The protective effects of procyanidin C-1 on bisphenol A-induced testicular dysfunction in aged mice
2022-06-20MsturAdMlekRzifDsimnNorAshikinMohmedNoorKhnSofeeMohmedAkhlkMohdfiziMhmud
Mstur Ad Mlek, Rzif Dsimn,,*, Nor-Ashikin Mohmed Noor Khn,Sofee Mohmed-Akhlk, Mohd-H fizi Mhmud
a Maternofetal and Embryo Research Group (MatE), Faculty of Medicine, Universiti Teknologi MARA, Selangor Branch,Sungai Buloh Campus, 47000 Sungai Buloh, Selangor, Malaysia
b Faculty of Health Sciences, Universiti Teknologi MARA, Selangor Branch, Puncak Alam Campus, 42300 Puncak Alam, Selangor, Malaysia
Keywords:
Apoptosis
Bisphenol A
Procyanidin
Mitochondrial dynamics
Testis
A B S T R A C T
The number and quality of sperm are decreased due to bisphenol A (BPA) exposure, an endocrine-disrupting chemical on the male reproductive system, especially in advanced paternal age (APA). Procyanidin C-1(PCY-1), an antioxidant from grape seed (Vitis vinifera L.), has demonstrated anti-viral, anti-melanogenic and immunostimulatory effects. Therefore, this study aims to determine the effects of PCY-1 intervention on sperm parameters, testis morphological changes, serum testosterone, oestradiol, and luteinizing hormone (LH)concentrations and the expression of apoptotic (Bax and Bcl-2) and mitochondria-related (Mfn1 and Opa1)genes in BPA-exposed aged mice. Results revealed that PCY-1 intervention improves aged male fertility in BPA-exposed conditions by decreasing abnormal sperms percentage and increasing spermatogenic cell diameter and epithelial height. PCY-1 also decreased oestradiol, and increased LH and testosterone levels.The gene expression of Bax was significantly down-regulated by PCY-1 intervention. In contrast, Bcl-2 was substantially up-regulated. Expression of Mfn1 and Opa1 genes were also significantly up-regulated in the PCY intervention group. Hence, it is demonstrated that PCY-1 was able to mitigate the adverse effects of BPA on reproductive parameters of aged mice. Collectively, we postulated that PCY-1 has a potential role in protecting the ageing male reproductive system against the damaging impacts of BPA.
1. Introduction
Infertility is a global problem that affects one in ten couples [1].According to the International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised Glossary ART, infertility is a reproductive disease. It is de fined as no conception after 12 months or more of regular unprotected sexual intercourse [2]. Studies have shown that the male partners contributed 50% of infertility cases because of low sperm count and poor sperm quality, or both [3-5].Male infertility may be attributed to numerous factors such as smoking [6], depression and anxiety [7], the influence of medical conditions such as diabetes [8]and heart disease, environmental toxicants [9], and lifestyle [10]. Ageing may also contribute to male infertility due to DNA damage to sperm [11].
Bisphenol A (2,2-bis (4-hydroxyphenyl) propane, BPA), an oestrogen-mimic endocrine disruptive chemical (EDC), is a ubiquitous pollutant used as a monomer in the production of polycarbonate plastics and epoxy resins. BPA is commonly used in food packaging,dental sealants, medical devices, and thermal receipts. Heat and either acidic or basic conditions speed up ester bonds’ hydrolysis that links BPA monomers to release BPA into the environment. BPA is toxic for male reproductive physiology in animal models. However, the adverse effects of BPA on human data have been argued to have disparities probably due to the exposure dosage, duration, route, and lifestyle that affect human reproductive health in various ways [12-14].Based on the evidence from laboratory animals and human exposure from 2007 to 2013, Peretz et al. [15]concluded that BPA had an impact on female reproduction at doses below the lowest observed adverse effect level (LOAEL) 50 mg/kg per day and also had possible adverse effects on the male reproductive system.
In the last few decades, delayed childbearing has led to older maternal and paternal generations. The age of fathers has increased due to increased life expectancy, increased divorce/remarriage rates,and increased access to the assisted reproductive technology (ART). In 2015, the birth rate among 35 to 49-year-old American men increased to 69.1 per thousand compared to 42.8 per thousand in 1980 [16].Khandawala and colleague also demonstrated that the mean paternal age in the US rose from 27.4 in 1974 to 30.9 in 2015 [17]. Vaughan and colleagues demonstrated a correlation between age and the DNA fragmentation index (DFI) of sperm from 17 000 samples which the sperm DNA fragmentation increased with increasing age [18].This shows that advanced paternal age (APA) does affect the fertility rate as increased DNA damage in sperm lowered male fertility in natural conception. Despite the well-established effects of advanced maternal age, the study on APA’s implication in male reproduction is still insufficient.
Procyanidin C-1 (PCY-1), a bioactive compound abundant in apple peel and grape seed, consists of three epicatechin units [19,20].Procyanidin C-1 exhibits high scavenging activities in DPPH(2,2’-diphenyl-1-picrylhydrazyl radical) assay, hydroxyl radical scavenging assays, lipid peroxidation-inhibiting and complementinhibiting activityin vitrofor antioxidative studies [21]. A study by Kin and colleagues [22]showed that PCY-1 has anti-metastatic or anti-epithelial-to-mesenchymal transition (EMT) effects on human lung adenocarcinoma A549 cells. Byun et al. [23]reported that PCY-1 effectively facilitated vasorelaxation on phenylephrine-constricted endothelium-intact thoracic aortic rings. Studies by Hori et al. [24]and Cary and Peterlin [25]demonstrated that PCY-1 has potential as HIV functional cure as it can reactivate latent HIV. Thus, the virus can be eliminated through the immune system. Bae et al. [26]illustrated the anti-cancer activity of PCY-1 on melanoma growth via the laminin receptor’s activation.
This study was designed to assess the impact of PCY-1 intervention on BPA-exposed aged male mice on sperm parameters,testes histomorphology, hormonal levels and gene expression. For this study, we selected genes that play essential roles in mitochondrial apoptosis and fusion. Mitochondrial fusion is vital in mitochondria equilibrium dynamics. Impaired fusion causes uneven mitochondrial elongation and leads to structural damage, which activates the mitochondria-mediated apoptosis pathway. We hypothesized that PCY-1 would improve sperm and testicular parameters and decrease apoptosis in BPA-exposed aged mice.
2. Materials and methods
2.1 Animals and administration procedure
A total of 24 male C57BL/6NTac mice (18-20 months old, (30 ± 2) g)were maintained at the Laboratory Animal Care Unit (LACU),Faculty of Medicine, Universiti Teknologi MARA Sungai Buloh,Selangor, Malaysia. The mice were divided into four groups (n=6 for each group): Group I (control) (ultra-purified deionized water),Group II (15 mg/kg BW BPA), Group III (PCY-1 20 µg/kg BW) and Group IV (BPA 15 mg/kg BW + PCY-1 20 µg/kg BW). Bisphenol A (CAS No. 80-05-7) was purchased from Sigma-Aldrich (Saint Louis, USA) and prepared as described by Bahariv et al. [27]. The PCY-1 was purchased from ChemFaces, Wuhan, China (CAS No.37064-30-5) and dissolved with ultra-purified deionized water as vehicle. Treatments were administered daily via oral gavage for 35 days, to cover a complete spermatogenesis cycle in mice [28,29].During the experiment, mice were housed in polyurethane cages at(24 ± 3) °C, humidity (50 ± 5)% in a controlled light environment(12 h light : 12 h dark). They were provided with water and standard rodent pelletsad libitum. The number of animals was estimated using the resource equation [30,31]. This study was approved by the Institutional Committee on Animal Research & Ethics (UiTM Care:169/2017).
2.2 Collection of samples for analysis
Each mouse was weighed weekly. They were anaesthetized on the Day 36 of treatment using a mixture of ketamine, xylazine and zoletil-50 (KTX agent) before collection of blood via cardiac puncture. The anaesthesia was administered intraperitoneal injection(i.p.) at a 13.5 mg/kg dose [32]. Then, the mice were euthanized by cervical dislocation. After allowing the collected blood to clot, the serum was separated via centrifugation at 2 500 ×gfor 10 mins at 4 °C. The serum was stored at -80 °C prior to hormone analysis.Testes were weighed and fixed in 10% neutral buffered formalin(Sigma, Saint Louis, USA). Reproductive organ coefficient (%) was calculated as the per cent of the ratio of the reproductive organ weight(mg) to body weight (g) [33,34].
2.3 Sperm concentration and morphology
The epididymides were excised, trimmed of fat, weighed and the cauda was punctured five times in 1 mL of warm M16 medium (Sigma,Saint Louis, USA) for 5 mins in a petri dish to allow for sperm swim out [35,36]. A small amount of sperm suspension was smeared onto a slide and stained with 1% Eosin Y (Sigma, Saint Louis, USA).Two hundred spermatozoa per mouse were examined [37]. Sperm abnormalities were recorded as a percentage of the total number of sperm counted. The sperm concentration was counted using a Makler Chamber (Se fiMedical Industries, Haifa, Israel) at 20X magnification.The total number of sperm were counted to represent the sperm concentration in millions per mL. The average of 3 readings was recorded [36,38].
2.4 Testes tissue preparation
The fixed tissues were serially dehydrated with alcohol, mixed with xylene, and subsequently infiltrated with paraffin wax using the automated Thermo Scientific™ STP 120 Spin Tissue Processor(Microm International GmbH, Thermo Fisher Scientific, Walldorf,Germany). The processed testes were paraffin-embedded, sectioned at 5 μm thickness using a rotary microtome (RM2125 Leica,Heidelberger, Germany) and stained with haematoxylin-eosin (H&E)(Sigma, Saint Louis, MO, USA) for histology evaluation.
2.5 Histomorphometry evaluation
The stained slides were analysed with light microscopy (Olympus BX35 fitted with a DP72 video camera) (Olympus Co., Tokyo, Japan)under 10X and 40X magnification. The seminiferous tubules diameter(STD) and seminiferous epithelial height (SEH) were measured using software Cell^D (Olympus Soft Imaging Solutions GmbH, Münster,Germany). A random evaluation was conducted from ten microscopic fields per section/mice. At least 50 circular and almost circular tubules were evaluated. SEH was calculated with the lumen diameter subtracted from the STD [39,40]. The testicular tissue assessment was carried out blinded to the experiment.
2.6 Serum hormonal analyses
Testosterone, oestradiol, and LH levels were determined in the serum using ELISA testosterone kit (Elabscience, cat no.:E-EL-M0518), oestradiol ELISA kit (Elabscience, cat no.: E-EL-0065)and LH ELISA kit (Elabscience, cat no.: E-EL-M0057) according to manufacturer protocol. A primary antibody was coated on the 96-well plates and, subsequently, 50 μL of the standard, control, or sample were added to each well, which was incubated following the manufacturer instructions. After washing, 100 μL of the enzymeconjugated solution was added and incubated. The substrate was added, and the reaction terminated with the addition of sulphuric acid. The absorbance of samples was measured on a microplate reader(Victor X5, PerkinElmer, Waltham, USA) at 450 nm. Data were expressed as nanogram of testosterone and LH per mL of serum (ng/mL)and picogram of oestradiol per mL of serum (pg/mL).
2.7 Total RNA extraction
Total RNA was extracted from the left testis of experimental and control animals. Freshly harvested samples were immediately stabilized in 5 mL RNAlater (Ambion, ThermoFisher Scientific,California, USA). The samples placed in RNAlater were stored at 4 °C overnight. Then, the samples were stored at -80 °C until further use. For RNA extraction, the NucleoSpin RNA XS kit (Macherey-Nagel, Germany) was used. Testes (10 mg) in RNAlater solution were used to extract total RNA. Samples were processed according to the manufacturer instructions. Isolated RNA was kept at -80 °C.The RNA quantity and purity were measured with a SpectraMax QuickDrop Micro-Volume Spectrophotometer (Molecular Devices,California, USA) described by Zatecka et al. [41].
2.8 Reverse transcription polymerase chain reaction (RT-PCR)
Reverse transcription into Complementary DNA (cDNA)synthesis was performed using Reverse Transcription Master Mix from Fluidigm (San Francisco, CA) according to the manufacturer protocol, with random primers in a final volume of 5 μL containing 80 ng total RNA. In a total volume of 5 μL, the reaction contained 1 μL of the pre-amplification master mix, 1.25 μL of cDNA, 0.5 μL of pooled Delta Gene Assays with a final concentration of each assay of 500 nmol/L (0.2-fold) and 1 μL of PCR water. The mixture was first incubated for 2 min at 95 °C, followed 11 cycles of 15 seconds at 95 °C, and finally 4 min at 60 °C. The pre-amplified cDNAs were diluted 10-fold.
2.9 Microfluidic quantitative real-time polymerase chain reaction (qRT-PCR)
High-throughput real-time PCR was performed using the highthroughput platform BioMark HD System, and the 192.24 GE Delta Gene Dynamic Arrays integrated fluidic circuit (IFC) qPCR (Fluidigm,San Francisco, CA). 3 µL of Fluidigm sample premix (SMM)consisted of 1.35 µL of 10-fold diluted pre-amplified cDNA, 0.15 μL of 192.24 Delta Gene Sample Reagent (Fluidigm, San Fransisco,CA), and 1.5 μL of 2X SsoFast EvaGreen Supermix with low ROX(BioRad, USA). 3 μL of 10-fold assay master mix (AMM) consisted of 0.15 µL 100 µmol/L each Delta Gene primers (forward and reverse combined), 1.35 µL of 1-fold DNA suspension buffer and 1.5 μL of 2-fold Assay Loading Reagent (Fluidigm, San Fransisco, CA). 3 μL of each SMM and each AMM premixes were added to the dedicated wells.The samples and assays were mixed inside the chip using the HX IFC controller (Fluidigm, San Fransisco, CA). QPCR’s thermal conditions were as follows: 95 °C for 60 seconds, 40 cycles of 95 °C for 15 seconds, and 60 °C for 1 min. Followed by a melting curve of 60–95 °C with a slope of 0.1 C/s. The data were obtained and analysed with BioMark 3.1.2 software and BioMark Real-Time PCR Analysis Software 4.3.1 (Fludigm, USA). The primer sequences details used in this study are presented in Table 1. Data were normalized with two housekeeping genes:β-actinandGAPDH. The fold change in expression was calculated using the 2−ΔΔCq method [42].The control was set at 100%, and experimental samples were correlated with the control [41].

Table 1Primer sequences.
2.10 Statistical analysis
All analyses were performed using GraphPad Prism version 8 for Windows (GraphPad Software, La Jolla California, USA). The results were expressed as mean ± SEM. The Shapiro-Wilk test was used before one-way ANOVA to analyse the normality of distribution.One-way ANOVA and the post-hoc test of Tukey were carried out to determine significant differences between control and treatment groups. Difference values were considered significant whenP< 0.05.
3. Results
3.1 Effects of PCY-1 on the body and reproductive organs coefficient weight of aged mice
We examined whether PCY-1 influences body and reproductive organs coefficient weight in BPA-exposed aged male mice. At the end of treatments, the bodyweight changes increased significantly(P< 0.05 andP< 0.01, respectively) in BPA and BPA+PCY-1 groups (Fig.1). However, the reduction of body weights in control and PCY-1 groups were not significant. There was no significant effect on the testis coefficient. Oral administration of PCY-1 alone increased epididymides coefficient (P< 0.01) significantly compared to BPA group (Fig. 2).
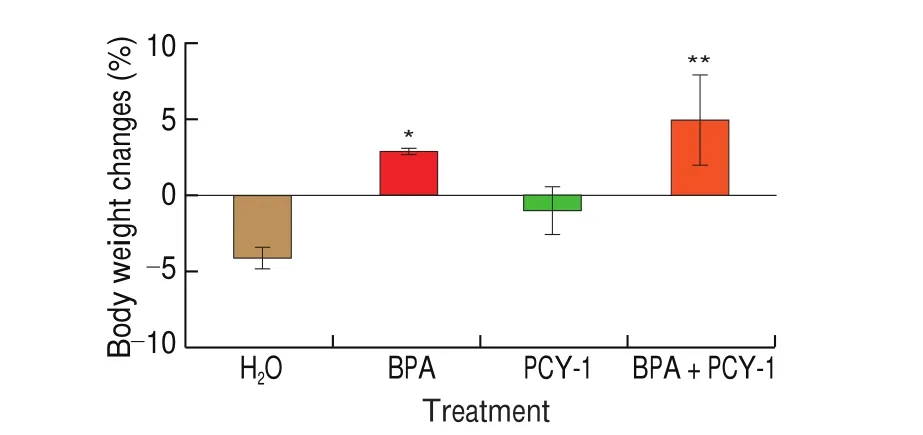
Fig. 1 Body weight changes of mice in control and experimental groups. Values are mean ± SEM (n = 6). **P < 0.01 and *P < 0.05 as compared to control group.

Fig. 2 Effects of PCY-1 on reproductive organ coefficients of the mice.Values are means ± SEM (n = 6). ##P < 0.01 as compared to BPA group.
3.2 Effects of PCY-1 on sperm parameters of aged mice
Epididymal sperm concentrations and abnormal sperm percentages are presented in Fig. 3, and the morphology of normal and abnormal sperms are shown in Fig. 4. There was no significant change in sperm concentrations in all groups. However, the treatment of PCY-1 in aged mice reduced the percentage of abnormal sperm significantly (P< 0.01 andP< 0.001, respectively) in comparison to control and BPA group. A significant (P< 0.05) reduction in the percentage of abnormal sperm was also determined in the BPA+PCY-1 group compared to the BPA group.
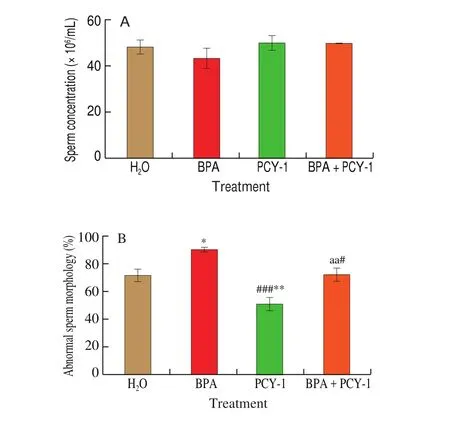
Fig. 3 The effect of PCY-1 on sperm concentration and abnormal sperm morphology. Values are mean ± SEM (n = 6). **P < 0.01 and *P < 0.05 as compared to control group. ###P < 0.001 as compared to BPA group. aaP < 0.01 as compared to PCY-1 group.

Fig. 4 Photomicrographs showing epididymal sperm morphology (1% Eosin Y, 100X): N = Normal, A = Abnormal; (a-e) types of sperm abnormalities;(a & b) Pinhead sperm (c) Sperm with bent tail (d) Folded sperm with amorphous head (e) Banana like sperm.
3.3 Effects of PCY-1 on testis histopathology of aged mice
Morphological characteristics of the testes are shown in Fig. 5.The results showed that the BPA group demonstrated expanded atrophy, as shown by an increased interstitial space and tubular disarrangement compared to the control group. Furthermore, the BPA group also exhibited lower luminal spermatozoa, disorganization,and degeneration of spermatogenic cells, with a damaged basement membrane. The STD and SEH in the BPA group (P< 0.001)decreased significantly compared to the control group, as shown in Table 2. No apparent histopathological changes were observed in the administration of PCY-1 alone. However, in contrast to the control group and the BPA group, PCY-1 significantly increased STD and SEH (P< 0.001). A significant (P< 0.001) enhancement was observed in the BPA+PCY-1 group with increased STD and SEH compared with the BPA group. In the centre of the tubules, the BPA + PCY-1 group showed more spermatid, and reduced lumen diameter, compared to the BPA group.
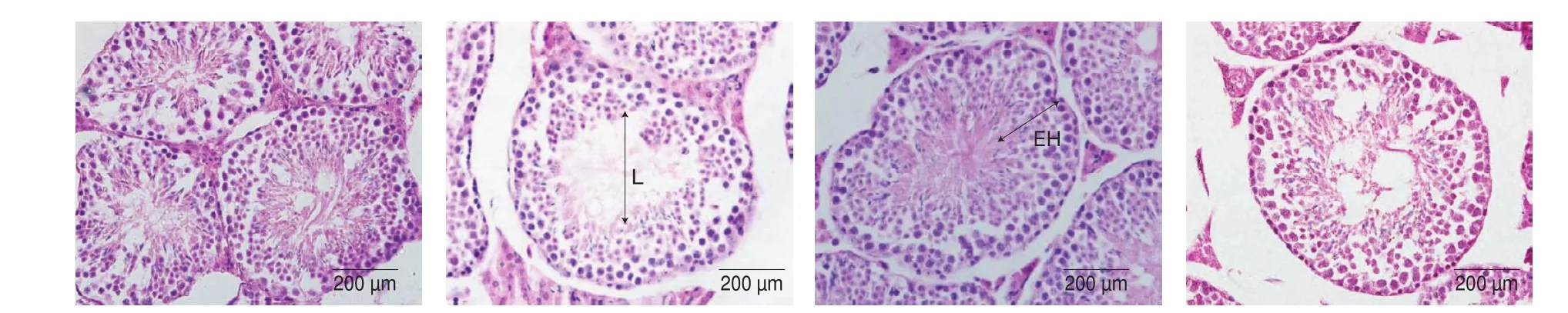
Fig. 5 Photomicrographs showing effects of (A) control (B) BPA (C) PCY-1 and (D) BPA+PCY-1 on testicular histology (H&E, 40X). L: Lumen, EH: Epithelial height.

Table 2STD and SEH in testes of mice in different groups.
3.4 Effects of PCY-1 on serum testosterone, oestradiol, and LH levels of aged mice
ELISA was conducted to determine serum concentrations of testosterone, oestradiol, and LH after BPA exposure for 35 days to test the effect of PCY intervention on BPA upon the reproductive endocrine system. Changes in the serum levels of testosterone, oestradiol and LH of aged mice are presented in Fig. 6. Co-administration of PCY-1 (BPA+PCY-1 group) significantly restored serum testosterone level (P< 0.05) compared to the control group (Fig. 6A)and lowered oestradiol level (P< 0.01) compared to the BPA group (Fig. 6B). The serum LH level in BPA+PCY-1 group increased significantly (P< 0.01) compared to BPA group (Fig. 6C). Administration of PCY-1 alone significantly elevated the serum testosterone (Fig. 6A)(P< 0.05 andP< 0.01, respectively) and LH levels (Fig. 6C) (P< 0.01 andP< 0.001, respectively) in comparison to the control and BPA group,respectively. However, the reduction of the serum oestradiol level in PCY-1 group was not significant (Fig. 6B).
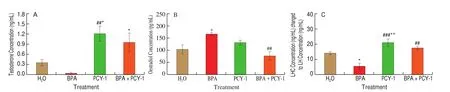
Fig. 6 The effect of PCY-1 on serum (A) testosterone, (B) oestradiol and (C) LH concentration levels. Values are mean SEM (n = 6). **P < 0.01 and *P < 0.05 as compared to control group. ###P < 0.001, ##P < 0.01 and #P <0.05 as compared to BPA group.
3.5 Effects of PCY-1 on the expression of selected apoptosisrelated genes in aged mice testis
There was significant up-regulation (P< 0.05) of Bax level in the BPA group compared to the control group. Co-administration of PCY-1 significantly down-regulatedBaxexpression (P< 0.05)(Fig. 7A), and up-regulated the expression ofBcl-2in the BPA+PCY-1 group (P< 0.05) (Fig. 7B), compared to BPA group.The intervention of PCY-1 in BPA+PCY-1 led to a substantial decreased in the ratio ofBax/Bcl-2compared to BPA group (P< 0.01)(Fig. 7C). TheBaxandBcl-2expression changes in the PCY-1 group were not significant compared to the control group. However, as shown in Fig. 7C, the administration of PCY-1 alone significantly reduced theBax/Bcl-2ratio, compared with the BPA group (P< 0.05).

Fig. 7 The effect of PCY-1 on the relative gene expression of selected apoptosis-related genes in the testis of mice in different groups. The control group represents 100% and the percentage in the other groups represents the ratio of gene expression between the treatment groups and the control group. (A) Expression of Bax. (B) Expression of Bcl-2. (C) Ratio of Bax/Bcl-2.Values are means ± SEM (n = 6). **P < 0.01 and *P < 0.05 as compared to control group. ##P < 0.01 and#P < 0.05 as compared to BPA group.
3.6 Effects of PCY-1 on the expression of selected mitochondrial dynamic-related genes in aged mice testis
Results showed that the administration of PCY-1 alone in aged mice significantly up-regulated the expression ofMfn1(Fig. 8A)andOpa1(Fig. 8B) genes (P< 0.01 andP< 0.001, respectively)compared to BPA group. Meanwhile, in the BPA+PCY-1 group,expression ofOpa1(Fig. 8B) was significantly up-regulated (P< 0.01)compared to the BPA group. However, up-regulatedMfn1expression was not statistically significant (Fig. 8A).

Fig. 8. The effect of PCY-1 on the relative gene expression of mitochondrial dynamic-related genes in the testis of mice in different groups. The control group represents 100% and the percentage in the other groups represents the ratio of gene expression between the treated groups and the control group.(A) Expression of Mfn1. (B) Expression of Opa1. Values are means ± SEM (n = 6).**P < 0.01 and *P < 0.05 as compared to control group. ###P < 0.001 and ##P < 0.01 as compared to BPA group. aP < 0.05 as compared to PCY group.
4. Discussion
Male infertility is a global problem affecting about 4%-12% of men; it contributes to 20%-70% of cases worldwide with Africa and Eastern Europe having the highest rates [43]. There is growing evidence that one of the causes of male reproductive disorders and infertility is due to exposure to endocrine-disrupting chemicals such as bisphenol A (BPA) [13,44,45]. Due to BPA strength and stability at high temperatures, it is used widely in the industry, especially in the production of polycarbonate plastics and food packaging. Prior studies have noted the adverse effects of BPA on male fertility include damage of testicular histoarchitecture [46], impaired spermatogenesis [12]and lowered testosterone and LH [47]. Findings suggest an essential correlation between paternal urinary BPA concentration and the couple’s high prevalence of infertility treatment failure [48]. The increased likelihood of infertility among older fathers has been observed due to DNA damage [49,50], and the decline of reproductive hormones and sexual function [51,52]. It has been noted that APA is adversely affecting the reproductive and fertility outcomes, including reduced implantation rate [53], higher risk of spontaneous miscarriage [54]and higher levels of stillbirths [55]. Besides, the continuous involuntary ingestion of BPA combined with APA factor may significantly disrupt the ageing male reproductive system’s functions and eventually lead to infertility.
The purpose of this study was to determine the potential of procyanidin C-1 (PCY-1), a natural compound from grape seed (Vitis viniferaL.), to mitigate the adverse effects of BPA on sperm, testes,and reproductive hormones in aged mice model. Procyanidin C-1,a water-soluble B-type procyanidin that can be found from various sources such as apple [56], black soybean seed coat [57], cinnamon [58]and cocoa nibs [59]has exhibited good bioavailabilityin vivo. Hence,it is an excellent compound that can be administered without any chemical vehicle for this study. Bisphenol A was supplemented for 35 days to C57Bl/6NTac mice in the BPA group and with the coadministration of PCY-1 in BPA+PCY-1 group. The control group was administered with ultra-purified deionized water, and PCY-1 group was supplemented with PCY-1 only. The dosage of PCY-1 which we have used in this study is based on the finding demonstrated by Yamashita and colleagues [60]. Even though at 10 μg/kg BW,PCY-1 showed a significant result in lowering the mice’s glucose level. Therefore, we speculated that PCY-1 at the dose of 20 μg/kg BW should be sufficient enough to negate the adverse effect of BPA on reproductive parameters in aged mice.
Our study evidenced that BPA significantly increased the final body weight in male mice, as was also reported in a study by Park et al. [61], where ICR mice were treated with 10 mg/kg BW of BPA for 48 days. The weight gain was probably due to dysregulation of endogenous reproductive hormones caused by BPA. The intervention of PCY-1 in BPA treated mice also caused weight gain(Fig. 1). Previous studies by Behmanesh et al. [39]and Grami et al. [62]showed contradictory results. The authors found that body weights of the animals were significantly reduced following exposure to BPA.The reduction of body weight of mice in the control and PCY-1 group were not significant. No statistically significant difference was observed for testes coefficient between all groups. Interestingly,the epididymides coefficient increased significantly in PCY-1 group compared to BPA group. Zhang et al. [63]identified that after being treated with BPA at 20 mg/kg BW for seven days in adult male mice, the weights and coefficients of testes and epididymides in BPA group were significantly decreased. Differences in body weight and testicular and epididymal coefficients may be due to the varying doses, BPA administration routes, length and exposure periods, the strain of mice used and food composition.
Several studies have shown the adverse effects of BPA exposure on sperm parameters such as sperm concentration and percentage of abnormal sperms in adult mice or rats. As documented by Kaur et al. [64],oral administration of BPA to adult male BALB/c mice at a 1 mg/kg BW concentration showed significant sperm count and motility reductions.This is possibly due to the reduction of the type A spermatogonia,spermatocytes and spermatids caused by BPA-generated oxidative stress. Grami et al. [62]also reported a significant reduction of sperm count and sperm motility, as well as an increase in the number of dead sperms in male Wistar treated with 100 mg/kg BW BPA for four weeks. Our data revealed that no changes in sperm concentrations.However, the co-administration of PCY-1 resulted in a substantially reduced percentage of abnormal sperm. This finding is similar to the study by Park et al. [61], which demonstrated that the ethanol extract ofLespedeza cuneatedecreased the percentage of abnormal sperm morphology in ICR mice-BPA exposed. The high total phenolic and flavonoid content of the extract could be the justification for this finding [65].
The present study indicated that STD and SEH were reduced following exposure to BPA, compared to the control group (Table 2).These results are consistent with the histological evaluation of seminiferous tubules by Behmanesh et al. [39], which exhibited decreased luminal spermatozoa in rats exposed to BPA. Güleş et al. [66]also demonstrated a significant decrease in STD and SEH in adult male Wistar rats exposed to 100 mg/kg per day of BPA. In contrast,intervention with PCY-1 improved STD and SEH parameters significantly compared to the BPA group, probably due to the antioxidant property of this polyphenolic compound [21,67]. In the BPA group, atrophy and separation of the germinal epithelium were observed in most seminiferous tubule vacuoles. Meanwhile, there was a reduction in these vacuoles’ count, and size in the BPA+PCY-1 administrated group.
Testosterone plays a crucial role in regulating germ cell growth and preserving male fertility. It is produced by Leydig cells in response to LH stimulation. The hypothalamic-pituitary-testicular axis regulates testosterone production. It is a paracrine factor that diffuses into seminiferous tubules and Sertoli cells to initiate spermatogenesis [68,69]. Although oestradiol in a small amount is vital for maintaining Sertoli cell activity [70], high levels cause a detrimental effect on sperm production by suppressing the formation of spermatocytes and spermatids [71]. In this study, exposure to BPA (15 mg/kg per day) for 35 days led to a significant decrease in serum LH, but not in serum testosterone levels. However, BPA group showed significant (P< 0.05) elevation of serum oestradiol.Our observations are in line with the finding of Zang et al. [72], in which the decrease of testosterone caused by BPA also suppressed sexual behaviour of adult male C57BL/6 mice. Bisphenol A has been demonstrated to promote sperm dysfunction by causing hormone level alterations [14]. As Wisniewski et al. [73]reported, exposure to BPA at 5 mg/kg and 25 mg/kg in Wistar rats disrupted the hypothalamicpituitary-gonadal axis by lowering testosterone and increasing oestradiol levels, thus, causing hypogonadotropic hypogonadism.BPA also caused oxidative damage to the Leydig cells and induced alteration in morphophysiology of the Leydig cells [73].
The intervention of PCY-1 resulted in significant amelioration of testosterone (P< 0.05) and LH (P< 0.01) levels, as well as a noticeable decline of oestradiol level (P< 0.001) (Fig. 6).Administration of PCY-1 alone was also able to elevate testosterone and LH serum levels in aged mice. A study conducted by Hasona [74]revealed that at a concentration of 400 mg/kg BW of grape seed extract (GSE) was able to attenuate the adverse effect of dexamethasone on reproductive hormones due to polyphenolic constituents of GSE. The ameliorative effect of PCY-1 on BPA might be due to the antioxidative properties of PCY-1 polyphenolic compounds in grape seed [21].
The regulation of apoptosis by specific genes and hormones in the testis dominates normal spermatogenesis. Inadequate or excessive apoptosis contribute to decreased sperm production and fertility.Mitochondrial pathway-mediated apoptosis, which is modulated byBcl-2family expression is vital in reactive oxygen species (ROS)-induced testicular injury. TheBcl-2family contains two main functional groups, which are the pro-apoptoticBaxand the antiapoptoticBcl-2. Bax induces apoptogenic molecules’ release from mitochondria’ intermembrane space, whereasBcl-2prevents it from occurring [75]. The intrinsic mitochondrial pathway is activated by BPA, resulting in increasedBaxexpression, which can inhibitBcl-2expression [76].
Our study demonstrated that Bax, the predominant pro-apoptotic gene, was up-regulated after BPA exposure, concomitantly with down-regulation of the anti-apoptotic protein Bcl-2. In concurrence with this finding, Ma et al. [47]presented that BPA exposure to the mother caused increased Bax and decreased Bcl-2 in testicular and ovarian tissues of offspring mice. The study by Ok et al. [77]reported that injection of 25 mg/kg BPA resulted in significant up-regulation ofBax, and down-regulation ofBcl-2protein expressions, compared to the control rats. We also observed significant down-regulation of Bax and up-regulation ofBcl-2in the BPA+PCY-1 group.Spermatocyte apoptosis or spermatogenesis inhibition is believed to be closely related to the increased ratio ofBax/Bcl2. An increase in theBax/Bcl-2ratio causes the release of cytochrome c from the mitochondria into the cytoplasm and activates a caspase cascade during apoptosis. In our experiments, a loweredBax/Bcl2ratio was seen in BPA+PCY-1. This suggests that PCY-1 was able to reduce the apoptotic effect of BPA.
Mitochondria are complex organelles that undergo continuous fission/fusion to reorganize their morphology, number and subcellular distribution to fulfil cellular energy requirements [78].The fusion of individual mitochondrion into a dynamic network may enhance mitochondrial function. Meanwhile, mitochondrial fission eradicates damaged mitochondria through mitochondrial autophagy(mitophagy) [78]. Mitochondrial fusion enables efficient mixing of mitochondrial content, forming a network of highly interconnected filaments based on membrane-anchored mitofusin 1 (Mfn1) and optic atrophy 1 (Opa1). Mfn1 mediates the external mitochondrial membrane’s fusion, while Opa1 mediates the internal mitochondrial membrane [78]. Disruption of mitochondrial fusion causes mitochondrial dysfunction, resulting in structural damage,mitochondrial fragmentation, and cytochrome c release that can activate the caspase cascade and induce apoptosis. Our results show that the intervention of PCY-1 in BPA+PCY-1 group up-regulated the expression ofMfn1andOpa1genes. This finding is similar to the study by Amanpour et al. [79], which demonstrated that vitamin E’s intervention up-regulated the expression of Mfn1 in cadmiuminduced testicular damage in rats. This study showed that PCY-1 exerted protective effects against BPA in the aged male reproductive system by reducing the ratio ofBax/Bcl-2and up-regulating theMfn1andOpa1genes.
5. Conclusion
Overall, this study illustrates that BPA may perturb the male reproductive system’s function in aged mice. PCY-1 intervention successfully attenuated these changes by restoring epididymis coefficient weight, reducing the percentage of abnormal sperm,lowering oestradiol, and elevating testosterone and LH levels in BPA-exposed aged mice. PCY-1 may play a role in protecting spermatocytes from apoptosis by decreasing the ratio ofBax/Bcl-2.To our knowledge, the current study is the first report to demonstrate PCY-1 protection against testicular damage caused by endocrine disruption chemical in the aged animal model. The studies indicate that PCY-1 may be a new possible alternative to improve fertility parameters. PCY-1 can be an efficient and effective manipulation to attenuate BPA- and APA-induced infertility in the future. However,human intervention trials are needed to corroborate these data.
Competing interest
The authors declare that they have no competing interests.
Acknowledgements
This research was supported financially by Universiti Teknologi MARA grants (600-IRMI/DANA KCM 5/3/LESTARI – 224/2017),(600-IRMI/MyRA 5/3/GIP-030/2017) and Ministry of Higher Education (MOHE) grant (600-IRMI/RAGS 5/3 - 72/2015).
杂志排行
食品科学与人类健康(英文)的其它文章
- Dietary bioactives and essential oils of lemon and lime fruits
- Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence
- Simultaneous quantification of 18 bioactive constituents in Ziziphus jujuba fruits by HPLC coupled with a chemometric method
- A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae)
- GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora
- Immunoregulatory polysaccharides from Apocynum venetum L.flowers stimulate phagocytosis and cytokine expression via activating the NF-κB/MAPK signaling pathways in RAW264.7 cells
