Syntheses,Crystal Structures and Properties of Coordination Polymers Based on 4,4′-Bis(imidazol-l-yl)-phenyl Sulphone or 4,4′-Bis(imidazol-l-yl)diphenyl Thioether
2022-06-14XUHanPANZhaoRuiJIANGRong
XU HanPAN Zhao-RuiJIANG Rong
(1School of Chemistry and Chemical Engineering,Key Laboratory of Inorganic Functional Materials,Huangshan University,Huangshan,Anhui 245041,China)
(2School of Environmental Science,Nanjing Xiaozhuang University,Nanjing 211171,China)
Abstract:Three new coordination polymers based on V-shaped ligands,namely{[Cd(BIDPS)(PA)(H2O)]·CH3OH}n(1),{[Zn(BIDPS)(p-bdc)]·H2O}n(2)and[Mn(BIDPT)(NBA)]n(3)(BIDPS=4,4′-bis(imidazol-l-yl)-phenyl sulphone,H2PA=pamoic acid,p-H2bdc=p-phthalic acid,BIDPT=4,4′-bis(imidazol-l-yl)diphenyl thioether,H2NBA=4,4′-azanediyl dibenzoic acid)have been hydrothermally synthesized and structurally characterized.Compounds 1 and 2 feature an undulate 2D layer structure.Compound 1 exhibits a rare 2D→3D inclined polycatenated structure,while compound 2 is further joined by intermolecular hydrogen bondings to form a 3D network.Compound 3 displays a 3-connected hexagonal layer hcb topology(honeycomb network)and is further assembled into a 3D network via C—H…π interaction.Compounds 1 and 2 showed excellent water stability and fluorescence,which were further confirmed as bifunctional fluorescent sensors for Fe3+and Cr2O72-with high selectivity,sensitivity,and anti-interference ability in the water.The mechanisms of fluorescence quenching were also studied in detail.CCDC:1919698,1;2085947,2;2117923,3.
Keywords:coordination polymer;crystal structure;fluorescence;detection
With the rapid growth of industrialization,environmental and water contamination problems have received growing attention because huge volumes of effluents containing high concentrations of heavy metal ions/anions have been discharged.Fe3+ion plays a vital role in humans or other living organisms due to its crucial role in muscle function,hemoglobin formation,and brain function[1-2].The deficiency or overload of Fe3+can cause serious physiological disorders such as skin diseases,insomnia,hepatic cirrhosis,and declining immunity[3-4].Similarly,Cr(Ⅵ)species are widely used in agriculture and industry.Long-term exposure to Cr(Ⅵ)can cause adverse health problems including hereditary genetic defects,lung cancer,and enzyme systems of the human body even at low concentrations[5-6].So Cr(Ⅵ)species have been listed as priority pollutants by the United States Environmental Protection Agency(EPA)[7].Therefore,rapid detection of Fe3+and Cr(Ⅵ)ions in a water system is essential for human health,food security,and the environment.Metal-organic frameworks(MOFs)have attracted considerable attention over the last couple of decades due to their potential application in gas adsorption and separation,catalysis,chemosensor,and so forth[8-12].Among them,chemists have been devoted to MOFs for chemical-sensing applications for their high sensibility,selectivity,quick response,and operability[13-14].Solution-phase detection of hazardous chemicals such as sensing of small molecules,cations,and anions by luminescent coordination polymers involvingd10metal ions/lanthanide cations has been well documented in the literature[15-16].Luminescent MOFs have been successfully applied in the sensing field toward inorganic ions or small organic molecules.
In this work,three new coordination polymers{[Cd(BIDPS)(PA)(H2O)]·CH3OH}n(1),{[Zn(BIDPS)(p-bdc)]·H2O}n(2)and[Mn(BIDPT)(NBA)]n(3)(BIDPS=4,4′-bis(imidazol-l-yl)-phenyl sulphone,H2PA=pamoic acid,p-H2bdc=p-phthalic acid,BIDPT=4,4′-bis(imidazol-l-yl)diphenyl thioether,H2NBA=4,4′-azanediyl dibenzoic acid)were synthesized.Their crystal structures,infrared(IR),thermal stability,and powder X-ray diffraction(PXRD)patterns have been investigated.Fluorescence testing results demonstrated that compounds 1 and 2 had excellent stability and fluorescence in water.So,we used compounds 1 and 2 to detect the representative pollutants of Fe3+and Cr2O72-in water,and the detection limits were all outstanding.The quenching mechanisms of compounds 1 and 2 toward Fe3+and Cr2O72-have also been studied in detail.
1 Experimental
1.1 Materials and measurement
All the chemicals except BIDPS and BIDPT were commercially purchased and used without further purification.BIDPS and BIDPT were synthesized according to the literature method[17].IR absorption spectra were recorded on a Nicolet(Impact 410)spectrometer as KBr pellets in a range of 400-4 000 cm-1.Elemental analyses(C,H,N)were carried out with a Perkin-Elmer model 240C elemental analyzer.Thermogravimetric(TG)analyses were conducted by using a Perkin-Elmer thermal analyzer.PXRD data were conducted on a Bruker D8 Advance X-ray diffractometer at room temperature with CuKαradiation(λ=0.154 18 nm,U=40 kV,I=150 mA,2θ=5°-50°).Fluorescence spectra were conducted on a SHIMAZU VF-320 X-ray fluorescence spectrophotometer at room temperature.UV-Vis absorption spectra were conducted on a UV-T9 spectrophotometer.
1.2 Synthesis of compound 1
A mixture of Cd(NO3)2·4H2O(30.8 mg,0.1 mmol),H2PA(38.8 mg,0.1mmol),and BIDPS(37.2 mg,0.1 mmol)was dissolved in 6 mL of DMF/CH3OH/H2O(2∶2∶2,V/V).The final mixture was placed in a Parr Teflonlined stainless-steel vessel(15 mL)and heated to 95℃for 72 h.The light yellow,block-shaped crystals were generated after cooling to room temperature naturally.The crystals were filtered off,washed with methanol,and dried in air(48.6 mg,54% yield based on BIDPS).Elemental analysis Calcd.for C42H34CdN4O10S(%):C,56.10;H,3.81;N,6.23.Found(%):C,56.05;H,3.78;N,6.26.IR(KBr,cm-1):3 406(m),3 189(m),1 646(m),1 597(s),1 406(s),1 296(s),1 234(m),1 183(w),862(m),816(m),683(m),564(w).
1.3 Synthesis of compound 2
A mixture of Zn(NO3)2·6H2O(29.8 mg,0.1 mmol),p-H2bdc(16.6 mg,0.1 mmol),and BIDPS(37.2 mg,0.1 mmol)was dissolved in 6 mL of DMF/H2O(4∶2,V/V).The final mixture was placed in a Parr Teflon-lined stainless-steel vessel(15 mL)under autogenous pressure and heated at 105℃for 72 h.Colorless prism crystals were obtained.The crystals were filtered off,washed with methanol,and dried in air(32.4 mg,36% yield based on BIDPS).Elemental analysis Calcd.for C26H20ZnN4O7S(%):C,52.23;H,3.37;N,9.37.Found(%):C,52.18;H,3.41;N,9.33.IR(KBr,cm-1):3 457(s),3 136(s),1 608(s),1 452(vs),1 257(m),1 089(m),1 024(w),839(w),730(w),557(w).
1.4 Synthesis of compound 3
A mixture of MnCl2(12.5 mg,0.1 mmol),H2NBA(25.6 mg,0.1 mmol),and BIDPA(34.0 mg,0.1 mmol)was dissolved in 5 mL of DMF/CH3CN/H2O(2∶2∶1,V/V),sealed in 15 mL Teflon-lined stainless-steel vessel and heated at 95℃for 72 h,then cooled to room temperature slowly.Brown block crystals were filtered off,washed with methanol,and dried in air(43.2 mg,45% yield based on BIDPT).Elemental analysis Calcd.for C32H23MnN5O4S(%):C,61.15;H,3.69;N,11.14.Found(%):C,61.12;H,3.42;N,11.17.IR(KBr,cm-1):3 436(s),3 097(s),1 594(s),1 507(s),1 473(s),1 415(m),1 302(w),1 126(w),832(m),739(m),722(m),545(w),522(w),505(w).
1.5 Crystal structure determination
Single-crystal X-ray diffraction measurements of compounds 1-3 were performed on a Bruker SMART CCD diffractometer with MoKαradiation(λ=0.071 073 nm).The structures were solved by direct methods and refined by full-matrix least-squares onF2using the SHELXL software package.All non-hydrogen atoms were refined anisotropically.The hydrogen atoms except those of water and methanol molecules were generated geometrically.The crystal parameters,data collection,and structure refinements for compounds 1-3 are listed in Table 1.Selected bond lengths and angles are summarized in Table 2.Hydrogen bond parameters of compound 2 are listed in Table 3.
CCDC:1919698,1;2085947,2;2117923,3.
2 Results and discussion
2.1 Crystal structure of{[Cd(BIDPS)(PA)(H2O)]·CH3OH}n(1)
Compound 1 crystallizes in the orthorhombic system withP212121space group.The single-crystal X-ray diffraction reveals that compound 1 consists of one Cd2+ion,one PA2-anion,one BIDPS molecule,one coordinated water molecule,and one crystalline methanol molecule.In the crystal lattice,the Cd(Ⅱ)ions have distorted octahedral geometry by bonding to O8 of the water molecule,three carboxyl oxygen atoms(O5,O6,and O3)of the PA2-anions,and two nitrogen atoms(N1 and N3#1)from two BIDPS ligands(Fig.1).The Cd1—O bond lengths are in a range of 0.221 8(5)-0.241 4(5)nm and those of Cd1—N bonds are from 0.229 9(6)to 0.230 9(6)nm,and the coordination angles around Cd ion vary from 55.34(2)°to 166.62(2)°,all of which are within normal ranges[18-19].

Fig.1 Coordination environment of Cd(Ⅱ)in compound 1
The carboxyl and hydroxyl groups of flexible PA2-adopt atransconformation so as to minimize the steric hindrance.We found that the twisted PA2-anions bridged the Cd2+ions to form a 1D infinite left-handed helical chain of[Cd(PA)]nwith a pitch of 2.562 1 nm.It is noteworthy that the V-shaped BIDPS linked two neighboring Cd2+cations to form another left-helical chain[Cd(BIDPS)]n2n+.As shown in Fig.2,the two lefthelical chains are further interconnected into a(4,4)undulated grid coordination polymer layer.The undulated grid has dimensions of 1.519 nm×1.686 nm with angles of 65.64°and 78.91°(defined by Cd…Cd and Cd…Cd…Cd angles).Topologically,the Cd(Ⅱ) cations can be regarded as 4-connected nodes,PA2-and BIDPS ligands as linkers.So,compound 1 can be represented as asqlnetwork with the point symbol of{44·62}.The packing of the layers forms two sets of layers oriented in different directions(Fig.3a).That is to say,these two sets of undulated layers catenate to each other in a parallel-parallel arrangement to form a 2D+2D→3D inclined polycatenation structure(Fig.3b).

Fig.2 Two-dimensional(4,4)network structure of compound 1,consting of1D[Cd(BIDPS)]n2n+and[Cd(PA)]nhelical chains
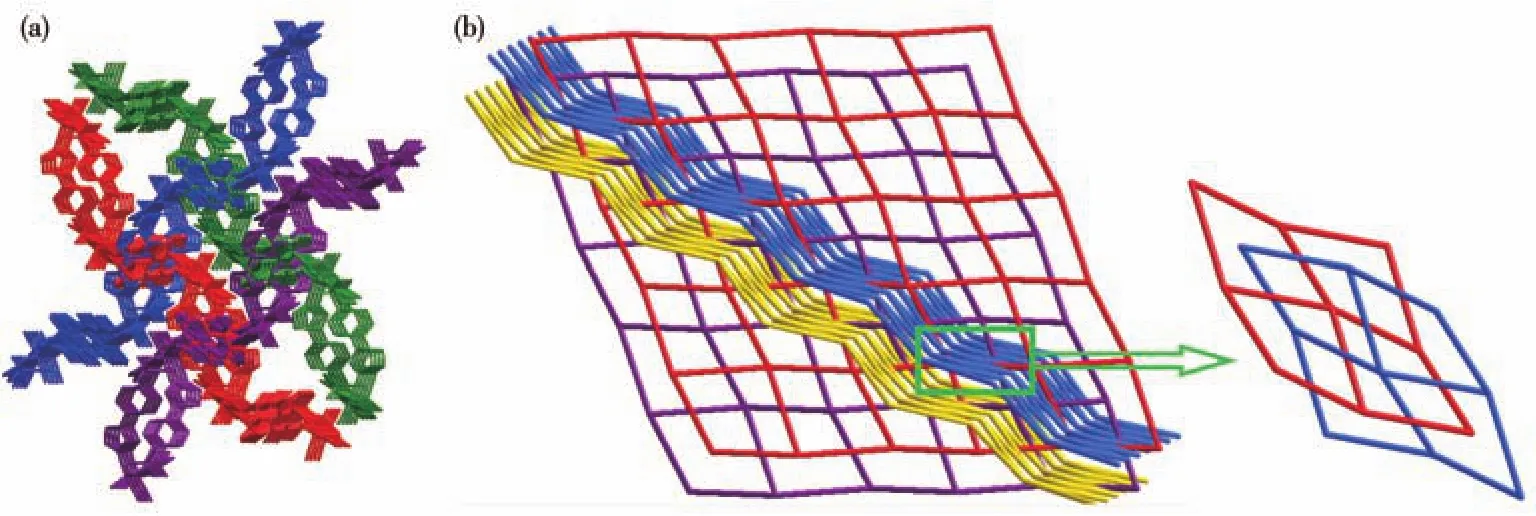
Fig.3 (a)Two sets of 2D undulated layers in a parallel-parallel arrangement of compound 1;(b)Schematic view of the sql topology,indicating a 2D+2D→3D inclined polycatenation structure of compound 1
2.2 Crystal structure of{[Zn(BIDPS)(p-bdc)]·H2O}n(2)
Compound 2 crystallizes in the triclinic system withPspace group.Its asymmetric unit comprises one independent Zn(Ⅱ)ion,one BIDPS molecule,onep-bdc2-anion,and a lattice water molecule.Zn(Ⅱ)is four-coordinated with coordination geometry of distorted tetrahedron,which is completed by two oxygen atoms(O2,O4)afforded by two monodentate carboxylates,and two nitrogen atoms(N1,N3#1)from two BIDPS ligands(Fig.4).The lengths of the Zn—O bonds are 0.193 8(2)and 0.195 6(2)nm,and Zn—N bond distances are 0.198 8(3)and 0.200 6(3)nm,which are within the normal values for the reported Zn complex[20-21].Notably,the twisted V-shaped BIDPS links two neighboring Zn2+ions to form right-and left-helical[Zn(BIDPS)]n2n+chains with a pitch of 1.488 9(1)nm along thec-axis,which are further interconnected with thep-bdc2-ligands into a 2D layer paralleled to theacplane(Fig.5).The adjacent 2D layers are connected together by hydrogen bonds interactions among O8—H8A…O5 and O8—H8B…O2 with O8…O5 and O8…O2 distances of 0.280 8(4)and 0.295 8(3)nm,respectively,generating a 3D supramolecular network(Fig.6).The same main ligands and metal salt Cd(NO3)2·4H2O as well as similar reaction conditions were adopted to synthesize another complex[Zn(L)2(p-bdc)]nwith the same 2D layer structure[22],while the 2D layers can′t form a 3D network for the absence of hydrogen bonds.

Fig.4 Coordination environment of the Zn(Ⅱ)in compound 2

Fig.5 Two-dimensional(4,4)network structure of compound 2,consisting of right-and left-helical[Zn(BIDPS)]n2n+chains

Fig.6 Three-dimensional supramolecular structure of compound 2 assembled through hydrogen bonds(black dashed lines)
2.3 Crystal structure of[Mn(BIDPT)(NBA)]n(3)
Compound 3 crystallizes in the monoclinicP21/cspace group and the coordination environment around Mn(Ⅱ)is shown in Fig.7.In the asymmetric unit,the Mn1 cation and its symmetry-related Mn1#1 are fivecoordinated,which are assumed an identical pseudo pentagonal bipyramid.The Mn1 cation is coordinated by N1 and N4#2 atoms from two BIDPT molecules in the axial positions(Mn—N0.2259(2)and0.2280(2)nm)and O1#1,O2,and O4#2 atoms from the carboxylate groups of threeunique NBA2-anions(Mn—O0.2104(2)-0.214 8(2)nm)and all in the reasonable range[23-24].Two deprotonated carboxyl groups of each NBA2-adopt different coordination modes (monodentate and bridging-bidentate)connecting two neighboring Mn(Ⅱ)cations to form an Mn2(CO2)2dinuclear cluster with an Mn…Mn distance of 0.423 0 nm.The NBA2-anion bridges Mn(Ⅱ)atoms through carboxylate groups,forming an infinite[Mn(NBA)]n2n+helical chain along theb-axis,with a pitch of 1.514 6 nm.Interestingly,there exist both left-and right-handed helices in the structure(Fig.8).The helical chains with opposite chirality are united alternately and connected by sharing Mn2(CO2)2units to compose(4,4)-net(the NBA2-anions are considered as connectors and Mn2(CO2)2units as four-connected nodes).

Fig.7 Coordination environment of the Mn(Ⅱ)in compound 3

Fig.8 Two-dimensional layer composed of Mn2(CO2)2and NBA2-of compound 3,showing the Mn2(CO2)2 clusters and the left-and right-[Mn(NBA)]n2n+helical chains with opposite chirality
More interest to us is that the imidazole group of BIDPT ligands links Mn(Ⅱ)cations to generate another type of[Mn(BIDPT)]nhelical chain with opposite chirality,which has the same pitch as[Mn(NBA)]n2n+helical chain(Fig.9).The left-and right-handed helices are hump alternately up and down from the(4,4)-net formed by Mn2(CO2)2and NBA2-.A topology analysis reveals that the binuclear manganese can be regarded as a network node,so the 2D coordination polymer can be described as a 3-connectedhcbnetwork with the point symbol of{63}(Fig.10).Noteworthy of mentioning here is that the edge-to-face C—H…πinteractions(0.262 nm)in an…ABAB… fashion between C atom(C9)and phenyl ring of NBA2-anions(C26-C31,Cg1)produce a 3D supramolecular network.The intramolecular C—H…π(0.312 nm)interaction between the carbon atom on BIDPT ligands and phenyl from the NBA2-ligands(C12 and carbon atoms C20-C25(Cg2))also existed in the compound(Fig.11).These C—H…πinteractions maybe increase the rigidity and make the molecule stable in the crystal.
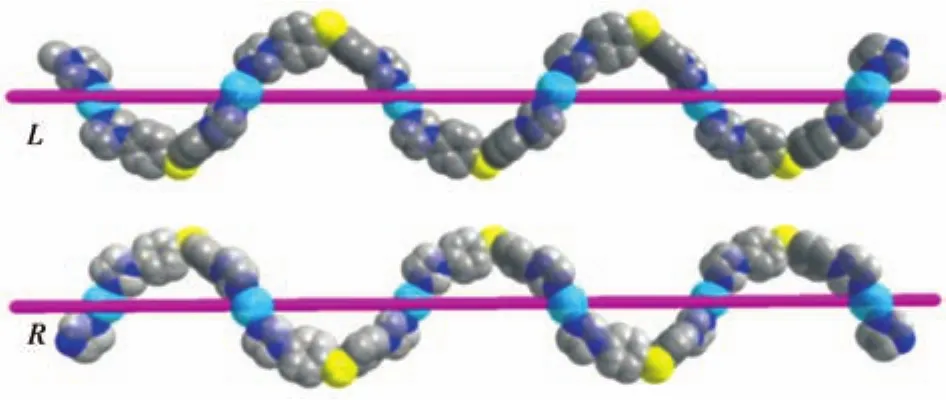
Fig.9 Left-and right-handed[Mn(BIDPT)]nhelical chains of compound 3

Fig.10 Schematic view of hcb topology of compound 3

Fig.11 Three-dimensional supramolecular framework and the C—H…π interactions of compound 3
2.4 PXRD and thermal stability analyses
PXRD analyses of compounds 1-3 were performed in order to check the phase purity.As shown in Fig.S4-S6(Supporting information),the experimental PXRD patterns were consistent with the simulated ones,demonstrating the phase purity of the samples.Furthermore,PXRD patterns of compounds 1 and 2 immersed in acidic and basic solutions(pH=4-10)for 2 h were obtained and the framework was maintained,indicating that compounds 1 and 2 remain stable in aqueous solution and can be candidates for contaminant sensing.
The thermal analyses curves of compounds 1-3 were performed under a nitrogen atmosphere.For compound 1,a gradual weight loss of 5.68%(Calcd.5.56%)from 132 to 155℃is due to the removal of the coordinated H2O molecule and lattice CH3OH molecule.The framework of compound 1 decomposed at 238℃,and the final residues were CdO(Obsd.14.27%,Calcd.14.38%,Fig.S7).For compound 2,along with heating,the weight loss of 3.13% from 136℃can be ascribed to the removal of the guest water molecule(Calcd.3.01%),then the framework gradually collapsed with a residual mass of ZnO(Obsd.13.70%,Calcd.13.61%,Fig.S8).Compound 3 started to lose its ligands at 85℃as a result of thermal decomposition.The final residual was presumed to be MnO(Obsd.11.42%,Calcd.11.31%,Fig.S9).
2.5 Fluorescence property
Coordination compounds,especially withd10metal centers,have been investigated for fluorescence properties because of their potential applications as luminescent materials[25-26].Free H2PA ligands showed the emission at 474 nm(λex=434 nm),which may be attributed to theπ→π*transition[27-28].The solid fluorescence spectra of BIDPS,compounds 1 and 2 were measured at room temperature(Fig.S10).The free BIDPS ligand showed fluorescence with the emission at 437 nm(λex=372 nm).The emission peaks of compounds 1(λem=418 nm,λex=366 nm)and 2(λem=410 nm,λex=363 nm)were blue-shift compared with the free H2PA and BIDPS ligands.This can be ascribed to ligand-to-ligand charge transfer(LLCT)transitions within the short distance between adjacent ligands[29].
Taking into consideration ofthe fluorescent response and the good water stability of compounds 1 and 2,as well as the industrial pollutants in wastewater,we selected the aqueous solutions of 1 and 2 to explore the fluorescent sensing activities toward a series of cations and anions.
2.6 Detection of Fe3+and Cr2O72-
The finely grounded samples of compounds 1 and 2(2 mg)were dispersed in 2 mL water and then subjected to 30 min of ultrasonication to disperse completely.Upon excitation at 363 nm for compound 1 and 360 nm for compound 2,the water suspension of compounds 1 and 2 emitted at 411 and 402 nm.The emission maximum displayed a little blue shift compared with its solid-state emission peak due to the solvent effect.The fluorescence sensing ability of 1 and 2 to varied metal cations was conducted with the gradual addition of an aqueous solution of nitrate salts M(NO3)x(Mx+=K+,Na+,Ca2+,Zn2+,Ba2+,Ni2+,Cu2+,Co2+,Cd2+,Fe3+)with a concentration of 5 mmol·L-1.Among the mentioned above cations,Fe3+showed a higher quenching effect than other cations.The fluorescent intensity of compound 1 decreased to 7.12% by gradual addition of 90 μL Fe3+solutions,but the other cations exhibited little effect on the fluorescence under the same test concentration.As shown in Fig.12a,the titration curves showed that the emission intensities of compound 1 gradually decreased with the increasing concentration of Fe3+.The fluorescence quenching efficiency of Fe3+can be analyzed by the Stern-Volmer(SV)equation:I0/I=1+KSVc,whereKSVis the quenching effect coefficient(L·mol-1),cis the concentration of Fe3+,andI0andIare the emission intensities before and after adding Fe3+,respectively.The SV plot for Fe3+was nearly linear at low concentration.However,the curve deviated from linearity and bent upward with the increasing concentration,which can be explained by self-absorption or an energy-transfer process[30].As shown in Fig.12b,theKSVwas calculated to be 1.18×104L·mol-1based on the linear part.The limit of detection(LOD)was calculated according to the equation reported in the literature[31].LOD of compound 1 was calculated to be 4.04 μmol·L-1.Similar to compound 1,compound 2 can selectively detect Fe3+.The fluorescent intensity of compound 2 decreased gradually with an increasing amount of Fe3+solution.And the emission of compound 2 was quenched by 91.2% after the addition of 90 μL Fe3+(Fig.12c).Furthermore,theKSVand LOD values for compound 2 were estimated to be 9.58×103L·mol-1(Fig.12d)and 5.38 μmol·L-1,respectively.TheKSVand LOD values for compounds 1 and 2 were comparable to the reported MOF-based fluorescent sensor toward Fe3+[32-33].Competitive experiments showed that the fluorescent intensities of compounds 1 and 2 suspensions in 1 mmol·L-1aqueous solution of individual cations(excluding Fe3+)did not cause significant quenching,while the addition of Fe3+(1 mmol·L-1)showed almost complete quenching(Fig.13a and 13b),demonstrating the excellent selectivity of compounds 1 and 2 toward Fe3+even in the presence of other competitive metal cations.

Fig.12 (a)Fluorescence spectra and(b)SV plot for compound 1 with gradual addition of Fe3+in water;(c)Fluorescence spectra and(d)SV plot for compound 2 with gradual addition of Fe3+in water
The sensing result of compounds 1 and 2 toward metal ions prompted us to further examine the detection of anions in an aqueous solution,which has relevance to environmental and security issues.The same procedure was conducted to examine the fluorescence sensing of inorganic anions.Various aqueous solutions containing 5 mmol·L-1NaxA(Ax-=F-,Cl-,Br-,NO2-,NO3-,IO3-,CO32-,SO42-,C2O42-,Cr2O72-)were added into the suspension of compounds 1 and 2.The gradual addition of 90 μL anions caused the intensity changes to a different extent.Strikingly,Cr2O72-afforded a noteworthy turn-off quenching effect with a quenching efficiency of 91.6% for compound 1 and90.2% for compound 2,respectively.While the other anions showed slight influence on the emission intensities.The titration curves showed that the fluorescent intensity of compounds 1 and 2 both decreased with the increasing concentration of Cr2O72-(Fig.14a and 14c).TheKSVand LOD were calculated to be 7.64×103L·mol-1,5.06 μmol·L-1for 1,and 6.89×103L·mol-1,6.15 μmol·L-1for 2(Fig.14b and 14d).Interestingly,the observedKSVand LOD values for compounds 1 and 2 are comparable to some previously reported fluorescent sensors for Cr2O72-[34-35].Interference by otheranions in the detection of Cr2O72-was studied by competitive experiments,which were performed in the same procedure as Fe3+,and the sensing response of compounds 1 and 2 toward Cr2O72-was unaffected even in the presence of added anions(Fig.15a and 15b).All these experiments results indicate that compounds 1 and 2 are highly selective and sensitive for Fe3+and Cr2O72-detection.

Fig.14 (a)Fluorescence spectra and(b)SV plot for compound 1 with gradual addition of Cr2O72-in water;(c)Fluorescence spectra and(d)SV plot for compound 2 with gradual addition of Cr2O72-in water

Fig.15 Quenching efficiency of compounds(a)1 and(b)2 in the presence of individual anions and mixed anions containing CrO2-27
2.7 Mechanism of fluorescence quenching
To deeply understand the selectivity of compounds 1 and 2 toward Fe3+and Cr2O72-,we studied their fluorescence quenching mechanism.The UV-Vis absorption spectra of Fe3+(260-400 nm)and CrO2-27(250-450 nm)had a large overlap with the excitation spectrum of compounds 1(λmax=363 nm)and 2(λmax=360 nm),while other ions have weak absorption in this wavelength range.It is very likely that Fe3+and CrO2-27in the solution can compete with compounds 1 and 2 for absorbing the excitation light and hinder the absorption of compounds 1 and 2,resulting in a decrease,or almost full quenching,of the fluorescent intensities.Furthermore,a small overlap between the emission spectra of compounds 1 and 2 with the tail end of the absorption spectra of Fe3+and Cr2O72-over 343 and 363 nm were observed,which can assist the resonance energy transfer from compounds 1 or 2(donor)to Fe3+and Cr2O72-(acceptor),and contributing a part to the quenching effect(Fig.16a and 16b).As shown in Fig.S11 and S12,PXRD patterns showed that the structural integrity of compounds 1and 2 could be well-retained before and after sensing experiments(immersed in Fe3+and Cr2O72-aqueous solution for 2 h),so fluorescence attenuation was not caused by the decomposition of compounds 1 and 2.Then we compared the fluorescence of compounds 1 and 2 with H2PA and BIDPS ligands in an aqueous solution.And H2PA,p-H2bdc,and BIDPS had no fluorescence,while compounds 1 and 2 had obvious fluorescence.All the above results confirm that the fluorescence quenching is caused by compounds 1 and 2,not the H2PA,p-H2bdc,and BIDPS ligands or the decomposition of crystalline structure.The quenching mechanism is consistent with other previously proposed mechanisms[36].

Fig.16 UV-Vis absorption spectra of the 1 mmol·L-1aqueous solutions of(a)cations and(b)anions along with the emission of compounds 1 and 2
3 Conclusions
In summary,three new compounds have been solvothermally synthesized based on 4,4′-bis(imidazol-lyl)-phenyl sulphone,pamoic acid,4,4′-bis(imidazol-lyl)diphenyl thioether,andp-phthalic acid,4,4′-azanediyl dibenzoic acid.The crystal structure analysis result shows that compounds 1-3 have 3D networks.Meanwhile,compounds 1 and 2 show highly selective detecting for Fe3+and Cr2O72-.Furthermore,the fluorescence quenching mechanisms for compounds 1 and 2 were studied by the UV-Vis absorption and PXRD techniques.This work indicates that compounds 1 and 2 have the potential for fluorescence detection.
Supporting information is available at http://www.wjhxxb.cn
