Improving electron and ion transport by constructing 3D graphene nanosheets sandwiched between porous carbon nanolayers produced from resorcinol-formaldehyde resin for high-performance supercapacitor electrodes
2022-06-13SUNBingTANGWenXIANGHuiXUWenli1CONGYe1YUANGuanming1ZHUHui1ZHANGQin1LIXuanke1
SUN Bing, TANG Wen, XIANG Hui, XU Wen-li1,, CONG Ye1,, YUAN Guan-ming1,, ZHU Hui1,, ZHANG Qin1,,, LI Xuan-ke1,,
(1. The State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology, Wuhan, 430081, China;
2. School of Chemistry and Chemical Engineering, Wuhan University of Science and Technology, Wuhan 430081, China)
Abstract: An ideal supercapacitor electrode should contain three-dimensional (3D) interpenetrating electron and ion pathways with a short transport distance. Graphene-based carbon materials offer new and fascinating opportunities for high performance supercapacitor electrodes due to their excellent planar conductivity and large surface area. 3D graphene nanosheets coated with carbon nanolayers of controllable thickness from resorcinol-formaldehyde (RF) resin are constructed and activated by KOH to develop pores. Such a sandwich structure provides abundant transport channels for ions with short paths. The porous carbon nanolayers accelerate ion transport, while the graphene networks improve the conductivity, boosting electron transport. As expected, the prepared porous carbon has a high surface area of 690 m2 g−1 and a high specific capacitance of up to 324 F g−1 in a 6 mol L−1 KOH aqueous electrolyte at a current density of 0.2 A g−1. More than 99% of the capacitance is retained after 8 000 charge-discharge cycles at a high current density of 5 A g−1, indicating good cycling stability. This research provides an effective strategy for the development of outstanding electrode materials for the enhanced transport of both electrons and ions.
Key words: Graphene-based nanosheet;Hierarchical porous structure;Controllable thickness;Supercapacitor
1 Introduction
Supercapacitors (SCs), a kind of the most prospective energy storage devices, have received considerable critical attentions owing to their merits of high power density, long cyclic life and rapid charge-discharge when they are served in power source and digital device applications[1-3]. Previous Studies have witnessed wide utilization of graphene equipped with high specific surface area (2 630 m2g−1) and superior conductivity in SCs electrodes[4]. Since the stacking and aggregation of graphene layers were induced by strong van der Waals interlayer interaction[5], graphene was partly restricted on specific surface area and electrochemical performance, failing to exert its theoretical outstanding capacitance.
Coating conductive polymers (CPs) on the surface of graphene oxide (GO), such as polyaniline[6,7],polypyrrole, and 3,4-ethylenedioxythiophene[8,9], were considered as an effective strategy to alleviate the aggregation and stacking of GO sheets. Moreover, a dramatic increase in specific capacitance of CPs-GO composite occurred, resulting from the contribution of pseudo-capacitive and electric double layer capacitive(EDLC) behavior[10]. However, slight aggregation of GO still exists during the postprocessing procedure. It becomes worse when CPs have low water solubility,especially. CPs cannot be well coated on the surface of GO. Furthermore, the huge volume expansion of CPs leads to a decay in their electrochemical performance during the charge-discharge process[11-13]. Considering this issue, microporous polymer networks,such as phenol-formaldehyde resin and resorcinolformaldehyde resin[14,15], were adopted to coat on the surface of GO to form a sandwich structure. For instance, Zhu and his co-workers reported a porous sandwich-like carbon/graphene nanosheets, possessing an ultrahigh surface area of 2 650 m2g−1[16]. Nonetheless, such a high surface area failed to exhibit an ideal capacitance (only 116 F g−1at 0.5 A g−1in 6 mol L−1KOH), owing to its unsuitable pore size distribution. Hao et al. also reported the microporous carbon nanosheets sandwiched by GO, which delivered a poor capacitance of 105 F g−1at 0.5 A g−1[17]. In the study, it has no knowledge of the relationship between thickness of carbon and ions, electrons transport. Obviously, controlling the thickness of carbon coating is a crucial factor for promoting electronic transportation and pore utilization of graphene-based sheets.Thus, it is no denying that constructing a graphenebased nanosheet with microporous carbon coating, as well as controlled thickness and pore size distribution,is of great significance to rapid ion and electron transportation.
Herein, a hierarchically porous carbon/graphene/carbon nanosheet, inspired by a cheese sandwich, was facilely prepared. RF-resin was well coated on both sides of GO via a controllable preparation process.GO and RF-resin were connected by electrostatic interaction and hydrogen-bond interaction, resulting in an effective restriction in stacking and aggregation of the graphene sheets. The abundant porous structure of the precursors was formed after pyrolysis and followed by KOH activation. High specific surface(690 m2g−1) and thin thickness (15 nm) 2D graphenebased nanosheets with narrow pore size distribution were prepared, which are favorable to the accessibility of the electrolyte and fast electrons transfer. Benefited from the effective synthesis strategy, the obtained composites exhibited high capacitance and excellent cycle stability.
2 Experimental
2.1 Synthesis of the porous C/G nanosheets
GO was prepared by a modified Hummer’s method[18], and then, it was dispersed into deionized(DI) water to get a homogeneous GO aqueous solution of 2.50 mg mL−1. The materials preparation process was described as follows. 2.72 mL of GO aqueous solution was dispersed into 2.28 mL of deionized water and 4 mL of anhydrous ethanol by ultrasonic, then the solution was mixed with 0.46 mL of resorcinol (AR, 99%, Aladdin Industrial corporation)aqueous solution of 250 mg mL−1. 60 μL of NH3•H2O(Sinopharm Chemical Reagent Co., Ltd), 0.38 mL of Pluronic F127 (Mw = 12 600, PEO106PPO70PEO106,Aldrich Corp, 100 mg mL−1) and 0.16 mL of formaldehyde (37 wt.%, Sinopharm Chemical Reagent Co.,Ltd) were mixed with the magnetic stirring at 25 °C for 1 h. The homogeneous emulsion was then sealed and transferred into an oven at 90 °C for 8 h.The precursor was dried in the vacuum freeze-drying and annealing at 650 °C for 2 h under N2atmosphere.And then, the hierarchically porous sandwich-like carbon/graphene/carbon nanosheets (donated CGS) were obtained.
The surface area is a key point for the high-performance of supercapacitor. Therefore, KOH chemical activation is the second step to enrich the pores of CGS. Typically, KOH (AR, Sinopharm Chemical Reagent Co., Ltd) was dissolved in 20 mL of deionized(DI) water and the dried CGSs were dispersed into the solution with sonication for 30 min. The mass ratio of KOH/CGS is 1∶1. The impregnated sample was dried at 100 °C in an oven and then activated at objective temperature (400, 600, 800 °C) for 2 h respectively. After it was cooled down, an excessive 0.5 mol L−1HCl was used to remove the KOH. Subsequently, samples were repeatedly washed with DI water to remove remaining inorganic salt with the pH7 of the filtrates. The product (denoted KACGS-T,whereTrepresents the activation temperature) was dried in an oven at 100 °C for 24 h.
2.2 Materials characterization
The micrographs were observed by scanning electron microscopy (SEM) using a Helios NanoLab 600i instrument. Transmission electron microscopy(TEM) images were obtained with a Tecnai G2 F20 electron microscope. X-ray diffraction (XRD) patterns were recorded with an Advance D8 using CuKαradiation. The Raman analyses of the samples were performed on a Lab RAM HR800 laser Raman spectrometer. The specific surface area and porous distribution were measured with an ASAP 2020 sorption analyzer.
2.3 Electrochemical measurements
The electrochemical performances were measured by using a three-electrode system at 25 °C. To make the working electrodes, 90 wt.% of active materials and 10 wt.% of poly-tetrafluoroethylene (PTFE) binder were dispersed in a mixed solution of DI water and ethanol (1/5, by volume) with the sonication for 30 min. The slurry was dried in the oven at 80 °C for 4 h and the mixture was laminated to a flake. Afterward, the as-electrodes (d= 1.2 cm) were dried in the vacuum oven at 80 °C for overnight and pressed it on a piece of nickel foam (1.2 cm×1.2 cm)under 5 MPa for 3 min. Each electrode contains about 5 mg of active materials. The electrodes were immersed in 6 mol L−1KOH solution under 60 Pa for 10 h to make sure the electrodes sufficient contact with electrolyte.
Cyclic voltammetry (CV), Galvanostatic chargedischarge (GCD), and electrochemical impedance spectroscopy (EIS) was measured by a Gamry interface 1000E electrochemical workstation. All the electrochemical performances were studied in a threeelectrode system using the above electrode as a working electrode, a platinum film as a counter electrode,and a mercuric oxide electrode as a reference electrode. The CV test was carried out with a potential window of −1~0 V vs Hg/HgO and the scan rate range of 5-200 mV s−1. EIS was measured in the frequency range of 0.01-105Hz at 5 mV amplitude. The GCD test had the same potential window of CV and the current densities range from 0.2 to 5 A g−1.
The specific capacitance was respectively calculated by the GCD and CV tests result using the following equation:
WhereC(F g−1) is the specific capacitance,Iis the discharge current (A g−1),tis the discharging time (s),m is the mass of active materials in one electrode (g)andVis the voltage drop upon discharging (excluding the IR drop).
WhereC(F g−1) is the specific capacitance,Iis the output current (A),Vis the potential (V),νis the potential scanning rate (mV s−1) andmis the mass of the active material in the electrodes (g).
3 Results and discussion
Fig. 1a shows the schematic illustration of the preparation process of sandwich-like hierarchical porous carbon/graphene/carbon nanosheets. GO was used as a substrate, NH3·H2O and tri-block co-polymer F127 were used as the effective binder for fixing RFresin on both sides of the substrate. Then, RF-resin was directly polymerized on the both sides of GO.The self-assembly of the micelle and RF oligomers induced the formation of micropores, mesopores and macropores. Meanwhile, a series of multilevel phase was separated in the polymerization process of RF resin. Subsequently, CGS was obtained after vacuum freeze-drying and pyrolysis under N2atmosphere.CGS was further activated by KOH to enrich their pore structures. Finally, the thin sandwich-like graphene-based nanosheets with rich microporous,plentiful mesoporous and macropores were formed.These features may shorten the pathway of the ion diffusion and enhance electron conduction. The macroscopic feature of CGS was shown in Fig. 1b. Threedimension carbon frameworks can be obtained and the mass density of CGS is about 14.86 mg cm−3, which is lightweight on the top of a flower. Fig. S1a shows the GO with the aggregated structure due to the van der Waals forces between the GO sheets. Obviously,without GO, the carbon derived from RF-resin (RFC)shows a typically self-assembly state of the regular balls (Fig. S1b)[19], and it is also confirmed by TEM images in Fig. S2. As seen in Fig. 1c, the obtained CGS shows a lamellar feature which has a smooth surface with interconnected structure. It indicates that the RFC is homogeneously coated on the surface of graphene, which can effectively avoid the stacking of thin graphene nanosheets. And no ball-like resin carbon was observed in CGS.
For supercapacitor, the specific surface area plays an important role in the electrochemical performances[20,21]. Therefore, the prepared CGS is activated by KOH chemical activation to enrich their pore structures. KACGS still exhibited graphene-like interlinked structure shown in Fig. 1d, indicating the stable structure of KACGS. The TEM images of KACGS ,shown in Fig. 1e and f, display the thin nanosheet morphologies, manifesting well dispersion of graphene after coating. Further, the hybrid features and detailed texture characteristics were observed by HRTEM. As shown in Fig. 1g, the interlayer with a distinct lattice fringe is GO, and both sides show the amorphous carbon texture with a large amount of micropores, illustrating a typical sandwich-like structure of KACGS.
The microstructure of the samples was further characterized by Raman spectroscopy and XRD analyses. Fig. 2a displays the Raman spectra of GO, CGS and KACGS. 2 peaks can be observed at 1 338 cm−1and 1 588 cm−1, corresponding to the defective/discorded sp3hybridized carbon (Dband) and the in-plane stretching of sp2carbon bonds in the graphite planes(Gband)[22], respectively. As is well known, the intensity ratio of theDband to theGband (ID/IG) is the reference of the disorder degree of carbon[23,24]. TheID/IGvalues of GO, CGS, and KACGS are 0.968,0.998 and 1.104, respectively. The result is consistent with the typical amorphous porous carbons, demonstrating that more defects are formed after the KOH activation. Distinctly, the formation of more micropores in the RF-resin carbon texture gives rise to an increasing degree of defects. The structure of GO,CGS, and KACGS was further verified by XRD spectroscopy measurement (Fig. 2b). The XRD pattern of GO shows a diffraction peak at around 2θof 8.2°, corresponding to the (001) diffraction peak of GO[25]. But the peak at 2θof 8.2° disappears in CGS after the GO coated with RF-base carbon, and a new broad peak at 2θof 24.9° is observed corresponding tod002spacing of 0.357 nm. Based on the analysis, the amorphous structure of carbon coating in KACGS can be confirmed. According to N2adsorption/desorption measurements results (Fig. 2c and d), the hierarchically porous structure of prepared CGS and KACGS was confirmed by the isotherms shown type Ⅳ characteristics[26]. The adsorbed volumes of both increase rapidly and reache saturation soon at the low relative pressurep/p0< 0.1, reflecting the rich micropores of CGS and KACGS[27]. The obvious hysteretic loop at the highp/p0region suggests the mesopores existing in samples. The BET specific surface area of CGS was promoted from 330 to 690 m2g−1(KACGS) after the activation with KOH. The pore size distribution is in the range of 0.8 to 125 nm. However, the main volume fraction is attributed to pores with diameters of 0.8 and 34.6 nm, confirming the hierarchical porous structure of the samples. In comparison with the pore volume of 0.59 cm3g−1of CGS, the pore volume of KACGS increases to 0.94 cm3g−1. The generation of such microporous features could be attributed to surface activation by KOH. A higher pore volume of KACGS manifests an increased exposure of active sites for ions adsorption in the micropores of electrode, and the mesopores contributes to the mass transportation, thus resulting in a high capacitance.
To explore the effect of RFC thickness on their electrochemical performance, CGSs with different thicknesses of RFC were prepared. The obtained CGSs (Fig. 3,) possess thin RFC with an average thickness of 5.5, 26 and 37 nm on one side, corresponding to the CGS, 4d-CGS and 8d-CGS, respectively. The thickness of RFC was controlled by adjusting the mass ratio of GO and RF-resin. When the mass ratio of RF-resin increases to 8 times of the original ratio, little RFC balls can be observed in 8d-CGS (Fig. 3e). Furthermore, the obvious sandwichlike nanosheets can be seen, and RFC was tightly coated on both sides of GO, showing the successful prepare strategy. As the primary factors for capacitive behaviors, ionic and electronic transportation are closely connected with the diffusion length and resistance, respectively, which are influenced by the thickness of nanosheets.
As mentioned above, attributed to the hierarchically porous structure and the well pronounced 2D morphology, the prepared composites would be anticipated to perform electrolyte-accessibility and fast electron transport with the aid of the graphene framework. The CGS would be a promising candidate for supercapacitor electrode material. Therefore, the electrochemical properties of the prepared materials were investigated by cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance spectra (EIS) techniques using a three-electrode system. The electrolyte of 6 mol L−1KOH aqueous solution was employed. Fig. S3 shows the GCD curves of CGSs with different thicknesses at a current density of 1 A g−1. The specific capacitance of CGS, calculated from GCD curves, delivers a capacitance of 173.8 F g−1at 1 A g−1, which is much higher than that of 4d-CGS (72 F g−1) and 8d-CGS (53 F g−1).The internal resistance voltage drop (IR drop) reflected the contact resistance and rate of ion transport,which can be calculated by the abrupt potential drop in the discharge progress[28]. The IR drop for CGS, 4d-CGS and 8d-CGS was 0.018, 0.218 and 0.335V, respectively, showing that there is a positive correlation between the thickness of CGSs and the IR drop. CV curves of CGSs (Fig. S4) show that the integral areas decreased with increasing of the thickness of RFC, indicating a high capacitance in CGS. Nyquist plots of the three samples (Fig. S5) show that CGS and 4d-CGS have capacitive behaviors because of the vertical curves in the low-frequency region. According to the equivalent circuits, the CGS exhibits the lowest ohmic resistance and charge transfer resistance.Therefore, a rational decreasing in the thickness of carbon coating benefits for electron and ion transport,resulting in a high capacitance.
Furthermore, the specific capacitances of GO,RFC, CGS, and KACGS were compared base on the GCD measurement at the current density of 1 A g−1(Fig. 4a). Their specific capacitances are calculated by Eq (1). The results show that the pure GO electrode exhibits much shorter discharging time and the lowest specific capacitance, indicating that the pores on GO nanosheets are useless caused by the aggregation of GO sheets. GO, RFC, CGS, and KACGS delivered specific capacitances of 23, 46, 172 and 223 F g−1at 1 A g−1, respectively. It can be proposed that the rich microporous carbon nanosheets with optimized thickness provide a short diffusion path for electrolyte penetration, and exert the conductivity of graphene for electron transport.
A wide range of prior studies proposed that high surface area and developed pore structure could ensure its superior capacitive behavior because the large surface area benefit for ion adsorption and the moderate pore structure promotes ion diffusion. Controlling the activation temperature is a crucial method to regulate surface area and pore structure. But excessive temperature causes the collapse of the microporous structure, resulting in a decrease in specific surface area. Therefore, the electrochemical performances of KACGS activated under varying temperatures were also investigated. As shown in Fig. S6, the shapes of the CV curves for KACGS and KACGS-800 exhibit a rectangular shape, indicating the EDLC behavior. The GCD curves (Fig. S7) for KACGSs were measured at a current density of 1 A g−1. The linear nature of the GCD curves also demonstrates the EDLC feature of the electrode materials. And a more symmetric GCD curve of KACGS is observed, it exhibits a specific capacitance of 223.5 F g−1,which is much higher than that of KACGS-400 of 141 F g−1and KACGS-800 of 199.3 F g−1. As the current density increases from 0.2 to 5 A g−1, the KACGS exhibits an excellent rate performance and retains 62.96% of its initial capacitance at 5 A g−1(170 F g−1), higher than that of KACGS-400(8.33%) and KACGS-800 (58.22%).
The CV curves of GO, RFC, CGS and KACGS(Fig. 4b) show analogous rectangular curves from −1 to 0 V. Depending on Eq (2), the area of CV curves can be calculated which are similar to GCD results.Electrochemical impedance spectra of GO, RFC,CGS, and KACGS are further analyzed by Nyquist plots, which can reflect the contact resistance, charge transfer resistance, and the diffusion resistance of the ion in pores under the corresponding frequency region[17,28]. For the Nyquist plot (Fig. 4c), in the lowfrequency region, the nearly vertical line reflects the capacitive behavior of an ideal capacitor. In the highfrequency region, the first intersection of semicircle andZ´ axis reveals the internal resistance (Rs), The small semicircle shapes reflects the small charge transfer resistance (Rct). An equivalent circuit model(inset of Fig. 4d) was shown to simulate the capacitive and resistive units in the supercapacitor system. It containsRs,Rct, the Warburg impendence (Zw), which results from the diffusion resistance for the ion transfer in the electrode, the capacitance of contact interface (CF), and the capacitance inside pores (CPE). All the samples have a smallRs(0.13-0.28 Ω). The RFC has a much largerRctvalue (11.6 Ω) and diffusion resistance than others. On the contrary, the GO and KACGS have a small diameter of the semicircle corresponding to the lowRctof 1.56 and 1.72 Ω, respectively. The CGS electrode shows a smallerRctthan GO and KACGS, due to the aggregation of GO and structural defect in KACGS by the activation of KOH. It indicates that the bulk conductive framework constructed from graphene and microporous carbons promotes the electrons transport, provides enough ionic transfer channel, and shortens ionic diffusion pathway during the electrochemical processes, and further gives rise to the outstanding energy storage performance.
To clearly understand the capacitive behavior of KACGS, the CV curves of different scan rates were shown in Fig. 5a. A rectangular shape curve can be observed at a low scan rate of 5 mV s−1, indicating a good capacitive behavior. However, the shape of curves can’t maintain with the increasing of scan rate because the charging-discharging process is mainly governed by reversible K+ion adsorption and desorption on the surface of the porous carbon nanosheets in the aqueous KOH electrolyte[29]. The galvanostatic charge/discharge cycling curves of KACGS at various current densities are shown in Fig. 5b. The quasi triangular shape of the curves at 0.2 A g−1reveals the EDLC behavior and the long discharging time suggests the high specific capacitance of 324 F g−1, calculated from the discharged curves by Eq (1). However,the little curved discharging curves indicate the contribution of pseudo-capacitance caused by the redox reaction of heteroatoms from nitrogen and oxygen in KACGS from the element analysis results, shown in Fig. S9. According to the pore size distribution of KACGS, the diameter of 0.8 nm is the optimum pore size for carbon-based electrodes for double-layer capacitance[30]. It is precisely that the pores with a diameter of 0.8 nm on the carbon nanosheets coated on both sides of graphene provide a suitable space for EDLC behavior and the hierarchical pore structure is beneficial for electrolyte penetration, thus resulting in such a high specific capacitance.
The triangular shapes of the curves have no change and the specific capacitance retains at a high level with the increasing of current density, suggesting a good rate capability. As shown in Fig. 5c, a very lowRsabout 0.13 Ω can be observed from the expanded high-frequency region of the plots, suggesting that the KACGS electrode has good conductivity, which is attributed to the good intrinsic conductivity of graphene. The small diameter of the semicircle impedance loop in the high-frequency region could reflect the small charge transfer resistance (Rct= 1.6 Ω)[31]. In the low-frequency region, the approximately vertical line shows the capacitive behavior of an ideal capacitor, due to the rapid ion diffusion in the electrolyte and EDLC behavior on the surface of electrode[32]. Stability is one of the most important properties of electrode materials for the supercapacitor. Cycling stability of KACGS was investigated at a current density of 5 A g−1, shown in Fig. 5d. The assembled electrode of KACGS exhibits a quite good cycling stability. The specific capacitance can retain about 99.8% of the initial capacitance after 8 000 cycles. The stability of CGS was also supplied in Fig. S10, it also shows a stability but a low capacitance of 160 F g−1at 1 A g−1.The present KACGS electrode material exhibited better performance than many previously reported carbon materials (Table 1)[33-41]. The superior electrochemical performance of KACGS may be attributed to the hybrid advantages of advanced hierarchical pore structure and conductivity of graphene framework, boosting ion and electron transportation. (Fig. 6).
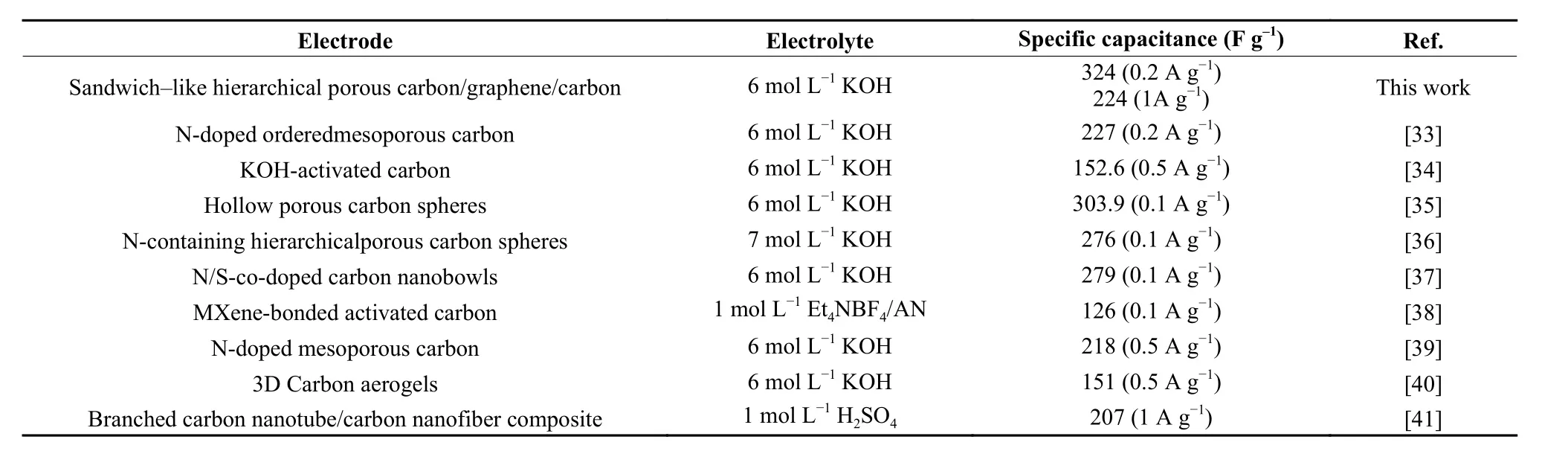
Table 1 Comparison of electrochemical performance of KACGS with some representative active carbon-based electrodesfor supercapacitors (All were tested in three electrode system).
4 Conclusion
In summary, the sandwich-like hierarchical porous carbon/graphene/carbon architectures with controllable thickness were prepared by a directional polymerization approach. Abundant micropores in RFC layers are beneficial to the rapid diffusion of electrolyte ions, which contribute to the double layer capacitance. Simultaneously, the graphene interlayers with good intrinsic conductivity could enhance the electrons transport during the charging and discharging processes. Such a unique integrated structure leads to excellent energy storage performance. The synthesized KACGS electrode delivers a high specific capacitance of 324 F g−1at 0.2 A g−1. Even at 5 A g−1, the capacitance of 175 F g−1can be still obtained after 8 000 cycles, showing a good cycling stability. It is believed that the hybrid structure can be applied to a wide range of applications requiring rapid electrons and ions transport.
Acknowledgements
National Natural Science Foundation of China(51902232, 52072275).
