Engineering the interface between separators and cathodes to suppress polysulfide shuttling in lithium-sulfur batteries
2022-06-13LONGXiangZHUShaokuanSONGYaZHENGMinSHAOJiaojingSHIBin
LONG Xiang, ZHU Shao-kuan, SONG Ya, ZHENG Min, SHAO Jiao-jing,, SHI Bin
(1. School of Materials and Metallurgy, Guizhou University, Guiyang 550025, China;
2. Mei Ling power sources Co. Ltd, Guizhou, Zunyi 563003, China;
3. State Key Laboratory of Advanced Chemical Power Sources, Zunyi 563003, China)
Abstract: Lithium-sulfur batteries have attracted extensive attention because of their high theoretical specific energy storage capacity and energy density. However, the shuttling of polysulfides greatly hinders their practical use. Many studies show that engineering the interface between separators and cathodes is an effective strategy to solve this problem. Ways to inhibit the shuttling can be divided into physical blocking, chemical adsorption, and catalysis. Among the interfacial materials, carbon materials have attracted enormous attention due to their high electrical conductivity, large specific area, and high pore volume. However, their non-polarity makes it impossible for them to bind polysulfides tightly and heteroatoms/functional groups are incorporated in them or highly polar materials are composited with them in the design of the interfacial materials. In addition, the catalytic effect of the carbon in the polysulfide conversion is believed to be very important in effectively suppressing the shuttling. This review focuses on the detailed strategies and functions of interfacial engineering in addressing the problems and challenges in the use of lithium sulfur batteries. Finally, practical applications of lithium sulfur batteries are proposed, based on a combination of various measures including interfacial engineering.
Key words: Carbon materials;Interfacial engineering;Polar materials;Polysulfides;Lithium-sulfur batteries
1 Introduction
The continuously growing demand for clean, sustainable power systems in electric vehicles (EV),household energy storage and consumer electronics is driving the research interest for electrochemical energy storage systems with better safety, lower cost,and higher energy density beyond current Li-ion battery[1]. Among alternative competitors, lithium sulfur(Li-S) battery has been regarded as one of the outstanding representatives considering its large theoretical capacity (Li, 3 860 mAh g−1and S, 1 675 mAh g−1),high energy density (Fig. 1), and lowest reduction potential of Li anode (−3.04 V vs NHE)[2], as well as the abundance, low price, environmental friendliness, and sustainability of the S cathode[3-6].
Li-S batteries are consisted of lithium metal anode, porous separator, a sulfur cathode, and electrolyte. During discharging, the active material S8is reduced to Li2S with fully discharged state, delivering a specific capacity of 1 675 mAh g−1based on S8. Upon charging, Li2S is oxidized back to S8. The underlying electrochemical conversion in the Li-S batteries is complex because of the multi-step reaction associated with various LiPSs (e.g., Li2S8, Li2S6, Li2S4and Li2S2)being generated. Moreover, the intrinsic properties of S8, Li2S and LiPSs cause many challenges during LSBs service, such as sluggish reaction kinetics due to the insulating nature of sulfur (S8) (conductivity of 5 ×10−30S cm−1at 25 °C) and lithium sulfide (Li2S) (conductivity ~10−13S cm−1at 25 °C), large volume variation of sulfur cathode during charge-discharge cycling, lithium polysulfides (LiPS) dissolution and the as-resulted “shuttle effect”, uncontrollable lithium dendrites growth and the as-leaded non-negligible electrolyte depletion[8], and largely impairing the electrochemical performance of LSBs in practical use.Among them, the “shuttle effect” is one of the most serious bottlenecks for the real application of LSBs.To solve this issue, various strategies have been attempted, such as the employment of conductive cathode host[9], binders[10-12], functional separators[13], and novel electrolytes[14].
Although the separators are not active components in batteries, they play the crucial role in influencing the cell cost, cycling life, safety, and so the forth[15]. The electrolyte-filled pores in separator allows lithium ion transfer from negative electrode (anode) to positive electrode (cathode) during the chargedischarge cycles, while preventing the short circuit.As essential parts of batteries, separators play certain roles in physically blocking polysulfides at the cathode side and preventing them from shuttling to the anode and reacting with lithium metal in the LSBs.Functionalizing separators could alter the surface chemistry and steric hindrance effect, which is beneficial for inhibiting the polysulfides shuttle and raising the S utilization. Furthermore, these functional separators generally contribute to the suppression of the Li dendrites and stabilization of SEI membrane on the surface of lithium metal by acting as a protective layer[16-20]. Overall, such interfacial engineering on the separator has been confirmed to be one of the effective ways to solve the shuttle effect and improve the battery performance.
In this review article, we first elaborate the working mechanism of lithium sulfur battery and the“shuttle effect” in details, and then we primarily pay attention to the recent progress in addressing the shuttle effect based on the functionalization of commercial porous separators, i.e., the separator interfacial engineering, mainly including[21-28]the construction of an interlayer[29,30]and the chemical modification of the commercial separators[31-33]. In the two interfacial engineering strategies, the main functions of the separator interfacial engineering, including physical adsorption, chemical interaction, and catalytic effect, are introduced. Finally, the prospects and challenges in the field of separator interfacial engineering are presented.
2 Working mechanism and the “shuttle effect” in LSBs
Unlike the ion-insertion charge storage mechanism of conventional lithium-ion batteries, Li-S batteries involve multielectron conversion reactions[34,35].The whole reaction consists of two steps: firstly, sulfur is reduced to long-chain lithium polysulfides(S8→ S82−→ S62−→ 2S42−) with 4e−transfer. And then, long-chain polysulfides are further reduced to short-chain lithium sulfides. In the reverse charging process, lithium sulfides are oxidized to polysulfides,which is then oxidized to sulfur (Fig. 2)[36].
A separator is an indispensable component for batteries and is placed between the cathode and anode to avoid direct contact of two active electrodes. The micro-nano pores provide the lithium ions with transportation channels during charging and discharging of the battery. Currently, most commercially available separators are semi-crystalline polyolefin membranes,such as single-layer polyethylene (PE), single-layer polypropylene (PP), PP/PE/PP three-layer composite membrane. These porous separators are typically less than 25 μm thick and have complex three-dimensional structures commonly with a porosity (ε) of around 40%. Such commercial porous separators are provided with highly flexibility, enough lithium ion passages,good mechanical strength and low cost[15,38]. Thus far,single-layer and three-layers polyolefin-based separators have been widely used in LIBs and LSBs. The pore size of commercial diaphragm is generally between 0.03-0.12 μm, and the pore size distribution is narrow and uniform. The discrepancy between the maximum pore size and the average pore size is no more than 0.011 μm[39,40]. Since the size of polysulfides is smaller than that of the commercial separator,both lithium ions and polysulfide anions could penetrate the porous separator from S cathode to Li metal anode and are reduced to solid LiS2/Li2S2on the surface of lithium metal, leading to the loss of active substances and fast capacity decay, forming the notorious “shuttle effect”[41-44]. In addition, these hydrophobic polyolefin-based separators would seriously affect the ability to retain electrolyte solutions. To address the above issues, many efforts have been devoted to the modification of separators by coating an interlayer with multifunctionalities on separator to inhibit the shuttle effect and improve its electrolyte wettability[45-48]. Carbon materials, metal oxides/sulfides/nitrites, etc. are often used to serve as the main components of the interlayers that play the role of physically entrapping, chemically adsorbing, and catalytically transforming the lithium polysulfides[49,50].
3 Separator interfacial engineering
In order to prevent the transport of polysulfides from the cathode side to the anode side through the porous separator, the main strategies relating to the separator interfacial engineering include the insertion of an interlayer between commercial separator and the active electrodes, as well as the chemical functionalization of the commercial separator. The interlayer could be inserted on one side of the commercial separator or on both sides of the separator. The chemical functionalization involves the creation of functional groups on the surface of commercial separators. Either way, the main functions contain three aspects: enhancing the physical blocking for the polysulfide diffusion, strengthening the chemical adsorption of separator for polysulfides, and offering catalytic sites for polysulfide conversion. Herein, the involved materials are classified to non-carbon polar materials, carbon-based composite materials, and carbon-based materials.
3.1 Carbon based materials
Because of the intriguing features, such as large specific surface area, high electronic conductivity, and easily-altered microstructures, carbon materials, such as carbon nanotubes[51], carbon fiber[52], graphene[53],porous carbon[54], porous carbon spheres[55], carbon black (Super P)[56], hierarchically porous carbon[57]and so forth, have been widely researched as the building blocks for the interlayer construction.
In 2012, Manthiram[30]inserted a carbon-based interlayer between the porous separator and the sulfur cathode to address the polysulfide shuttle (Fig. 3a).The conductive MWCNT interlayer not only retarded the charge transfer resistance of sulfur cathodes, but also accommodated the dissolved intermediate polysulfides by taking advantage of the porous interlayer matrix, which promoted the utilization of active substances and effectively inhibited the shuttle effect. Finally, high-rate performance and long-term cycling stability were achieved in the battery. Carbon blacks are a cost-effective carbon material with high specific surface area. Zhenget al.[58]coated ultra-light carbon black flakes derived from the carbonization of sodium citrate on commercial separator, leading to significantly-improved electrochemical performance for the Li-S cell due to the remarkable conductivity of the carbon black flakes and its strong immobilization capability for LiPSs (Fig. 3b). Kim and his co-workers reported that the dissolved polysulfides could be captured by introducing an acetylene black mesh, which brough about enhanced rate and cycling performance in the as-assembled Li-S battery (Fig. 3c)[59].
The porosity of carbon materials is very important for enhancing the physical adsorption for polysulfides[29,61-69]. Generally, porous carbon materials can hold more sulfur element and polysulfides due to the high specific surface area and large pore volume[70].Balachet al.[51]coated mesoporous carbon on a commercial separator, and the as-assembled Li-S battery with such a modified separator exhibited outstanding electrochemical performance, primarily due to that the mesoporous carbon interlayer provided effective place to trap the dissolution of polysulfides and raised the reutilization of active materials. The mesoporous structure worked as reservoir to effectively trap and confine the LiPSs, and it was able to accommodate the large volume change of sulfur during charge and discharge cycling. As a result, the shuttle effect was well suppressed and the active material utilization was improved, which finally enhanced electrochemical performance and prolonged cycle life in the Li-S battery using such mesoporous carbon interlayer (Fig. 3d).
Even though the large porosity of porous carbon materials contributes to strengthening the physical adsorption of long-chain polysulfides, it is noteworthy that porous carbon materials usually suffer from low electronic conductivity, and thus we need find a balance between the pore structure and electronic conductivity. In addition, the weak polarity of carbon materials also makes them hard to adsorb polar polysulfides effectively. Heteroatom (e.g., N, P, O and S) doping is considered as an effective way to address the above issues. Heteroatom-doped carbon materials not only have improved electronic conductivity but also show enhanced chemical adsorption ability for LiPSs compared with their pristine counterparts[71]. For example, Zhouet al.[72]fabricated a carbon-based interlayer composed of graphene and N, P-doped porous carbon, which was denoted as N-P-PC/G. The carbonbased interlayer was served as an excellent conductive framework, and the dual-doped porous carbon with a large number of interconnected meso-/micro pores could offere large pore volume and specific surface area that contributed to strong physical accommodation and chemical adsorption, which significantly suppressed the shuttle effect and finally leaded to improved battery performance (Fig. 4a).
Graphene, as a novel 2D carbon material, has many advantages including large surface area and high electrical conductivity, which is in favor of adsorbing polysulfides and facilitating electron transport, thus improving the S utilization and electrochemical reaction kinetics[76]. Additionally, the flexibility and mechanically robust of conjugated graphene sheets are well compatible with PP separators(Fig. 4b)[73]. However, it is quite difficult to manipulate graphene sheets into graphene-based macroforms directly with controllable microstructures in liquid phase owing to the highly hydrophobic nature.Graphene oxide (GO), as an oxidized derivative of graphene, is a water-soluble material that can be obtained by the modified Hummers’ method[77-80]. GO can be transformed to chemically modified graphene,also known as reduced GO (rGO) that has good electronic conductivity and large specific surface area.Chouet al.[74]inserted a self-supporting single-walled carbon nanotube (SWCNT)/rGO interlayer between the separator and the anode, accompanied by sulfonated polyetheretherketone (SPEEK) membrane with negatively charged ion nanochannels being integrated into the cathode. Such an interlayer blocked the shuttle of polysulfides and provided enough space for continuous electrochemical reaction. Finally the Li-S battery showed increased capacity and cycling life(Fig. 4c).
Because of the excellent conductivity and special interwoven or nonwoven structure as well as the much more reliable mechanical strength and flexibility than other carbon materials, carbon fibers have attracted extensive attention in the separator interfacial engineering to obtain enhanced inhibition towards the polysulfide shuttling and ensure the robust stability of Li-S batteries during cycling[81]. For example, Zhanget al.[75]prepared carbon fiber based paper of porous structure and used it as an interlayer in Li-S battery,leading to significantly improved electrochemical performance including large reversible capacity, excellent cycling ability, and rate capability (Fig. 4d). In addition, a lot of work has been done to introduce some functional groups and microcracks on carbon fibers to improve their adsorption capacity for LiPSs[82-87].
Currently, hollow carbon spheres have attracted enormous attention due to its capability of reserving a large amount of LiPSs and accommodating repeated volume changes. Songet al.[88]prepared hollow carbon spheres with silica spheres as templates, and the as-obtained hollow carbon spheres were coated onto separators to serve as interlayers that effectively suppressed LiPS shuttling due to the physical and chemical blocking effects as well as the good conductivity of the carbon spheres.
Overall, carbon materials usually have favorable conductivity and thus can serve as a second collector to promote fast electron transport and reduce the interfacial impedance between separator and cathode. In order to enhance the adsorption capability of carbon materials for LiPSs, heteroatom doping is a widelyused strategy, which can also create reactive sites for fast charge transfer. Finally, raised S utilization and increased discharging capacities can be achieved in the Li-S batteries.
3.2 Non-carbon polar materials
Due to the weak interaction between highly polar polysulfides and non-polar carbon layer, the physical confinement of the carbon interlayers is generally not enough to achieve effective suppression towards the polysulfide shuttle effect. In comparison with carbon materials, strong polar materials, such as polymers[89], metal oxide[13], metal sulfides[90], metal nitrides[91], etc., can provide stronger chemical interaction with polysulfides, and therefore, highly polar materials are currently often used to construct the interlayer (Fig. 5a). For instance, Shiet al.[92]fabricated a multifunctional interlayer with ordered microstructure by layer-by-layer self-assembly of covalent triazine framework (CTF) and negatively charged PEDOT: PSS with positively-charged polydi
allyldimethylammonium chloride (PDDA). In the interlayer, PEDOT: PSS, as a conductive polymer, expediated the electron transfer and offered polar groups that contributed to the formation of strong chemical interaction, and also CTF@PDDA showed strong LiPS-anchoring ability mainly due to its large specific surface area and porous structure, which finally effectively inhibited the LiPS shuttle and raised the sulfur utilization (Fig. 5b). Strong chemical bonding can be found between polar metal oxides/sulfides and polysulfides, which offers an excellent opportunity for such materials as the building blocks for constructing the polysulfide-suppressing interlayer[93]. For example,it is reported that Ti-S bonds can be formed between TiO and polysulfides, and the TiO interlayer serves as a highly effective polysulfide absorbent to inhibit the dissolution of polysulfides and improve the cycling performance of the battery (Fig. 5c)[94]. Wanget al.[95]chemically decorated commercial PP separator based on the oxidizing property of acidic KMnO4solution.The PP separator was first soaked in acidic KMnO4solution for 1 h and then the ultra-thin self-assembled MnO2layer was directly constructed on the surface of separator. Because of the strong Mn-S and Li-O binding effect, the self-assembled MnO2interlayer could effectively inhibit the polysulfide shuttle, finally leading to excellent electrochemical kinetics and effective recycling of active substances (Fig. 5d). Liuet al.[53]constructed a multifunctional interlayer through inserting Fe3O4nano-particles (NPs) in a porous graphene (PG) film. The porous graphene with optimized structure facilitated the ion transfer, and the polar Fe3O4NPs trapped sulfur species via strong chemical interaction, which finally leaded to effective immobilization towards the polysulfides (Fig. 6a). In contrast to metal oxides, metal sulfides and metal nitride can adsorb polysulfides through stronger Li-S interaction associated with the dipolar interaction of metal-sulfur bonds on the polarized interlayer surface.For example, Songet al.[96]prepared a vanadium nitride (VN)-modified separator. The thin yet dense VN interlayer formed by porous and intertwined VN nanobelts served as a physical barrier to block LiPSs whilst selectively sieved lithium ions. Also, the polar conductive VN could chemically anchor LiPS and maintained efficient charge transport. More importantly, the ample active sites of the VN catalytically accelerated the uniform deposition of Li2S (Fig. 6b).
Besides the strong chemical adsorption towards LiPSs, these transition metal-based compounds generally can work as the catalysts to promote the redox reaction kinetics for the polysulfide transformation,which is the reason why many attempts have been made to prepare transition metal-based interlayers.
Defect engineering is a novel strategy to design an interlayer with effective suppression effect towards polysulfide shuttle. Defects, such as vacancies,have been intentionally introduced in the material design. The defects generally can improve the electronic conductivity, adsorption ability, and catalytic activity of the materials. For example, ultra-thin and lightweight Bi2Te2.7Se0.3(BTS) with high-density inversion defects was used as the interlayer material, in which Bi vacancies and Te vacancies coexist. Such BTS interlayer could not only improve the utilization of active substances, but also enhance the capacity retention of the batteries. Finally, the battery with high sulfur loading still exhibits excellent electrochemical performance (Fig. 6c)[97]. Wanget al.[98]coated mixedvalence cerium-based MOF with isolated Ce (IV, III)arrays and abundant oxygen vacancies (OVs) on the separator. The spontaneously recycled CeIV/CeIII redox couple could performe as an effective mediator to improve the redox catalytic activity of the interlayer (Fig. 6d). Furthermore, oxygen vacancies (OVs),one of the most common defects in metal oxides, can work as highly efficient active sites for various heterogeneous catalysis, including electrocatalysis for accelerating the redox reaction kinetics for the transformation of polysulfides and Li+transfer rate in the Li-S battery system. The separator interlayer remarkably accelerated the redox kinetics of the polysulfide conversion in the Li-S battery. Sb2Se3-x/rGO microspheres rich in metal atom defects prepared by a simple spray drying method were used as the multifunctional interlayer for the LiPS inhibition, and such a porous electron/ion conductive framework provided rich catalytic active interfaces for the conversion of LiPS due to the existence of atomic defects, leading to excellent Li-S battery performance.
In the separator interfacial engineering, the commonly-used non-carbon polar materials involve the transition metal atoms, due to that the strong transition metal-sulfur bindings can significantly enhance the adsorption of the interlayer towards LiPSs, and these transition metal-based materials can serve as the catalysts to accelerate the LiPS conversion, which is beneficial for smooth adsorption-diffusion-conversion of LiPSs and efficient suppression towards the shuttle effect.
3.3 Carbon based composite materials
Even if the electronically-conductive carbon materials including graphene, carbon nanotubes, and acetylene black have weak polarity, they are often mixed with some high polar materials to prepare composite interlayers, since these carbon materials could work as the secondary current collectors to expediate the electron transport, while these high polar materials can endow the interlayer with strong chemical affinity to LiPSs and/or high catalytic activity for the LiPS conversion. Thus far, various metal oxides/carbon (such as V2O5/graphene, MgO/acetylene black(AB), TiO2/AB, Fe3O4/graphene, etc.) and hydroxides/carbon (such as FeOOH/G-C3N4/KB)composite interlayers[53,56,57,99-103]are designed to take the advantages of both the carbon matrix and metal oxides/hydroxides. For example, Wang’s group[104]reported a sandwich interlayer by loading MnO2nanoparticles and graphene oxide (GO) sheets on superoriented carbon nanotubes (CNT), and the as-obtained ultrathin interlayer with thickness of 2 μm and areal density of 0.104 mg cm−2inhibited the polysulfide shuttling thanks to the strong chemical adsorption between MnO2/GO and polysulfides, as well as the high electronic conductivity of the CNT, leading to the high-performance Li-S battery (Fig. 7a). In comparison with metal oxides, metal sulfides generally have higher electrical conductivity (from 6.7 × 10−5to 1.36 S m−1). Various cobalt sulfides, especially polar CoS2and Co9S8, have been developed and applied in Li-S batteries to inhibit the shuttle effect. In addition to strong absorption capability, CoS2and Co9S8not only have strong adsorption capacity, but also have good catalytic activity, which can accelerate the redox reaction of polysulfide. For example, the battery using an acetylene black (AB)-CoS2separator has a lowcapacity decay rate of 0.09% per cycle. In addition,the vertical Co9S8arrays grown in-situ on the PP separators (Co9S8-Celgard) acts as a catalytic layer to enhance the conversion of polysulfides and reduce the barrier toward interfacial electron/ion transport(Fig. 7b)[105]. In general, metallic nitrides are highly conductive and exhibit metal-like properties. A multifunctional catalytic interface consisted of niobium nitride/N-doped graphene (NbN/NG) was designed by Fanet al[106]. Such an interlayer possessed long-distance electron transfer passages, strong chemical adsorption ability, and abundant catalytic sites, the asassembled Li-S batteries with NbN/NG interlayer have excellent rate performance (621.2 mAh g−1at 3C) and high stable cycling life (81.5% capacity retention after 400 cycles) (Fig. 7c). In addition, the conversion of polysulfide can be also catalyzed by metal selenides/phosphides/carbides/borides as well as metal-organic frameworks, which have also been composited with carbon-based materials to construct the interlayers.
Ranaet al.[107]reported a multifunctional separator interlayer consisted of SO3-containing Nafion (NNGN) and nitrogen-doped multilayer graphene(NGN). Nafion was used as a binder, providing ―SO3to chemically bind PS, while highly conductive NNGN provided efficient pathways for electrons, which accelerated the electrochemical conversion and finally leaded to a high areal capacity LSB (Fig. 7d).
Zhanget al.[60]prepared a Ni/SiO2/graphene modified separator, in which graphene provided electron transport passages, while SiO2and Ni displayed strong affinity towards the LiPSs, and more importantly Ni played a role of catalyst that realized the rapid conversion of LiPSs to Li2S2/Li2S (Fig. 7e).
Since it is difficult to integrate high conductivity,strong adsorption capability, and effective catalytic function in one material, it is a simple but effective way to fabricate composites. Therefore, carbon-based composites have already attracted enormous research interests in the separator interfacial engineering. Even though the introduction of an interlayer generally can effectively suppress the polysulfide shuttling, it is notable that the interlayer is an inert component in the Li-S batteries, and thus its mass loading and thickness need to be strictly controlled to avoid the reduction of gravimetric/volumetric energy densities for the batteries. Based on this consideration, Table 1 compares some previously reported literatures involving the separator interfacial engineering, and the gravimetric energy densities of the coin cells are calculated and provided as follows.
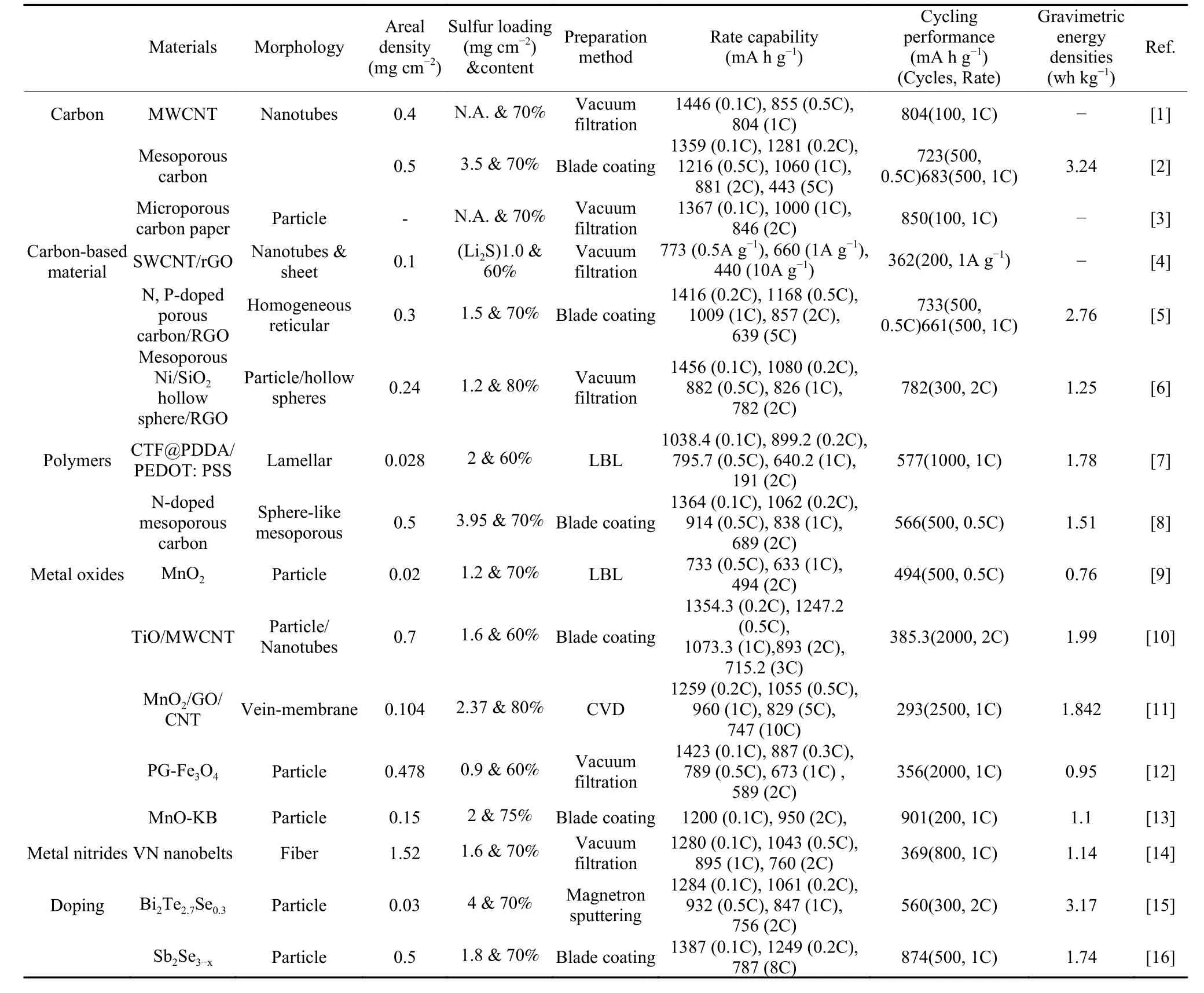
Table 1 Comparison of some previously reported literatures involving the separator interfacial engineering in the Li-S batteries (1 C=1 675 mA g−1).
4 Characterization methods
Investigation on structure, morphology, and properties of the key components in LSBs are of great significance to understand the transformation mechanism of polysulfides in-depth, inhibit the shuttle effect,and achieve high-performance Li-S batteries.
Scanning electron microscopy (SEM) is usually employed to observe the surface morphology of the materials including the interlayer, cathode, and lithium metal anode before and after cycling, as well as characterize the thickness of the interlayer. By combining with the corresponding energy dispersive X-ray spectroscopy (EDS), the suppression effect of the interlayer towards the polysulfide shuttle can be evaluated by observing the roughness and the elemental distribution on the surface of the material surface. The thickness of the interlayer is closely related to the overall electrochemical performance of LSB. Generally, much thick interlayer brings large battery impedance, which would also reduce the gravimetric and volumetric specific energy density of the battery,while much thin interlayer hardly plays the role of inhibiting the shuttle effect effectively, and thus it is important to looking for the optimum interlayer thickness.
Transmission electron microscopy (TEM) has higher resolution than SEM and thus can reveal the lattice fringes of crystals. Like SEM, high-resolution TEM (HR-TEM) combined with EDS can also be used to analyze the element distribution on a specific area with nano-scale size. The corresponding selected region electron diffraction (SAED) or electron energy loss spectroscopy (EELS) can obtain various intrinsic information about materials including crystal structure. The electrode reaction in LSBs is a multi-electron process. In-situ TEM can provide more detailed information about the electrochemical reaction kinetics. For instance, the real-time structure evolution of catalytic active center can be provided in each step,which has great potential in exploring the mechanism of catalytic reaction.
Contact angle measurement is often used to study the electrolyte wettability of the interlayer material.Better electrolyte wettability contributes to reduce electrolyte/electrode interfacial resistance. The adsorption ability of the interlayer materials towards the polysulfides is very crucial for effectively suppressing the shuttle effect. Static adsorption test, polysulfide penetration experiment and ultraviolet-visible spectroscopy (UV-Vis) are simple but reliable characterization methods to evaluate the adsorption ability.The adsorption between the interlayer materials and polysulfides can be classified to physical adsorption/confinement and chemical interaction. The physical adsorption/confinement ability is high related with the pore structure that can be characterized by nitrogen adsorption-desorption measurement and mercury intrusion method. The chemical interaction of a sample towards polysulfides can be analyzed by performing X-ray photoelectron spectroscopy (XPS)before and after the polysulfide adsorption. Usually,formation of the chemical interaction can be confirmed according to the shift of some binding energies. Currently, theoretical simulation calculation based on density function theory (DFT) is widely used to further evaluate the chemical interaction ability between the materials of interest and polysulfides by calculating the adsorption energy[29,74,110].
Electrochemical measurements, including galvanostatic charge-discharge (GCD), electrochemical impedance spectroscopy (EIS), cyclic voltammetry curve (CV), etc., are indispensable characterization methods to study the electrochemical conversion process of polysulfides in LSBs. For instance, the redox peaks in the CV profiles of LSBs correspond to the solid-liquid-solid conversion of the polysulfides, and thus the peak potential separation and current density can reflect the electrochemical reversibility and reaction kinetics, respectively. Fast lithium-ion transport is very crucial for achieving improved electrochemical reaction kinetics and high-rate Li-S batteries, which can be estimated by lithium-ion diffusion coefficient(DLi+).DLi+can be calculated according to the CV curves of LSBs at different scan rates. HighDLi+represents the fast lithium-ion diffusion and is beneficial for promoting the conversion reaction kinetics of polysulfides. Galvanostatic charge-discharge (GCD)curves can display discharge capacity, voltage platform, and the polarization of the batteries. Initial discharge capacity is rather critical for preliminary evaluation of battery performance. In addition, the cycling performance profiles of the batteries can be plotted based on the change trend of discharge capacity with the increasing of cycle number, which can give the capacity decay rate per cycle after long cycling at a certain current density. The voltage separation between the charge and discharge platforms reflects the battery polarization, smaller polarization suggests promoted redox reaction from LiPSs to Li2S. Besides the long cycling stability, excellent rate performance is very critical for the practical application of LSBs.Rate performance of Li-S batteries can also be obtained based on the GCD profiles. Better rate capability often represents the higher ion conductivity in the battery. In Li-S batteries, the electrochemical impedance spectroscopy (EIS) usually contains three parts,the high frequency intercept on the real axis that represents the ohmic resistance (Re) of the electrolyte and electrodes, the semicircle at medium-to-high frequency corresponding to the interface charge transfer resistance (Rct), and the inclined line at the low-frequency region indicating the Warburg impedance(W1) and is related to the semi-infinite diffusion of soluble LiPSs in the electrolyte. In LSBs, the smaller semicircle in the EIS indicates smaller Rct, often revealing the lower internal impedance of the cell as the result of the introduction of some high conducting materials, which contributes to the enhanced polysulfide conversion and more effective suppression toward the LiPS shuttling. In Li-S batteries, it is very important to evaluate the liquid-solid transformation kinetics from LiPSs to Li2S/Li2S2. Chronoamperometry is employed to investigate the deposition process, in which the relationship between nucleation time and current is obtained. The higher Li2S deposition capacity and earlier peak rising time represent the enhanced reaction kinetics. The slope of Tafel plots of Li-S batteries can also be used to estimate the electrochemical conversion kinetics of polysulfides according to the exchange current densities in both the reduction/oxidation processes.
To better understand the polysulfide conversion,ex-situ SEM observation combining with EDS is often conducted on the Li-S batteries after cycling. The surface morphology and elemental analysis of the sulfur cathode, separator, and lithium metal anode can reflect the inhibition effect of the interlayer towards the polysulfide shuttling. For example, smoother surface and less dendrites on the Li metal anode correspond to more effective suppression. Optical spectroscopy techniques, including Raman spectroscopy, Fourier transform-infrared spectroscopy and UV-vis, have been extensively used to study the mechanism of polysulfide shuttling due to their ease of operation,nondestructive nature, high time and spatial resolutions, ample information, etc. The LiPS conversion process during cycling can be tracked by combining the optical spectroscopy and in situ techniques. Currently, in-situ/operando Raman and X-ray diffraction(XRD) are widely used in the field of Li-S batteries to monitor the transformation of polysulfides in real time during the charge-discharge cycling.
The self-discharging behavior of Li-S batteries can cause serious performance attenuation due to the spontaneous dissolution of LiPSs in the electrolyte. It is necessary to acquire the self-discharging data for propelling the practical application of Li-S batteries.The self-discharge of Li-S batteries can be examined by comparing the capacity attenuation or the potential change of the batteries before and after resting for several days or even months[111].
Overall, the detailed transformation mechanism of LiPSs can be better understood with the development of more advanced characterization technologies,which contributes to promoting the practical application of high-energy-density Li-S batteries.
5 Conclusions, prospects and challenges
The notorious shuttle effect of polysulfides is one of the main challenges that limits the real application of Li-S battery. Although there are several strategies to address the polysulfide shuttle issue, herein we pay our attention to the separator interfacial engineering,including the insertion of an interlayer and the chemical modification on the commercial separator. It has been confirmed that the interfacial engineering is a very simple but effect way to inhibit the polysulfide shuttle and improve the battery performance. The main functions of the interfacial engineering involve physical blocking, chemical interaction, and catalytic effect, towards the polysulfides. Furthermore, both the intrinsic properties and the microstructures of the interlayer materials are equally important in realizing the effective suppression towards the polysulfide shuttle. Noteworthy, the separator interfacial engineering can also regulate the uniform Li plating/stripping deposition thanks to the improved Li transmission channels on the modified separator, which contributes to resolving another thorny issue, i.e., the lithium dendrites, and promotes the real application of lithium sulfur battery.
Notably, two-dimensional (2D) materials (such as graphene and MXene) have been investigated and used as the building blocks for the construction of separator interlayer because of their extraordinary physiochemical properties, including atom-scale thickness, highly anisotropic dimensions, and large specific surface area. 2D materials easily form ultrathin interlayer that is very crucial for an ideal interlayer,as we know, the thick interlayers would impair the volumetric/gravimetric energy density of the batteries,which is contrary to the original intention of obtaining high energy density Li-S battery. Furthermore, it has been reported that the nanochannels among the negatively-charged 2D sheets can work as cation-selective ion transport passages, and thus it is suggested that the 2D material-based interlayers probably can allow the free transport of lithium ions and repel the transmission of polysulfide anions.
Even though much-improved performance in Li-S batteries has been achieved through the separator interfacial engineering, Li-S pouch cells are still far from commercial application because of many other limitations, such as the low sulfur loading, flooded electrolytes, lithium dendrites, etc. Researchers are continuously devoting to raising the sulfur loading (≥70 wt%, ≥5 mg cm−2), lowering the electrolyte/sulfur ratio (E/S≤ 4 μL mg−1), increasing the electrode size(≥1 cm2/cell), and improving the safety, to facilitate the commercialization of LSBs[112,113]. By combining other measures, such as synthesizing new electrolytes,introducing electrolyte additives, designing cathode materials, employing lithium alloy anode, and using solid electrolytes, it is expected that the practical application of lithium sulfur battery can be finally realized in the near future.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (51972070 and 52062004), Key Project of Guizhou Provincial Science and Technology Foundation ([2020]1Z042), Cultivation Project of Guizhou University(GDPY[2019]01), Science and Technology Support Project of Guizhou Province (QKHZC[2021]YB317),and Graduate Innovation Research Fund of Guizhou Province (YJSCXJH[2020]028).
