Severe acute respiratory syndrome coronavirus 2 infection: Role of interleukin-6 and the inflammatory cascade
2022-06-16MohaddesehBahmaniRojinCheginiElhamGhanbariElhamSheykhsaranParisaShiriAghbashHamedEbrahimzadehLeylabadloEhsanMoradianAmirMasoudKazemzadehHoujaghanHosseinBannazadehBaghi
Mohaddeseh Bahmani, Rojin Chegini, Elham Ghanbari, Elham Sheykhsaran, Parisa Shiri Aghbash, Hamed Ebrahimzadeh Leylabadlo, Ehsan Moradian, Amir Masoud Kazemzadeh Houjaghan, Hossein Bannazadeh Baghi
Mohaddeseh Bahmani, Department of Virology, Student Research Committee, Tabriz University of Medical Sciences, Tabriz 15731, Iran
Rojin Chegini, Department of Medical Science, Metabolic Liver Disease Research Center,Isfahan University of Medical Sciences, Isfahan 81745-33871, Iran
Elham Ghanbari, Department of Medical Science, Fertility and Infertility Research Center,Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah 67159-59167, Iran
Elham Sheykhsaran, Department of Microbiology, Student Research Committee, Tabriz University of Medical Sciences, Tabriz 15731, Iran
Elham Sheykhsaran, Parisa Shiri Aghbash, Immunology Research Center, Tabriz University of Medical Sciences, Tabriz 15731, Iran
Parisa Shiri Aghbash, Department of Virology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz 15731, Iran
Hamed Ebrahimzadeh Leylabadlo, Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz 15731, Iran
Ehsan Moradian, Department of Medical Science, Medical Faculty, Tabriz University of Medical Sciences, Tabriz 5165665931, Iran
Amir Masoud Kazemzadeh Houjaghan, Department of Internal Medicine, Medical Faculty,Tehran University of Medical Sciences, Tehran 14155-6559, Iran
Hossein Bannazadeh Baghi, Department of Virology, Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz 15731, Iran
Abstract Since December 2019, a novel coronavirus that represents a serious threat to human lives has emerged. There is still no definite treatment for severe cases of the disease caused by this virus, named coronavirus disease 2019 (COVID-19). One of the most considered treatment strategies targets the exaggerated immune regulator, and interleukin (IL)-6 is a crucial pro-inflammatory mediator. Severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) cases show an elevated level of IL-6 related to disease severity. IL-6 activity can be inhibited by the following: IL-6 itself, IL-6 signaling pathways such as Janus kinase and signal transducer and activator of transcription (JAK-STAT), gp130, IL-6R, and downstream activated ILs, such as IL-17 and IL-6 cytokine. Currently, according to these studies and their results, IL-6 blockade with anti-IL-6 or its receptor antibodies such as tocilizumab in COVID-19 is beneficial in severe cases and may reduce the mortality rate. JAK-STAT inhibitors block the cytokine storm by inhibiting several crucial pro-inflammatory mediators such as TNF-α and IL-6 and have shown various results in clinical trials. IL-6 induces IL-17 secretion, and IL-17 is involved in the pathogenesis of inflammatory processes. Clinical trials of anti-IL-17 drugs are currently recruiting, and anti-gp130 antibody is preclinical. However, this agent has shown positive effects in inflammatory bowel disease clinical trials and could be tested for SARS-CoV-2. This study aimed to review the role of IL-6 in the cytokine storm and studies regarding IL-6 and blockade of its inflammatory pathways in COVID-19 to determine if any of these agents are beneficial for COVID-19 patients.
Key Words: Anti-interleukin-6; COVID-19; Inflammation; Interleukin-6; Interleukin-6 receptor; SARS-CoV-2
INTRODUCTION
In December 2019, an epidemic of secretive pneumonia which started in Wuhan city, Hubei province, China, quickly spread to many other countries and finally resulted in a pandemic[1]. The causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a single-stranded enveloped RNA virus belonging to Nidovirales and the family Coronaviridae. The analysis of SARS-CoV-2 genome structure has shown that this virus is related to the beta-coronavirus genus, containing bat SARS-identical coronavirus and two previous invasive coronaviruses Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and SARS-CoV[2]. Universally, as of September 2021, there have been 226,844,344 recognized cases of SARS-CoV-2, including 4,666,334 victims[3]. The disease caused by the novel coronavirus, coronavirus disease 2019 (COVID-19), is similar to these previous viruses, which is mainly pulmonary disease[4], and all of them have a zoonotic origin. In addition to pulmonary involvement, various organs such as the kidney, gastrointestinal system, nervous system, liver, and coagulation system, may be targets of the virus, leading to serious complications such as acute kidney injury (AKI), acute pulmonary failure, and disseminated intravascular coagulation (DIC) that may lead to death[5]. Currently, this virus is a serious global concern with enormous social and economic damage to societies worldwide[6].
Moreover, the fatality rate is high in severe cases[7]. At present, we do not have any definite treatment for severe cases of this disease, and the management of severe SARS-CoV-2 patients is still challenging. Therefore, various treatment options have been assumed according to the different levels of viral pathogenesis, including viral entry, replication, and effects of the virus on target cells. The antiviral agent remdesivir is the only treatment with Food and Drug Administration (FDA) approval for this disease, and dexamethasone is the only drug to reduce mortality in hospitalized patients with decreased oxygen saturation but not in others[8]. However, the World Health Organization (WHO) has suggested mortality trials for some repurposed anti-viral drugs, including lopinavir, interferon beta 1a (INF-β1a), and hydroxychloroquine in hospitalized patients with SARS-CoV-2[9].
In this regard, IL-6 is known as a crucial inflammatory mediator with essential roles in the pathogenesis of inflammatory diseases in addition to several chronic disorders such as diabetes mellitus[10]. This cytokine is widely expressed by different immune cells and affects immune function[11]. Thus, the disease has a wide range of symptoms. Clinical deterioration in COVID-19 is mainly due to the effects of inflammatory cytokines such as IL-1, IL-6, IFN-α, and tumor necrosis factor (TNF) that are increased in the cytokine storm phase, and the role of immune cells including neutrophils[12-15]. In this process, when a neutrophil encounters a pathogen, the extensive release of cytokines such as IL-1 and IL-6 may become harmful to the body and lead to multi-organ damage[13]. In this rationale, targeting the cytokine release syndrome (CRS) symbolizes a possible therapeutic goal in managing SARS-CoV-2 related cytokine storms and IL-6[16].
In this study, we aim to review the role of IL-6, the rationale of IL-6 blockade in COVID-19, and the results of recent studies on this topic to determine whether any available anti-IL-6 agents or any other drugs with the ability to inhibit inflammatory pathways induced by this cytokine have shown efficacy in improving patient prognosis in SARS-CoV-2 infection.
STUDY METHOD
PubMed, Google Scholar, Scopus, and the Web of Science were searched with the following keywords or their combinations, without any time limits: COVID-19, IL-6, IL-6 receptor, SARS-CoV-2, anti-IL-6, Inflammation. Related articles of any type were selected and reviewed. Extracted information included: SARS-CoV-2 pathophysiology and characteristics, IL-6 activities in the immune system and associated pathways, studies focused on the concept of anti-IL-6 antibodies in the treatment of COVID-19, and other methods of IL-6 inhibition [Janus kinase and signal transducer and activator of transcription (JAKSTAT) inhibition and anti-IL-17 therapies] and are discussed further.
SARS-COV-2 PATHOPHYSIOLOGY AND CHARACTERISTICS
In the last two decades, the third most common coronavirus to cause a pandemic of acute respiratory disease in humans is SARS-CoV-2. These viruses enter the body through respiratory aerosols and are attached to the nasal or paranasal epithelial cells[17]. Angiotensin-converting enzyme 2 (ACE-2) is the major receptor for these viruses to enter host cells, which is expressed in nasal epithelial cells[18,19].
The virus, along with the infection of ciliated cells in the airways, undergoes local replication and dissemination. This stage lasts a few days, and a slight immune response is produced during this process. Despite having a low viral load at this time, infected individuals are highly contagious, and the virus can be identified following a nasal swab[20].
Virus entry into the host cell
Through its spike (S) protein, the virus enters the host cell by binding to ACE-2 on the cellular surface. Transmembrane serine protease 2 (TMPRSS2), then mediates S protein cleavage, and the virus enters the cell[21]. A high virus infectivity rate is associated with mutations in the binding domain of the receptor and the acquisition of a furin cleavage site in the S protein. The association of the virus with ACE-2 can decrease anti-inflammatory function and increase angiogenic activity[22]. The virus migrates from the nasal epithelium to the upper respiratory tract within the conducting airways[23]. The disease presents various signs and symptoms such as fever and dry cough due to involvement of the upper respiratory tract[24].
At this stage, a higher immune response occurs due to the virus-infected cells and results in the secretion of C-X-C motif chemokine ligand 10 (CXCL-10) and interferons (IFN-β and -λ). As a result of the sufficient immune response to control the spread of infection, the majority of patients do not advance beyond this point[25]. About one-fifth of infected individuals advance to this point and may experience severe symptoms. The virus,viathe host receptor ACE-2, targets alveolar epithelial cells type 2 and continues to undergo replication to create more and more viral nucleocapsids[26].
Many distinct cytokines and inflammatory markers are now produced by virus-laden pneumocytes such as ILs (IL-1, IL-6, IL-8, and IL-12), tumor necrosis factor-alpha (TNF-α), IFN-λ and IFN-β, monocyte chemoattractant protein-1 (MCP-1), CXCL-10, and macrophage inflammatory protein-1 alpha (MIP-1α). This 'cytokine storm' serves as a chemoattractant to neutrophils, CD4 helper, and CD8 cytotoxic T cells, and these cells then become sequestered in the pulmonary tissue[27,28]. In addition to being crucial in fighting the virus, these cells cause inflammation and damage to the lungs and other organs. The host cell undergoes apoptosis and releases new viruses, which will then infect the neighboring type 2 alveolar epithelial cells in the same way. Diffuse trauma to the alveoli eventually results in an acute respiratory syndrome and finally respiratory distress, owing to the recurrent injuries triggered by the sequestered immune cells and viral replication, contributing to the annihilation of both type 1 and type 2 pneumocytes[29,30].
COVID-19 spreads mainly by the transmission of respiratory droplets from person to person and occurs when someone is in close contact with an infected individual who is coughing or sneezing violently. This occurs as the host's mucosal surfaces,i.e., the eyes, nose, and mouth, are exposed to the infected respiratory droplets[31]. Virus transmission may also occur by fomites, such as bedsheets, towels, kitchen utensils, thermometers, and stethoscopes, used by or used on the infected person. Airborne transmission of COVID-19 can occur especially in situations where aerosol-generating procedures are conducted,i.e., endotracheal intubation, bronchoscopy, open suction, oxygen nebulization, bronchodilators, or steroids, ventilation using a bag and mask, tracheostomy, and cardiopulmonary resuscitation[32]. In this way, the incubation time for SARS-CoV-2 (between the onset of symptoms and exposure to the virus) is about 5 to 6 d. However, it can be up to 14 d. During this time, also known as the 'pre-symptomatic' phase, the affected individual can be contagious and transmit the virus to the healthy population[33,34]. The most frequent symptoms include fever, muscle aches, shortness of breath, malaise, and a dry cough.
While patients can remain asymptomatic or develop a mild, moderate, or severe illness, gastrointestinal manifestations such as stomach pain, vomiting, and loose stools can also occur. Many of the complications seen in SARS-CoV-2 infected individuals are attributed to the CRS[35,36].
Cytokine storm
The cytokine storm was historically referred to as an influenza-like syndrome that occurred during systemic diseases such as sepsis and after immunotherapies such as Coley’s toxins.Yersinia pestis(causative agent of plague or black death) infection has led to extreme pandemics; it induces alveolar macrophages to produce disproportionate quantities of cytokines, resulting in the cytokine storm and has subsequently caused massive pandemics[37]. An intensive inflammatory response and fast release of various cytokines (such as TNF-α-1, 2, IL-6, and IFN-γ) to the circulation are activated by pathogen infection (Figure 1). Patients with viral infections are especially vulnerable to acute respiratory failure due to the cytokine storm[38]. For instance, in other coronaviruses (SARS and MERS), cytokine cascades and low lymphocytes are positively linked to the course and severity of the disease. Recent experiments have supported this conclusion in most cases of SARS-CoV-2, indicating low lymphocyte counts and heightened levels of inflammatory mediators[12,39]. Furthermore, it has been shown that pro-inflammatory cytokines such as IL-6 play an essential role in the progression of COVID-19.

Figure 1 The mechanism of the inflammatory storm. ① Antigen presenting; Dendritic cells activate T-cells by processing the antigen and delivering it to these cells; ② Start reproducing; Native T cells become activated by receiving antigens from dendritic cells; ③ A significant quantity of cytokines is secreted during the activation of T cells. These cytokines can activate B cells, macrophages, and NK cells; ④ Activated T cells also release cytokines and further activate macrophages, B cells and NK cells; ⑤ Cytokines secreted; These activated cells, in turn, lead to the secretion of inflammatory and pro-inflammatory cytokines; the resulting cytokine storm leads to the development of clinical signs of infection.
IMMUNE SYSTEM AND ROLES OF IL-6
IL-6 is a soluble mediator with various functions in the immune system[40]. For example, controlling the differentiation and migration of immune cells, apoptosis of target cells[41], and assembly of acute-phase proteins such as C-reactive protein (CRP), haptoglobin, and fibrinogen. In contrast, IL-6 reduces the production of other proteins such as albumin. Human IL-6 comprises 212 amino acids (28-amino-acid signal peptide), and its controlling gene is located on chromosome 7p21[40]. This interleukin contributes to hypothalamic-pituitary-adrenal axis regulation and glucose homeostasis. It induces the differentiation of T-helper cells, which secrete IL-17. These cells are related to the pathogenesis of chronic inflammatory diseases[42]. IL-6 is produced in the immune system by various cells including endothelial cells and contributes to the pathogenesis of chronic inflammatory diseases such as rheumatoid arthritis, atherosclerosis, and systemic lupus erythematosus[41]. This cytokine acts by binding to its receptor on the target cells that consist of CD126 (IL-6 Receptor-α) and glycoprotein 130 (gp130). Therefore, it activates signaling pathways such as JAK-STAT[43] and mitogen-activated protein kinase[11]. Conformational alterations in the gp130 cytoplasmic domain when IL-6 binds to the IL-6 receptor induces activation of JAK-STAT[43], and JAK-STAT signaling pathway activation leads to cytokine release[44]. However, these signaling pathways downregulate IL-6 expression[11].
While the membrane-bound receptor (IL-6Rα) is expressed only on the surface of a small number of cells such as leukocytes and hepatocytes (known as IL-6 classic signaling), IL-6 can affect many other cells through its soluble receptor (sIL-6Rα). It was recently discovered that endothelial cells also express IL-6R. This receptor forms a complex with IL-6 that binds to gp130. This complex then mediates a signal known as IL-6 trans-signaling through which pro-inflammatory responses are mainly mediated. In contrast, the classic signaling pathway is related to anti-inflammatory pathways[41]. Furthermore, IL-6 is produced by the innate immune cells after encountering a pathogen and is critical in the body's defense against the respiratory syncytial virus and influenza virus in the early infection phases[45]. However, in CRS, IL-6 and IL-5 can induce coagulation cascade and complement system overactivation, capillary leakage, hypotension, and myocardial dysfunction[46].
In severe SARS-CoV-2 infection, high levels of pro-inflammatory mediators are present, such as IL-6. Although one study showed that monocytes were a source of IL-1β and IL-8, the exact source of IL-6 remains unclear[47]. In the presence of immune dysregulation, in addition to a non-sufficient anti-viral response, there is also a continuous secretion of pro-inflammatory mediators such as IL-6 that resembles the macrophage activation syndrome and lead to multi-organ damage[45]. Also, in COVID-19, multifocal interstitial pneumonia is the chief reason for pulmonary failure and death. In this process, there are inflammatory infiltrates in the interstitial tissue of the lungs, which lead to alveolar damage[48]. These infiltrates consist of mononuclear cells that will be induced after the pro-inflammatory pathways are activated by trans-signal transduction of IL-6[45]. In this way, one study showed that patients with high levels of ACE-2 expression experience more severe tissue damage by IL-6 and the cytokine storm after infection with SARS-CoV-2. These individuals also have a suppressed immune system to fight against the virus[7]. In summary, IL-6 is crucial in both pro-inflammatory and antipathogen responses, and trans-signaling is the critical pathway of inflammatory processes conducted by IL-6. A diagram of the significant roles of IL-6 and its location in the immune cascade is summarized in Figure 2.
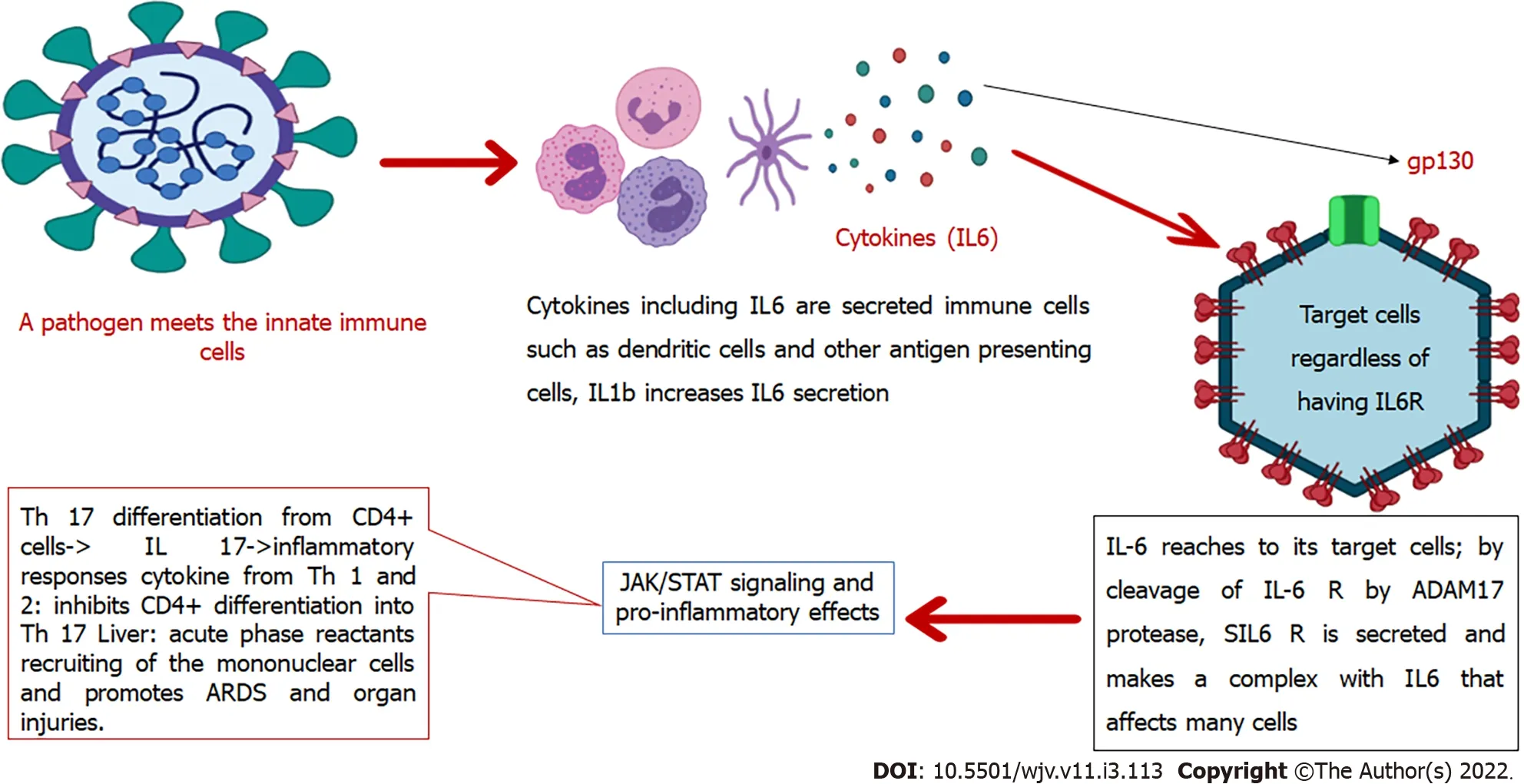
Figure 2 Interleukin-6 in the immune system. IL: Interleukin; JAK-STAT: Janus kinase and signal transducer and activator of transcription.
DRUGS AVAILABLE TO INHIBIT IL-6 ACTIVITY
According to the signaling pathways induced by IL-6 and its components, IL-6 activity can be inhibited by the following: IL-6 itself, IL-6 signaling pathways such as JAK-STAT, gp130, IL-6R, or the IL-6/sIL-6R complex[49]. Two main drugs in the class of IL-6 receptor blockers are tocilizumab (TCZ) and slumab, which are FDA approved monoclonal antibodies for rheumatoid arthritis, and TCZ is also approved for juvenile idiopathic arthritis (JIA) and giant cell arteritis[50].
TCZ blocks both soluble and membrane-bound receptors and accordingly blocks signal transductionviaJAK-STAT[51]. JIA, a chimeric antigen receptor (CAR)-T cell-induced CRS, giant cell arteritis, rheumatoid arthritis, and Still’s disease are examples of the conditions in which TCZ has been used to control the disease[52]. Siltuximab is an anti-IL-6 agent that has shown more effectiveness than TCZ in some aspects, and although it is not FDA approved, it is used in refractory CRS cases. Data regarding Siltuximab in COVID-19 are currently restricted[46].
The specific gp130FC named Olamkicept specifically blocks the trans-signaling pathway. In animals, it showed more effectiveness in controlling the hyper-inflammatory status due to sepsis than anti-IL-6 antibodies. Significantly, it did not impair the anti-inflammatory responses of IL-6viaclassic signaltransduction[45]. JAK-STAT inhibition is another option. Some of these agents are currently on COVID-19 clinical trials, such as ruxolitinib. A list of these drugs is shown in Table 1.

Table 1 Drugs with anti-interleukin-6 activity and their side effects with examples of clinical trials in coronavirus disease 2019
EXPERIENCE OF IL-6 BLOCKADE IN COVID-19
The cytokine storm is associated with disease intensity in SARS-CoV-2, as also shown in SARS-CoV-1 and MERS-CoV. Although the reports from different studies focused on IL-6 blockade in COVID-19 are inconsistent, it was first shown to reduce the mortality rate in critically ill patients[53].
Considering the presence of lymphopenia in SARS-CoV-2 patients, administration of immunosuppressive agents might increase the risk of secondary fungal or bacterial infections[54]. In a previous study, TCZ induced necrotizing fasciitis and candidemia[55]. Accordingly, the exact place for immunosuppression and anti-IL-6 agents in COVID-19 is crucial. The possible effects of TCZ on management of the COVID-19 related cytokine storm first originated from observational studies that showed it to be effective in the clinical improvement of COVID-19 patients[56]. In a recent clinical trial, the effect of a single dose of 8 mg/kg TCZ administrationviathe intravenous route in addition to the standard of care in the management of COVID-19 was investigated. In this study, 46 adult patients who were positive for SARS-CoV-2 and had multifocal interstitial pneumonia on imaging studies were enrolled soon after showing clinical worsening. The drug was influential in the clinical improvement of severely ill patients and patients in the early clinical worsening state. However, it did not show significant efficacy in reducing the mortality rate and was accompanied by adverse effects[48].
According to a recent observational study, having an IL-6 level of more than 30 pg/mL is related to the disease severity and need for respiratory support in COVID-19 patients. This study showed the positive effects of TCZ in patients with higher IL-6 levels at baseline, but no positive trends were seen in the group with low IL-6 levels[51].
A recent case series showed the efficacy of subcutaneous TCZ in three severely ill COVID-19 patients in reducing inflammatory-related indices and improving the clinical condition[57]. The results of a prospective phase two cohort study (TOCIVID-19) showed that TCZ effectively reduced the mortality rate at 30 d, especially in severe patients who did not require mechanical ventilation. This effect was independent of corticosteroids and was not accompanied by significant adverse events[58].
One of the concerns regarding the use of anti-immune drugs in SARS-CoV-2 is that they may interfere with the proper immune response to the virus. Cytokines, especially IL-6, play a significant role in the host's fight against viruses through the humoral and cellular responses by affecting helper and cytotoxic T cells. Accordingly, a cohort study conducted in Spain found that these drugs do not pose a problem in the body's fight against the virus. Although the study found that patients treated with anti-cytokines had a longer viral clearance time, they initially had higher virus levels, and their disease was more severe[59]. A preprint study that showed an unexpected increase in inflammatory mediators after TCZ administration supports the fact that IL-6 blockade alone may not be effective in the management of COVID-19[60]. Recently, two studies showed a transient elevation in the D-dimer level in SARS-CoV-2 patients receiving TCZ[61,62]. A recent meta-analysis also demonstrated that IL-6 blockade alone does not lower the mortality rate, although it may effectively reduce the risk of respiratory failure in hospitalized patients[63]. According to another study, administration time is another crucial factor, and treatment with TCZ ten days after disease onset is more beneficial[64]. In contrast, other studies, including the RECOVERY trial, have shown that early administration of TCZ in severe cases before intensive care unit (ICU) admission and the need for mechanical ventilation is effective in reducing the mortality rate,[65,66] and when the patient requires mechanical ventilation, it will not have much effect[66,67]. In general, different methods and inclusion criteria in studies do not result in the same conclusions. A list of recent studies in this regard is summarized in Table 2. According to some clinical trials, TCZ, when added to a corticosteroid, markedly reduces the mortality rate compared with corticosteroids (CSs) alone. Treatments that include agents to target more ILs in addition to IL-6 have more efficacy than only IL-6 blockade[63,68]. IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF, IL-1, and IL-8 are the primary inflammatory mediators that could be targeted in CRS. IL-1 is proximal to IL-6 in the inflammatory cascade, and its blockade has recently been considered. A recent study compared the effectiveness of IL-1 and IL-6 blockade with the standard of care, and it was observed that IL-1 inhibition is more effective in reducing the mortality rate, while positive effects of IL-6 antibodies were restricted to a group of severely ill patients with high CRP levels[50]. Another clinical trial of TCZ in COVID-19 patients with a hyperinflammatory state also stopped recruiting as it failed to reach its primary endpoints (improving the patient's clinical status or reducing the mortality rate)[69]. In general, despite the effect that IL-6 blockade has on the suppression of inflammation, it cannot completely control inflammation as it does not affect the distal inflammatory pathways[70]. However, in severe and critical SARS-CoV-2 patients with a hyperinflammatory state, IL-6 blockade with monoclonal antibodies seems to be effective in reducing the mortality rate, reducing the risk of mechanical ventilation, and improving the clinical condition[67,71-74]. Although all of these studies have been performed in adult patients, the effect of TCZ in the treatment of COVID-19 in children is also being investigated in the RECOVERY trial[67].

Table 2 List of recent clinical trials and observational studies regarding interleukin-6 blocker monoclonal antibodies in severe acute respiratory syndrome coronavirus 2

SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; ICU: Intensive care unit; TCZ: Tocilizumab; CSs: Corticosteroids; COVID-19: Coronavirus disease 2019.
To date, several clinical trials have failed to show the efficiency of TCZ in COVID-19 treatment. However, the RECOVERY trial and some other clinical trials showed positive results[67,75,76]. Although meta-analysis had previously demonstrated an 11% reduction in 28-d mortality following TCZ administration in patients with severe SARS-CoV-2 infection, this reduction was significant when the results of the RECOVERY trial were added[67]. In conclusion, this drug can effectively improve the prognosis in extreme cases.
TCZ inhibits both classic and trans-signal transduction through IL-6, thus interfering with this cytokine's anti- and pro-inflammatory functions. As mentioned previously, IL-6 signaling pathways involve the JAK-STAT that could be targeted with drugs such as ruxolitinib, a JAK 1 and 2 inhibitor. This drug lowers the levels of IL-6 and is currently being evaluated for SARS-CoV-2 and had positive effects in one study[77]. However, RUXCOVID, a phase 3 clinical trial of ruxolitinib, revealed no significant efficacy in reducing the death rate and serious complications[78]. Another JAK inhibitor is baricitinib. A recent clinical trial (ACTT2) that evaluated baricitinib in hospitalized patients with SARSCoV-2 infection indicated that it reduced the recovery time when added to remdesivir, compared with remdesivir alone[79,80]. Another study also investigated the potency of the anti-myeloproliferative agent ruxolitinib and included the patients requiring supplementary oxygen but not with respiratory failure. This study found that inflammatory mediators significantly reduced after ruxolitinib administration which also improved clinical conditions. These successes were not accompanied by any severe effects[81]. Another effect of JAK inhibitors in hampering the cytokine storm is related to TNF, the other crucial inflammatory mediator in the cytokine storm that uses JAK signaling and can be inhibited by JAK inhibitors. A recent study evaluated the concurrent administration of an IL-1 blocker antibody and ruxolitinib in critical patients with SARS-CoV-2. The preliminary report of this study demonstrated that this combination was beneficial in clinical improvement, and the lymphocyte count increased after this treatment[82]. In addition, no treatment-related severe complications were observed. Tofacitinib is another JAK inhibitor that was shown to reduce adverse outcomes and mortality in COVID-19 patients in a previous retrospective cohort study[83]. Another exciting intervention for IL blockade with positive effects in patients on ECMO in previous research was extracorporeal cytokine adsorption which showed a significant decrease in IL-6 in treated patients[84,85]. Other agents with anti-IL-6 properties have not yet been entered in clinical trials of COVID-19. However, targeting the trans-signaling pathway seems more efficient than non-specific IL-6 blockade with monoclonal antibodies.
IL-6 INDUCES TH17 LINEAGE DIFFERENTIATION
Th17 is related to inflammatory processes. As mentioned in Figure 2, when the IL-6-sIL-6R complex reaches CD4+ T cells, it causes them to differentiate into Th17 cell lineage. This action is mediated through the JAK-STAT signaling pathway (IL-6 recruits JAK 1 and 2). These cells can secrete IL-17, 21, and 22 and GM-CSF, and therefore contribute to the pathogenesis of inflammatory processes and chronic diseases. Viral diseases also promote Th17 related responses, and severe cases show higher Th17-related cytokines. Accordingly, Th17 blockade seems to be another way to fight against COVID-19, especially in extreme cases. One study showed that fedratinib reduced Th17 related cytokines in mouse models. Fedratinib is a JAK 2 inhibitor[86].
It was shown that the Th17 subgroup of T cells is increased relative to the other subgroups in severe COVID-19 cases. The role of these cells in SARS-CoV-2 patients with lung injuries has been revealed. Drugs with anti-IL-17 activities include ixekizumab, secukinumab, and brodalumab, and they are used in moderate to severe cases of psoriasis[87,88]. Ixekizumab is an anti-IL-17 antibody and is currently being evaluated in a COVID-19 clinical trial. Inclusion criteria in this study are those with high serum levels of IL-6 and not admitted to the ICU[89]. When IL-17 is secreted from Th17 cells, it causes target cells to produce inflammatory mediators, including IL-6, TNF-α, chemokine C-C motif 2 (CCL2), and IL-1β. These procedures lead to CRS and clinical worsening in SARS-CoV-2[87]. IL-17 is also related to the cutaneous manifestations of COVID-19[90]. However, recent evidence has shown undetectable quantities of IL-17A expression in COVID-19 patients[91]. In a previous study, secukinumab, an anti-IL-17A selective antibody, resulted in clinical improvement in severe SARS-CoV-2 patients[92].
CONCLUSION
According to the above-mentioned data, IL-6 blockade alone with anti-IL-6R monoclonal antibodies has no significant benefits in improving the prognosis of patients, except for those in a critical condition and in the hyper-inflammatory state before mechanical ventilation. Many factors are related to a patient's response to IL-6 blockade, such as baseline IL-6 level and disease severity. It may also be associated with some worrying side effects. According to recent data, a combination of anti-inflammatory agents is more effective than any one agent alone. Other ways to inhibit IL-6, such as a selective trans-signaling pathway and JAK-STAT inhibition, should be investigated further.
ACKNOWLEDGEMENTS
The authors would like to thank the Clinical Research Development Unit and Tabriz University of Medical Sciences Faculty of Medicine for providing the expertise that greatly assisted in this work.
FOOTNOTES
Author contributions:Bahmani M, Bannazadeh Baghi H, and Chegini R contributed to the conceptualization; Bahmani M, Chegini R, and Ghanbari E contributed to writing - original draft; Shiri Aghbash P, and Bannazadeh Baghi H contributed to writing - review and editing; Shiri Aghbash P, Bannazadeh Baghi H, Chegini R, and Sheykhsaran E contributed to the visualization; Leylabadlo HE, Bannazadeh Baghi H, Shiri Aghbash P, Ghanbari E, and Sheykhsaran E contributed to the supervision; Bannazadeh Baghi H and Shiri Aghbash P contributed to the project administration; Moradian E, and Kazemzadeh Houjaghan AM contributed to the language editing.
Conflict-of-interest statement:The authors declare that there are no conflicts of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Iran
ORCID number:Mohaddeseh Bahmani 0000-0002-7438-7653; Rojin Chegini 0000-0001-8505-1728; Elham Ghanbari 0000-0001-6040-1912; Elham Sheykhsaran 0000-0002-9273-433X; Parisa Shiri Aghbash 0000-0001-6733-4556; Hamed Ebrahimzadeh Leylabadlo 0000-0002-3790-9176; Ehsan Moradian 0000-0003-1218-1059; Amir Masoud Kazemzadeh Houjaghan 0000-0001-5456-3835; Hossein Bannazadeh Baghi 0000-0002-2513-5361.
S-Editor:Ma YJ
L-Editor:Webster JR
P-Editor:Ma YJ
杂志排行
World Journal of Virology的其它文章
- Impact of COVID-19 on mental health and emotional well-being of older adults
- SARS-CoV-2 Omicron variant (B.1.1.529): A concern with immune escape
- Omicron variant and change of electrostatic interactions between receptor binding domain of severe acute respiratory syndrome coronavirus 2 with the angiotensin-converting enzyme 2 receptor
- Educational, psychosocial, and clinical impact of SARS-CoV-2(COVID-19) pandemic on medical students in the United States
