常数app高于β-PbO2/Ti-Ti电极体系,TN去除表观速率常数低; BDD/Si-Ti电极体系COD去除指数能耗Eeo低,TN去除指数能耗高.在处理实际印染行业反渗透浓水时β-PbO2/Ti-Ti电极体系能实现COD和TN同步去除,适用于COD和TN超标的废水,而BDD/Si-Ti电极体系更适合用于污染物完全矿化处理.
3.3 β-PbO2/Ti-Ti电极体系在5mA/cm2条件下电解15min可以将B/C从0.18提升至0.42(提升1.33倍)而BDD/Si仅提升0.78倍.相比之下,使用β-PbO2/ Ti-Ti电极体系,在低电流密度下作为预处理可进一步降低处理全过程所需能耗.
[1] Joo S H, Tansel B. Novel technologies for reverse osmosis concentrate treatment: A review [J]. Journal of Environmental Management, 2015, 150:322-335.
[2] Weng J, Jia H, Wu B, et al. Is ozonation environmentally benign for reverse osmosis concentrate treatment? Four-level analysis on toxicity reduction based on organic matter fractionation [J]. Chemosphere, 2018,191:971-978.
[3] Lin X H, Li S F Y. Impact of the spatial distribution of sulfate species on the activities of SO42−/TiO2photocatalysts for the degradation of organic pollutants in reverse osmosis concentrate [J]. Applied Catalysis B: Environmental, 2015,170-171:263-272.
[4] Barreto-Rodrigues M, Silva F T, Paiva T C B. Combined zero-valent iron and fenton processes for the treatment of Brazilian TNT industry wastewater [J]. Journal of Hazardous materials, 2009,165(1):1224-1228.
[5] Chen M, Pan S, Zhang C, et al. Electrochemical oxidation of reverse osmosis concentrates using enhanced TiO2-NTA/SnO2-Sb anodes with/without PbO2layer [J]. Chemical Engineering Journal, 2020, 399:125756.
[6] Radjenovic J, Bagastyo A, Rozendal R A, et al. Electrochemical oxidation of trace organic contaminants in reverse osmosis concentrate using RuO2/IrO2-coated titanium anodes [J]. Water Research, 2011, 45(4):1579-1586.
[7] 李金城,宋永辉,汤洁莉.电化学氧化法去除兰炭废水中COD和NH3-N [J]. 中国环境科学, 2022,42(2):697-705.
Li J C, Song Y H, Tang J L Removing of COD and NH3-N from blue-coke wastewater by electrochemical oxidation [J]. China Environmental Science, 2022,42(2):697-705.
[8] Onn S W, Bashir M J K, Sethupathi S, et al. Colour and COD removal from mature landfill leachate using electro-persulphate oxidation process [J]. Materials Today: Proceedings, 2020,31:69-74.
[9] Olvera-Vargas H, Gore-Datar N, Garcia-Rodriguez O, et al. Electro-Fenton treatment of real pharmaceutical wastewater paired with a BDD anode: Reaction mechanisms and respective contribution of homogeneous and heterogeneous OH [J]. Chemical Engineering Journal, 2021,404:126524.
[10] Pereira G F, Rocha-Filho R C, Bocchi N, et al. Electrochemical degradation of the herbicide picloram using a filter-press flow reactor with a boron-doped diamond or β-PbO2anode [J]. Electrochimica Acta, 2015,179:588-598.
[11] Garcia-Segura S, Ocon J D, Chong M N. Electrochemical oxidation remediation of real wastewater effluents — A review [J]. Process Safety and Environmental Protection, 2018,113:48-67.
[12] Zhou X, Wang s, Ma C, et al. Effect of Ag Content and β-PbO2Plating on the Properties of Al/Pb-Ag Alloy [J]. Rare Metal Materials and Engineering, 2018,47(7):1999-2004.
[13] Sirés I, Low C T J, Ponce-de-León C, et al. The characterisation of PbO2-coated electrodes prepared from aqueous methanesulfonic acid under controlled deposition conditions [J]. Electrochimica Acta, 2010,55(6):2163-2172.
[14] Abaci S, Yildiz A. Electropolymerization of thiophene and 3- methylthiophene on PbO2electrodes [J]. Journal of Electroanalytical Chemistry, 2004,569(2):161-168.
[15] Zhou X, Zhou Q, Chen H, et al. Influence of dimethylphenol isomers on electrochemical degradation: Kinetics, intermediates, and DFT calculation [J]. Science of The Total Environment, 2021,794:148284.
[16] Pérez G, Saiz J, Ibañez R, et al. Assessment of the formation of inorganic oxidation by-products during the electrocatalytic treatment of ammonium from landfill leachates [J]. Water Research, 2012,46(8): 2579-2590.
[17] Panizza M, Kapalka A, Comninellis C. Oxidation of organic pollutants on BDD anodes using modulated current electrolysis [J]. Electrochimica Acta, 2008,53(5):2289-2295.
[18] He Y, Huang W, Chen R, et al. Anodic oxidation of aspirin on PbO2, BDD and porous Ti/BDD electrodes: Mechanism, kinetics and utilization rate [J]. Separation and Purification Technology, 2015,156: 124-131.
[19] Panizza M, Cerisola G. Application of diamond electrodes to electrochemical processes [J]. Electrochimica Acta, 2005,51(2):191-199.
[20] Martı́nez-Huitle C A, Ferro S, De Battisti A. Electrochemical incineration of oxalic acid: Role of electrode material [J]. Electrochimica Acta, 2004,49(22):4027-4034.
[21] Li A, Weng J, Yan X, et al. Electrochemical oxidation of acid orange 74using Ru, IrO2, PbO2, and boron doped diamond anodes: Direct and indirect oxidation [J]. Journal of Electroanalytical Chemistry, 2021, 898:115622.
[22] Santos J E L, de Moura D C, Cerro-López M, et al. Electro- and photo-electrooxidation of 2,4,5-trichlorophenoxiacetic acid (2,4,5-T) in aqueous media with PbO2, Sb-doped SnO2, BDD and TiO2-NTs anodes: A comparative study [J]. Journal of Electroanalytical Chemistry, 2020,873:114438.
[23] Xing X, Ni J, Zhu X, et al. Maximization of current efficiency for organic pollutants oxidation at BDD, Ti/SnO2-Sb/PbO2, and Ti/SnO2-Sb anodes [J]. Chemosphere, 2018,205:361-368.
[24] 邓冬莉,吴明珠,李 应,等.电催化氧化处理邻苯二甲酸酯类物质阳极材料的研究进展 [J]. 化工新型材料, 2021,49(4):267-271.
Deng D L, Wu M Z, LI Y. Advance on anode material for electrocatalytic oxidation of phthalate ester [J]. New Chemical Materials, 2021,49(4):267-271.
[25] Song S, Fan J, He Z, et al. Electrochemical degradation of azo dye C.I. Reactive Red 195by anodic oxidation on Ti/SnO2–Sb/PbO2electrodes [J]. Electrochimica Acta, 2010,55(11):3606-3613.
[26] 叶志平,周丹飞,刘梓锋,等.对甲基苯磺酸在Ti/PbO2电极上的电氧化反应信息. [J]. 化工学报, 2021,72(5):2810-2816.
Ye Z P, Zhou D F, Liu Z F, et al Electro-oxidation information of p-toluene sulfonic acid on Ti/PbO2electrode [J]. CIESC Journal, 2021,72(5):2810-2816.
[27] Zhang C, Lu X, Lu Y, et al. Titaniumboron doped diamond composite: A new anode material [J]. Diamond and Related Materials, 2019,98:107490.
[28] Santos J E L, Gómez M A, Moura D C d, et al. Removal of herbicide 1-chloro-2,4-dinitrobenzene (DNCB) from aqueous solutions by electrochemical oxidation using boron-doped diamond (BDD) and PbO2electrodes [J]. Journal of Hazardous materials, 2021,402:123850.
[29] Chen X, Gao F, Chen G. Comparison of Ti/BDD and Ti/SnO2–Sb2O5electrodes for pollutant oxidation [J]. Journal of Applied Electrochemistry, 2005,35(2):185-191.
[30] Sun J, Lu H, Du L, et al. Anodic oxidation of anthraquinone dye Alizarin Red S at Ti/BDD electrodes [J]. Applied Surface Science, 2011,257(15):6667-6671.
[31] Deborde M, von Gunten U. Reactions of chlorine with inorganic and organic compounds during water treatment—Kinetics and mechanisms: A critical review [J]. Water Research, 2008,42(1):13-51.
[32] Zhou Q, Zhou X, Zheng R, et al. Application of lead oxide electrodes in wastewater treatment: A review [J]. Science of The Total Environment, 2022,806:150088.
[33] Huang Y K, Li S, Wang C, et al. Simultaneous removal of COD and NH3-N in secondary effluent of high-salinity industrial waste-water by electrochemical oxidation [J]. Journal of Chemical Technology and Biotechnology, 2012,87(1):130-136.
[34] Labiadh L, Barbucci A, Carpanese M P, et al. Comparative depollution of Methyl Orange aqueous solutions by electrochemical incineration using TiRuSnO2, BDD and PbO2as high oxidation power anodes [J]. Journal of Electroanalytical Chemistry, 2016,766:94-99.
[35] Santos J E L, Antonio Quiroz M, Cerro-Lopez M, et al. Evidence for the electrochemical production of persulfate at TiO2nanotubes decorated with PbO2[J]. New Journal of Chemistry, 2018,42(7): 5523-5531.
[36] Zaidi S Z J, Harito C, Walsh F C, et al. Decolourisation of reactive black-5at an RVC substrate decorated with PbO2/TiO2nanosheets prepared by anodic electrodeposition [J]. Journal of Solid State Electronics, 2018:1-12.
[37] Kuang P, Natsui K, Einaga Y, et al. Annealing enhancement in stability and performance of copper modified boron-doped diamond (Cu-BDD) electrode for electrochemical nitrate reduction [J]. Diamond and Related Materials, 2021,114:108310.
[38] Kapałka A, Joss L, Anglada Á, et al. Direct and mediated electrochemical oxidation of ammonia on boron-doped diamond electrode [J]. Electrochemistry Communications, 2010,12(12):1714-1717.
[39] Yao J, Mei Y, Yuan T, et al. Electrochemical removal of nitrate from wastewater with a Ti cathode and Pt anode for high efficiency and N2selectivity [J]. Journal of Electroanalytical Chemistry, 2021,882: 115019.
[40] Su L, Li K, Zhang H, et al. Electrochemical nitrate reduction by using a novel Co3O4/Ti cathode [J]. Water Research, 2017,120:1-11.
[41] Kokabian B, Bonakdarpour B, Fazel S. The effect of salt on the performance and characteristics of a combined anaerobic–aerobic biological process for the treatment of synthetic wastewaters containing Reactive Black 5 [J]. Chemical Engineering Journal, 2013, 221:363-372.
[42] Abou-Elela S I, Kamel M M, Fawzy M E. Biological treatment of saline wastewater using a salt-tolerant microorganism [J]. Desalination, 2010,250(1):1-5.
[43] Asaithambi P, Sajjadi B, Abdul Aziz A R, et al. Ozone (O3) and sono (US) based advanced oxidation processes for the removal of color, COD and determination of electrical energy from landfill leachate [J]. Separation and Purification Technology, 2017,172:442-449.
[44] Daneshvar N, Aleboyeh A, Khataee A R. The evaluation of electrical energy per order (EEo) for photooxidative decolorization of four textile dye solutions by the kinetic model [J]. Chemosphere, 2005, 59(6):761-767.
[45] Long Y, Li H, Jin H, et al. Interpretation of high perchlorate generated during electrochemical disinfection in presence of chloride at BDD anodes [J]. Chemosphere, 2021,284:131418.
[46] He Y, Zhu Y, Chen J, et al. Assessment of energy consumption of municipal wastewater treatment plants in China [J]. Journal of Cleaner Production, 2019,228:399-404.
[47] Yao J, Pan B, Shen R, et al. Differential control of anode/cathode potentials of paired electrolysis for simultaneous removal of chemical oxygen demand and total nitrogen [J]. Science of The Total Environment, 2019,687:198-205.
致谢:审稿专家提出修改意见和建议,浙江省科学技术厅提供的支持,此一并致谢.
Performance comparison of β-PbO2and BDD electrodes for treating reverse osmosis concentrate in printing and dyeing industry.
LIU Zi-feng, ZHENG Rui-hao, ZHOU Qing-qing, WANG Jia-de*, SHI Xue-ru
(College of Environment, Zhejiang University of Technology, Hangzhou 310014, China)., 2022,42(6):2671~2679
Two electrode systems of β-PbO2/Ti-Ti and BDD/Si-Ti were systematically compared for the performance in treating reverse osmosis concentrate (ROC) from a real printing and dyeing industry. Kinetics of simultaneous remove chemical oxygen demand (COD) and total nitrogen (TN), as well as the improvement of the biochemical properties of the effluent were also investigated. It is shown that the BDD/Si-Ti electrode system has a high anodic oxygen evolution reaction (OER) potential (2.45V) and chlorine evolution reaction (CER) potential (1.90V), and the absolute values of anodic oxidation potential and cathodic reduction potential are higher than those of the β-PbO2/Ti-Ti electrode system; the degradation of COD and TN followed pseudo-first-order kinetics. The BDD/Si-Ti electrode system is more effective in COD removal and current efficiency while the β-PbO2/Ti-Ti electrode system is more effective in TN removal. Electrolysis at a low current density (5mA/cm2) for 15min resulted in a rise in BOD/COD from 0.18 to 0.42 (a 1.33-fold enhancement) for the ROC for the β-PbO2/Ti-Ti electrode system, while the BDD/Si-Ti electrode system only showed a 0.78-fold enhancement. As a result, the BDD/Si-Ti electrode system is suitable for pollutant mineralization, and the β-PbO2/Ti-Ti system is more suitable for the improvement of wastewater biochemical properties.
reverse osmosis concentrate;electrochemistry;printing & dyeing wastewater;kinetics
X703.1
A
1000-6923(2022)06-2671-09
刘梓锋(1997-),男,浙江湖州人,浙江工业大学硕士研究生,主要从事电化学处理反渗透浓水研究.
2021-11-01
浙江省科技厅重点研发项目(2019C03094);浙江省“万人计划”人才培养项目(2017R52018)
* 责任作者, 教授, jdwang@zjut.edu.cn
