Design of active sites in carbon materials for electrochemical potassium storage
2022-06-13GENGChaoCHENYaxinSHILiluoSUNZongfuZHANGLeiXIAOAnyongJIANGJiangminZHUANGQuanchaoJUZhicheng
GENG Chao, CHEN Ya-xin,, SHI Li-luo, SUN Zong-fu, ZHANG Lei, XIAO An-yong, JIANG Jiang-min, ZHUANG Quan-chao, JU Zhi-cheng,
(1. School of Materials Science and Physics, China University of Mining and Technology, Xuzhou 221116, China;
2. School of Materials and Chemical Engineering, Xuzhou University of Technology, Xuzhou 221018, China;
3. School of Materials Science and Engineering, Northeastern University, Shenyang 110819, China)
Abstract: Carbon materials have attracted considerable attention as anodes for potassium ion batteries owing to their low-cost,nontoxicity, and controllable structures. The potassium storage behavior of carbon materials is highly associated with their active sites. In recent years, significant advances have been made in designing the active sites of carbon materials to meet the requirements of different potassium-based storage devices. Here, potassium storage mechanisms (intercalation and adsorption) for guiding the rational design of carbon materials are discussed. Based on these mechanisms, the review provides fundamental insight into the relationship between the structures and potassium storage performance of different carbon materials, including graphite, soft carbon, hard carbon, porous carbon, heteroatom-doped carbon, hybridized carbon and composited carbon. The structural design principles of carbon anode materials for potassium-ion full cell and potassium-ion capacitors are summarized based on the initial coulombic efficiency, capacity, potential plateau, rate performance, and cyclic stability. Finally, the problems and future research directions for the design of active sites in carbon materials for electrochemical potassium storage are considered.
Key words: Carbon materials;Active site;Potassium-ion battery;Anode materials
1 Introduction
The rapid consumption of traditional fossil fuel resources and serious global environmental pollution has caused many issues for the development of contemporary society[1,2]. Therefore, the development of clean energy is a good choice to alleviate fossil fuel dependence, especially the electrochemical energy storage (EES) system has received extensive attention[3-5]. In the EES system, lithium-ion battery(LIB) is the most mature one and has been widely used in electronic products, automotive and aerospace fields[1,6]. However, large-scale lithium-based energy storage equipment is restricted due to the scarce resources and their uneven distribution, and arduous recycling of lithium[7-9]. Thus, potassium-ion batteries(PIBs) with attractive/unique properties, such as low cost, abundant resources and relatively low reduction potential of potassium, have gained extensive attention in recent years[4,10,11]. At the same time, K+possesses the smallest Stokes’ radius (0.36 nm) compared to Li+(0.48 nm) in propylene carbonate solvents, indicating that it has the highest ion mobility and ion conductivity[12,13]. The above advantages make potassium-based energy storage equipment an ideal candidate for green energy storage.
Carbon materials are considered to be the most promising anode materials for realizing K+storage owing to the obvious advantages of low cost, good electrical conductivity, controlled surface chemistry and structure[14-16]. The potassium storage active sites are highly correlated with the electrochemical potassium storage behavior and even performance[17]. Based on the different potassium storage sites, carbon materials have two typical potassium storage mechanisms,including intercalation and adsorption[1]. In general,the sloping region in the charge-discharge curve is determined by surface-driven K+storage, and the lowpotential plateau region is driven by K+intercalation[14,18,19]. Graphite is a typical K+host by intercalation to form compound KC8, which has a theoretical capacity of 279 mAh g−1and an intercalation potential platform of about 0.3 V[20,21]. As a PIB anode material, graphite exhibits low discharge potential and high initial coulomb efficiency (ICE), which is beneficial for the construction of high energy density full-cells. However, during the charging and discharging process, the large size of K+(0.138 nm) produces volume expansion in graphite, resulting in cycle instability, capacity fading and poor rate performance[22,23]. Carbon structural engineering of graphitic materials can improve the K+storage capacity and cycling stability[24]by expanded interlayer spacing[25,26], heteroatom doping[27]and building composite structures[28,29].
Enhancing the K+adsorption capability at the active sites on the material surface by producing abundant carbon defects or large specific surface area(SSA) is an effective strategy to improve the potassium storage performance of carbon materials[30,31].Abundant active sites on the carbon surface can increase the K+accumulation space and fast kinetics,resulting in high capacity and excellent rate performance of carbon anode materials[32,33]. Based on these significant advantages, much progress has been achieved in the design and development of various types of anode materials, including morphology adjustment (porous, nanosheets, nanofibers)[16,34-37],structural design (disordered structures, hybridized structures)[2,10,38], and heteroatom doping[39,40]. But, the low ICE, high-potential sloping curves, and low material density should be cautiously considered for practical applications[41]. It is found that the variation of active potassium storage sites leads to different potassium storage mechanisms, and further influences the potassium storage performance. Therefore, the intercalation and surface-driven potassium storage of different active sites should be fully considered in the design of carbon structures to optimize the performance. At the same time, it should also be focused on the assembly of full device corresponding to the appropriate mechanisms and electrodes.
In this review, a detailed summary of the relationship between active sites and potassium storage mechanism/performance of carbon materials has been discussed (Fig. 1). The influence of carbon structure evolution during potassium storage is introduced in detail in terms of graphite, soft carbon and hard carbon materials. Based on that, we review the design strategies, including porous structure, heteroatom doping, hybrid structure and composite structure for carbon materials aiming at boosting electrochemical performance. Finally, the potassium storage devices with different carbon electrode materials are summarized,and the challenges and prospects for further development of carbon anodes are put forward.
2 Potassium storage mechanism of active sites
Active sites of carbon materials are particularly important for K+storage in carbon materials. Potassium storage active sites mainly include graphitic layers, edges and defects, which can be divided into two mechanisms: intercalation/deintercalation and adsorption/desorption[22]. The K+intercalation/deintercalation mechanism occurs mainly in the carbon interlayers[42]. Due to the low discharge potential and high ICE of interlaminar materials, PIBs can possess high energy density in practical applications. However, the K+intercalation in carbon material will produce volume expansion, resulting in the loss of contact between the active material and the collector, increasing interface resistance and deteriorating battery performance[9,43]. The adsorption/desorption of K+is mainly on the surface, pores and defects of carbon materials[44]. The storage of K+by adsorption is not limited by the electrode structure damage and the theoretical capacity of KC8, showing high potassium storage capacity, superior rate performance and cyclability[18]. Therefore, the diffusion kinetics and adsorption capacity of K+can be boosted by increasing the specific surface area (SSA) and creating more defects. However, the large surface area and high defects will consume a large amount of the electrolyte to form a solid electrolyte film (SEI), resulting in high irreversible capacity and low ICE during the initial cycle. What’s more, the active sites on the carbon surface break the integrality of the π conjunction of the carbon plane, which decreases the intrinsic conductivity[32]. As shown in Fig. 2, two typical potassium storage mechanisms are specifically compared in terms of their potassium storage performance.
Many efforts have been devoted to the development and design of carbon structures, which have revealed the potassium storage mechanisms in different carbon structures and achieved excellent electrochemical performance. The electrochemical performance of carbon materials, including capacity, potential platform, rate capability, ICE and cycle stability is shown in Table 1.
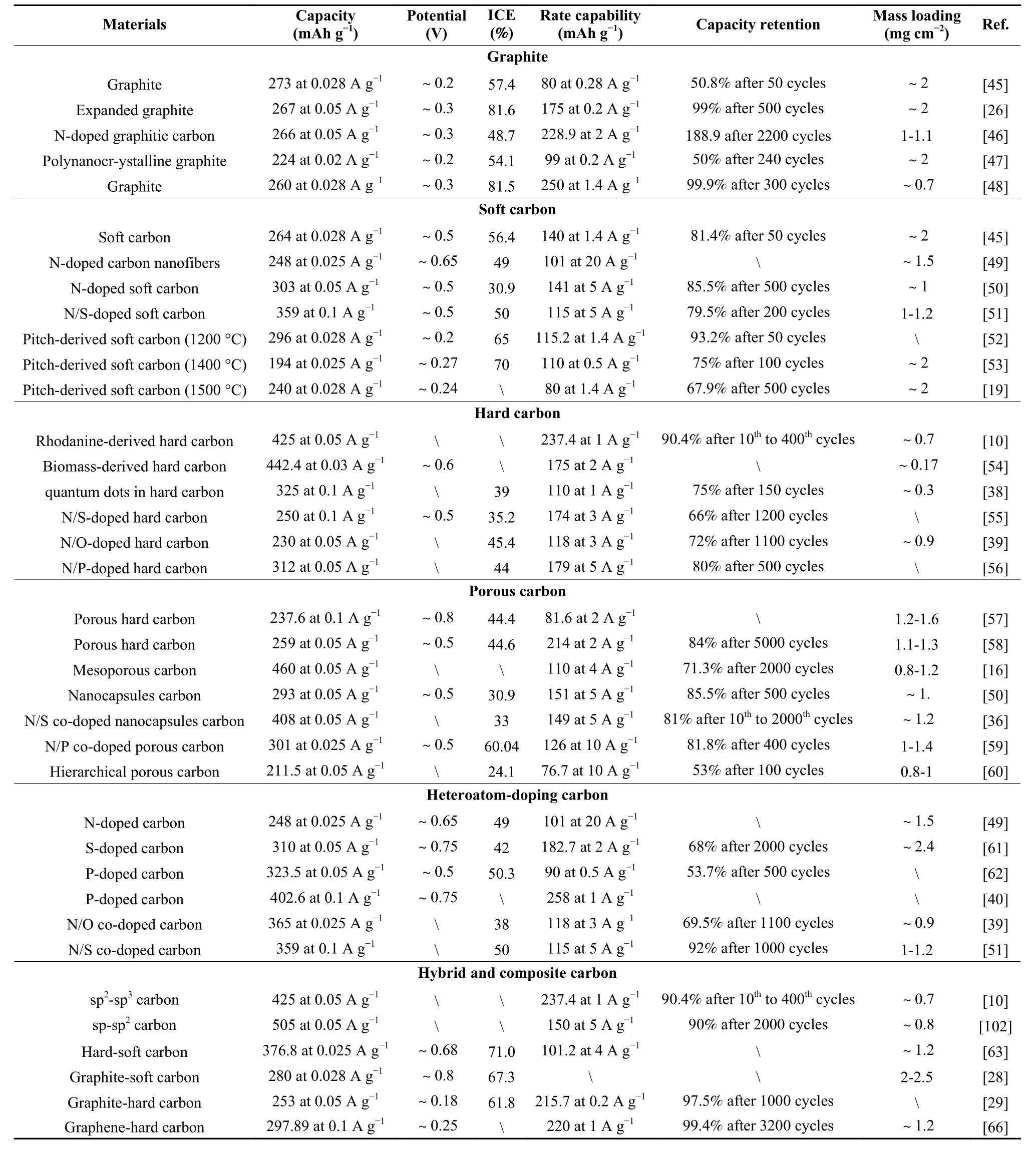
Table 1 A comparison of the K+ storage performance for different carbon materials.
3 Effect of carbon structure evolution on the potassium storage behavior
An in-depth understanding of the potassium storage mechanism at different active sites is necessary for designing material structures for performance requirements. Rationally controlling the intercalation and adsorption behavior of the carbon structures to achieve a balance of material properties is essential yet challenging to the reasonable allocation of the two mechanisms. The carbon structure evolves with the heat treatment temperature (HTT), which significantly affects the potassium storage behaviors[67]. For graphite, the structure stability and the kinetics during cycling can be boosted by adjusting the graphite interlayer distances, further enhancing the cyclic stability and the rate performance. For both hard and soft carbons, regulating the carbon structure by controlling the pyrolysis temperature is an effective way to improve the K+storage performance (Fig. 3). Generally,K+storage behavior exhibits a tendency from adsorption on carbon surfaces or edges at low HTT to intercalation in the carbon layers at high HTT. Correspondingly, the electrochemical properties of potassium storage also change with HTT, such as the capacity, rate performance, cycling stability, ICE and potential plateau of the carbon material.
3.1 Graphite
Graphite is composed of stacked graphene layers, which is a typical representative of the intercalation reaction in anode materials. K+can intercalate into the interlayers of graphite to form a series of intercalated compounds with the theoretical capacity of 279 mAh g−1[68]. Moreover, the formation sequence of these intercalated compounds is closely related to the process of potassium intercalation. Ji et al.[45]investigated the K+intercalation/deintercalation mechanism in the interlayers of graphite byex-situXRD,revealing the formation stage of KC36- KC24- KC8(Fig. 4a, 4b). On this basis, Fan et al.[69]discovered firstly the KC48peak at 23°/29° byin-situXRD,which clarifies the types of KCxintercalation compounds in the charge/discharge process (Fig. 4c, 4d).In addition, four peaks of 0.37 V, 0.34 V, 0.24 V and 0.16 V were identified, corresponding to the transition stage of KC48, KC36, KC24and KC8(Fig. 4e).
Graphite possesses a low potential plateau during charging/discharging. However, the large K+has sluggish kinetics and produces volume expansion ofca.61% when it is intercalated into the interlayers of graphite, which leads to serious capacity fading and low-rate performance[45]. To enhance the stability of graphite structures during intercalation/deintercalation of K+, a highly graphitic carbon nanocage (CNC)was designed by Cao and co-workers[64]. Benefiting from the hollow cage structure to buffer the volume change and reduce the K+diffusion length, CNC exhibited superior depotassiation capacity of 175 mAh g−1at 35 C (1 C = 279 mA g−1). Feng et al.[26]reported that commercial graphite (CG) had a discharge capacity of 202 mAh g−1and 60% ICE (Fig. 4f). The reversible capacity decreased from 169 to 61 mAh g−1after 200 cycles. Regulating the structure of graphite material alleviates the structural instability caused by K+intercalation. As shown in Fig. 2f, the expanded graphite (EG) with wide interlayer distances possessed a discharge capacity of 267 mAh g−1with a CE of 80.83% during the initial cycle. After 200 cycles,the reversible capacity remained at 228 mAh g−1with a capacity retention rate of 105.07%. Tai et al.[65]ex-panded the interlayer spacing from 0.334 to 0.358 nm by activating the graphite with KOH, resulting in a graphite electrode with a high reversible capacity of 100 mAh g−1in 100 cycles at 200 mA g−1. Moreover,Shen et al.[70]concluded that N-doping in graphite foam facilitated K+storage by theoretical calculations(Fig. 4g). Benefiting from more K+adsorption and faster charge transfer (Fig. 4h), capacity retention of 247 mAh g−1after 200 cycles was accomplished(Fig. 4i). The above methods can improve the diffusion kinetics of K+between graphite layers by optimizing the graphite structure, which leads to the graphite anode materials with superior electrochemical potassium storage performance.
3.2 Soft carbon
Soft carbon with a turbostratic structure that is convertible into graphite at high temperatures and is beneficial to K+storage due to the large interlayer spacing and multiple carbon edges[52,71]. The turbostratic carbon structure possesses a good arrangement of carbon layers, resulting in superior electrical conductivity[18]. Moreover, soft carbon can be converted into graphite after annealing at HTT higher than 2 500 °C,which is of significant value for studying the storage mechanism and electrochemical properties of K+through structural evolution. Zhang et al[53]. investigated the correlation between the order degree of soft carbon and the corresponding K+storage as shown in Fig. 5a. Two main K+storage mechanisms were identified in soft carbon, adsorption at carbon edge/defect sites and intercalation into the interlayers. On this basis, Lu et al.[19]determined that the capacity in the voltage range of 0.45-1.1 V originates from the K+adsorption at the edge/defect sites, while capacity below 0.45 V is attributed to the insertion of K+(Fig. 5b). In addition, the proportion of sloping regions gradually decreases with the increase of HTT, and plateau regions appear at 0.23 V at 1 500 °C. When HTT is further increased to 2 800 °C, the sloping region disappears and the charging/discharging potential platform is below 0.23 V (Fig. 5c). Fig. 5d shows the intercalation mechanism of K+in soft carbon[52]. With the beginning of the discharge process, the (002) peak disappears rapidly and the graphitization characteristics decrease due to K+intercalation. The preparation of soft carbon from pitch by carbonization at 1 200 °C(SC-1200) shows shoulder peaks with no impurity phase and the emergence of tiny KC8peaks at around 0.14 V. Furthermore, the electrochemical behavior of three typical samples (SC-1200, hard carbon and graphite) is compared, among which SC-1200 displays advantages as PIB anodes with a high reversible capacity of 296 mAh g−1and 93.2% of the initial capacity remained at 0.1 C (1 C = 279 mA g−1) after 50 cycles (Fig. 5e). Ou et al.[72]found that the potential plateau is closely correlated to non-uniformity in the interlayer distance and defect density in soft carbon (Fig. 5f). The widely distributed scattering peaks in the wide-angle X-ray scattering (WAXS) pattern(Fig. 5g) indicate that the non-uniformity of the interlayer distance is closely associated with the defects.With the increase of HTT, the non-uniformity in the interlayer distance decreases and the potential platform is enhanced. Moreover, as shown in Fig. 5h, a soft carbon with a higher defect density possesses a higher discharge potential platform as demonstrated by DFT calculation.
Regulating the carbon structure by adjusting the HTT is an effective way to reveal the intercalation/adsorption hybrid mechanisms of K+. The K+storage mechanisms of the carbon nanofibers (CNFs) were explored from 650, 1 250 to 2 800 °C using polyacrylonitrile as the precursor (Fig. 6a)[67]. As shown in Fig. 6b, partially ordered carbon displays intercalation and adsorption hybrid behavior of K+at 1 250 °C in contrast to the single potassium storage mechanism of disordered carbon treated at 600 °C and the intercalation mechansim of graphitic carbon treated at 2 800 °C. Thein-situRaman (Fig. 6c) andin-situXRD (Fig. 6d) spectra show that the peak of CNF-1250 is well maintained without significant positional shifts during discharging/charging process. In contrast, CNF-2800 displays a clear peak of the KC8compound (Fig. 6e). The above results indicate that the K+storage mechanism in CNF-650 is dominated by surface adsorption, and that in CNF-2800 is driven by interlayer intercalation. Based on this hybrid potassium storage mechanism, the authors further investigated the sensitivity of the anode material to the test temperature. When the temperature drops to 0 °C, the capacity retention of CNF-1250 is 79.7%, while that of CNF-650 and CNF-2800 is 75.2% and 60.8%(Fig. 6f), respectively, indicating that the hybrid mechanism is more insusceptible to the working temperature.
3.3 Hard carbon
The carbon microcrystals within the hard carbon possess a low accumulation of carbon lamella along the c-axis (Lc) and exhibit an overall random orientation arrangement[42]. As hard carbon materials are difficult to graphitize even above 2 500 °C, many nanographitic domains such as edges and defects exist in the hard carbon[14]. Thus, the potassium storage mechanism in hard carbon materials is complex. In this regard, many efforts have been contributed to exploring the relationship between the microstructures of hard carbon materials and potassium storage behaviors.Komaba et al.[73]directly observed the temperature-dependent microstructure of hard carbon and the corresponding selected area electron diffraction (SAED) using TEM (Fig. 7a, 7b). Compared with the HC700(hard carbon prepared at 700 °C) sample, the HC2000(hard carbon prepared at 2 000 °C) sample possesses advanced structural ordering and a relatively clearer diffraction ring. As shown in Fig. 7d, 7e,d002decreases from 0.402 to 0.364 nm andLcincreases fromca.0.7 to 1.3 nm in the HTT range of 700-2 000 °C.Simultaneously, the results reveal that the microstructure, morphology and elemental parameters of a hard carbon are strongly dependent on the HTT (Fig. 7c).Wang's work illustrated the relationship between the structure and potassium storage performance of hard carbon materials at different HTTs[74]. As the HTT rises from 1 000 to 1 600 °C, the plateau region capacity (II-region) increases from 92 to 165 mAh g−1(Fig. 7f). Meanwhile, the slope region capacity (I-region) decreases from 164 to 86 mAh g−1. Correspondingly, the oxidation peak varies from 0.32 to 0.27 V,and reduction peak varies from 0.20 to 0.14 V with increasing HTT (Fig. 7g). Thus, the dominant potassium storage mechanism of hard carbon gradually varies from a surface-driven to an intercalation-controlled one as HTT rises, which is ascribed to the production of micro-graphite structures. It's worth noting that the hard carbon at 1 200 °C (SP-HC 1200) possesses a capacity of 284 mAh g−1, which exceeds the theoretical capacity of graphite (279 mAh g−1). This is because K+not only intercalates/deintercalates in microcrystalline graphite, but also adsorbs/desorbs on its surface and edges. As shown in Fig. 7h, the adsorption-dominated slope region (I-region) delivers additional potassium storage capacity, leading to superior rate capacity and excellent cycling performance of hard carbon. Similar results were also obtained by Kim and co-workers[75], K half-cell delivered the obvious sloping region (above 0.25 V) and quasi-plateau region (0.25 V). From 1 000 to 1 500 °C, the slope capacity decreases and the plateau capacity increases for hard carbons (Fig. 7i). Moreover, Wen et al.[38]reported a carbon quantum dots@hard carbon (CQDHC)composite electrode material for PIBs (Fig. 7j). It is found that randomly distributed carbon quantum dots(CQDs) can boost the disorder extent, enhancing the capacitive storage process driven by adsorption on the hard carbon surface.
Anode materials with K+adsorption behavior generally possess a larger SSA and higher defects than intercalation/deintercalation ones. High SSA and defect-rich materials have higher capacity yet inevitably lower ICE[76]. Balancing the capacity and ICE is the key to overcoming this issue. Wang et al.[77]prepared carbon spheres with a nano-size and porous structure(SPCS) by the sol-gel method. SPCS displayed a higher SSA (398.85 m2g−1) compared to carbon spheres (CS, 76.02 m2g−1). However, SPCS with a high SSA delivers a higher ICE (68.2%) and capacity(232.6 mAh g−1) than CS with a lower ICE (60.3%)and capacity (121.5 mAh g−1). Mai et al.[2]developed a hard carbon with a high ICE by constructing interconnected mesoporous carbon (meso-C) nanowires. Compared with the SSA of micro-C (706.6 m2g−1), the SSA of meso-C (22.4 m2g−1) is reduced byca.32 times, which exhibited a high ICE of 76.7% and an excellent capacity of 231 mAh g−1simultaneously.The above works demonstrate that the improvement of both ICE and capacity can be achieved through the structural design of hard carbon materials.
4 Structure design strategies of carbon materials
Based on the understanding of the potassium storage mechanism of different active sites, the rational design of a carbon anode material can effectively improve its electrochemical performance[78]. This paper specifically discusses the design strategies of several carbon structures, including porous structure, heteroatom-doping, hybrid structure and composite structure.
4.1 Porous structure
Introducing pores into the carbon materials is an effective strategy to enhance the electrochemical performance of carbon anodes. The introduction of pores will create defects in the carbon materials, which can induce the local charge density rearrangement of the carbon network to enhance the potassium storage capability[79,80]. Generally, the carbon materials can be designed to obtain a rich pore structure to meet the potassium storage requirements[81]. Furthermore, many efforts have been contributed to the relationship between porous structures and K+storage behavior as well as electrochemical properties.
To reveal the K+storage mechanism in porous hard carbons (HCs), Li et al.[57]developed HCs with controlled micro/mesoporous structures by a templateassisted spray pyrolysis method (Fig. 8a). TEM images showed that the author successfully obtained three products, which were spherical carbon (HC-A,Fig. 8b), bowl-like carbon shell (HC-B, Fig. 8c) and flake-like composites (HC-C, Fig. 8d). Among them,HC-B possessed the largest pore volume (0.58 cm3g−1)and SSA (955.1 m2g−1) owing to the unique bowl-like structure, which contributed to the improved K+storage performance. Fig. 8g exhibits that the initial reversible capacities of HC-A, HC-B and HC-C are 78.6, 237.6 and 285.2 mAh g−1, with corresponding ICE of 27.7%, 44.4% and 47.2%, respectively. It is concluded that ICE strongly depends on the mesopore volume (Fig. 8f). There is no evident correlation between ICE and SSA, suggesting that the effective potassium storage active sites are on mesopore wall.What’s more, the authors provide insight into the K+storage mechanism byin-situRaman characterization(Fig. 8h). HC-B manifests two distinct regions at about 0.8 V on theID/IGratio curve. They correspond to two different K+storage mechanisms: surface-driven capacitive storage at high potentials and intercalation storage mechanism at low potentials. The capacitive contribution dominates throughout the cycle in HC-B as revealed by quantifying the capacity of the two different storage mechanisms (Fig. 8i). Meanwhile, the schematic diagram of the K+storage mechanisms in porous HCs are presented, including absorption on the active sites, trapping at micropores and intercalation between the graphene layers (Fig. 8j). Recently, Yuan et al.[58]also unraveled the correlation between micro/mesopores and K+migration behavior in HCs. It was confirmed that the micropores provided additional active sites for K+adsorption. A large number of mesopores could also provide pathways for K+intercalating, thereby improving ICE and intercalation capacity. Moreover, Zhang et al.[16]concluded that the introduction of mesopores can create a large number of defects, such as graphene edges and voids,which are active sites for K+storage.
The preparation methods of porous carbons are simple and diverse, which have promising applications in PIB anode materials[82]. The template method is a common method to obtain porous carbons due to its low cost, simple method and accurate adjustment[83,84]. As shown in Fig. 9a, Qiu group[36]synthesized 3D N/S co-doped carbon nanocapsules(NSCN) by ZnO templating. TEM image (Fig. 9b)confirms that NSCN possesses carbon nanocapsules with a 3D morphology. Moreover, the nanocapsule size can be varied by the sizes of the ZnO template.Similarly, Liu et al.[50]designed a carbon negative electrode of a nanocapsule-like shape (NSCN) by MgO templating, resulting in excellent rate performance(151 mAh g−1at 5 A g−1). In addition, metal organic frameworks (MOFs) have become effective precursors for porous carbons. For example, Liu et al.[82]developed N-rich hollow carbon-onion-constructed nanosheets (HCONs) from Co-hexamine coordination frameworks as high-performance potassium storage anode materials. However, all of the above hard templates require harmful acid solutions to etch away.NaCl templates avoid this problem by using water as the leaching solvent. Hence, Qiu and co-workers[59]prepared a N/P co-doped porous carbon (NPPC) by carbonizing the mixture of coal tar pitch and NaCl(Fig. 9c). As shown in Fig. 9d, NPPC has a pore structure composed of carbon sheets, which are distributed with a large number of mesopores of 10-30 nm. What’s more, our group[60]reported the successful preparation of a hierarchical porous carbon (HPC) including micropores, mesopores and macropores by multiple templates (PAANa, NaCl and Zn nanoparticles). HPC benefits from the hierarchical porous structure that accelerates electrolyte penetration and K+migration rate,achieving an excellent rate performance (76.7 mAh g−1at 10 A g−1).
Different from the hard template method, the self-template with specific morphology is formed during the polymerization process without use of any additional templates[85]. Recently, Song's group[114]constructed carbon nanotube microspheres (CNTMs) by one step injected pyrolysis using thiophene and ferrocene as both catalysts and carbon sources. Qiu et al.[86]synthesized nitrogen-doped hierarchical porous hollow carbon spheres (NHCSs) by polymerization of 3-aminophenol with formaldehyde followed by carbonization (Fig. 9e). NHCSs exhibit a monodisperse hollow spherical shell structure, which possess an average diameter of about 330 nm and shell thickness of about 50 nm (Fig. 9f). Further, Tao et al.[87]developed hollow carbon nanospheres (HCNs) that have a controllable cavity size and shell thickness by a self-templating method (Fig. 9g). The morphology of the precursor and cavity size are adjusted by changing the polymerization reaction time and the solvent used for sample washing (Fig. 9h).
4.2 Heteroatom-doping
Currently, heteroatom-doping is mainly implemented through the direct pyrolysis of organic precursors containing heteroatoms or the reaction of carbon materials with substances containing heteroatoms at high temperatures. In addition, the post-annealing in NH3, H2S and other gases is also widely used due to their simplicity and controllability. Heteroatomic doping can increase the defect sites in carbon materials,thus providing more potassium storage active sites,which is an effective method to improve the electrochemical performance of carbon materials[88]. The doped elements mainly include non-metallic elements such as nitrogen, sulfur, boron and phosphorus.
Among them, N-doping is one of the main research directions, and the deep insights in the potassium storage mechanism of N-doped carbons have been gained. Share et al.[89]have demonstrated that N-doping in graphite can increase the potassium storage active sites between layers, boosting from a theoretical capacity of 278 to over 350 mAh g−1. Moreover, most of the N-doped atoms are in the forms of pyridine N(N-6), pyridine N (N-5) and graphite N (N-Q), which are the different potassium storage sites[49]. Shen et al.[90]found that N-5 and N-6 can not only enhance the electrical conductivity but also increase the numbers of the defect sites and active sites. The adsorption energy (ΔE) of N-5 and N-6 are −2.63 and −2.86 eV, respectively as calculated by Discrete Fourier Transform DFT, which are much higher than that of N-Q(0.14 eV). This further indicates that the N-5 and N-6 dopings have significantly higher K+adsorption capacities than that of N-Q doping. On this basis, Zhang et al.[91]developed a highly nitrogen-doped (26.7 at.%)accordion-like carbon anode with a N-6 ratio of 48.4%, and a N-5 ratio of 43.7%. The results indicate that the dominant N-6 and N-5 dopings in anode materials lead to a high reversible capacity of 346 mAh g−1and superior cycling stability. Moreover, Guo and co-works[92]reported the preparation of a single pyrrole nitrogen configuration doped carbon material(SPNCM) to provide a guide for investigating the energy storage mechanism of N-doped carbon materials.Electrochemical test results show that more pseudocapacitance can be delivered with increasing the single pyrrole nitrogen content.
The use of large radius heteroatoms such as S[61,93]and P[40,62]to expand the carbon layer spacing for K+storage has also been widely reported. Zheng et al.[61]expanded the carbon layer spacing from 0.37 to 0.38 nm by preparing sulfur-doped multichannel carbon fiber (S-MCCF) composites. S-MCCF possesses super-rate capability (exhibits 182.7 mAh g−1at 2 A g−1), and long-cycle stability (maintains 150 mAh g−1after 2 000 cycles) as an anode material for PIBs. Furthermore, Li et al.[93]examined the storage mechanism of K+in S-doped carbon materials byex-situXPS at different charge/discharge depths during the first and second cycles. It is concluded that there is a strong binding force between C and S after doping. The C-S bond can react with K to form a C-SK bond, which displays a K+storage capacity higher than of KC8. By mixing red phosphorus with graphite,our group[62]successfully doped P into the carbon skeleton to form P-C and P-O-C bonds (Fig. 10a). As an anode material, both capacity (323.5 mAh g−1at 0.05 A g−1) (Fig. 10b) and rate performance(90 mAh g−1at 0.5 A g−1) (Fig. 10c) are enhanced as compared with a pure graphite electrode. On this basis, our group[40]further studied the effects of the chemical bonding states between P and carbon matrix on the K+storage performance (Fig. 10e) by compositing red phosphorus with different types of carbon nanotubes (Fig. 10d). Compared with the P-C bond(264 kJ mol−1), the P-O-C bond (585 kJ mol−1) displays a stronger binding energy. As a result, carbon anode with a P-O-C bond (P-CGCNT) performs an outstanding reversible capacity (402.6 mAh g−1over 110 cycles at 0.1 A g−1) (Fig. 10f) and excellent rate performance (258 mAh g−1at 1 A g−1) (Fig. 10g).
Due to the synergistic effect between two different heteroatoms, diatomic doping has also been shown to be a way of boosting the potassium storage performance for carbon anode materials[88,94]. Our group[39]produced a N/O co-doped graded porous carbon by carbonizing and acidizing the NH2-MIL-101(Al) precursor. Benefiting from the large layer spacing (0.39 nm), high SSA (1 030 m2g−1) and abundant pores, the anode material delivers a high reversible capacities of 365 mAh g−1at 25 mA g−1. But from a commercial perspective, it is necessary to develop a cost-effective and manipulable strategy. In this regard,Cui et al.[95]realized a N/O self-doping hard carbon(NOHC) by simply carbonizing sorghum stalks. As a PIB anode material, NOHC exhibits a large average interlayer spacing (0.411 nm), enabling a high reversible capacity (304.6 mAh g−1at 0.1 A g−1) and superior cycle stability (189.5 mAh g−1at 1 A g−1after 5 000 cycles). In recent years, N/S doping has received increasing attention due to the fact that it can simultaneously obtain excellent conductivity and significantly enlarge the interlayer spacing[96]. Liu et al.[51]prepared N/S double-doping porous soft carbon nanosheets(NSC) using coal tar pitch, urea and sublimed sulfur as the carbon precursors, nitrogen source and sulfur source, respectively. N/S double doping boosts the interlayer distance, electronic conductivity and charge storage active sites (Fig. 11a). As a result, the NSC accomplishes a high capacity (359 mAh g−1at 0.1 A g−1), a high rate (115 mAh g−1at 5.0 A g−1) and a long cycle life (92.4% capacity retention after 1 000 cycles at 1.0 A g−1). Moreover, our group[10]researched the effect of N and S elements on K+storage mechanism byex-situXPS (Fig. 11b, 11c). It is found that the content of N5 and N6 would gradually transform to NQ with increasing the pyrolysis temperature(Fig. 11b). N5 (ca.398.5 eV) shows high activity at 600 °C, and the peak values of NQ-K (ca.399.1 eV)and N6-K (ca.398 eV) reach the maximum values at 1 000 °C. As shown in the S 2p XPS spectra(Fig. 11c), initial S 2p1/2(165.0 eV) and S 2p3/2(163.8 eV) are converted to new peaks related to -K2Sx(162.5 and 161.5 eV) and thiosulfate/-K2Sx(ca.166.9 and 169.0 eV) during the discharge process. At the pyrolysis temperature of 1 000 °C, 66% of S is retained, in contrast to 600 and 800 °C where only 24%and 26% are retained during discharge and charge, respectively.
To sum up, various studies have shown that experimental parameters (such as the carbonization temperature) and heteroatomic precursors have a great influence on the content of the dopants. Generally speaking, the lower the carbonization temperature and the higher the heteroatomic content of the precursor,the higher the heteroatomic content of the products.At the same time, the type of heteroatom has a significant effect on K+storage performance. For example,Wang et al.[97]comprehensively compared the abilities of N, P and S dopants to capture K+in PIB anode materials by DFT calculation. Pyridine-N, pyrrole-N and P doping produce active sites in the carbon skeleton that are more inclined to capture K+. However,both graphite-N and sulfur doping show poorer affinities to K+.
4.3 Hybrid structure
Hybrid structures can enhance potassium storage performance by taking the advantage of different carbon structures. At present, many researches on carbon-based materials mainly focus on the sp2-sp3hybrid structure: sp2hybrid carbon represented by graphite and graphene, and diamond and diamond-like sp3hybrid carbon. Our group[10]reported the successful preparation of sp2-sp3hybrid carbon nanosheets(CNSs) by constructing an ordered graphite-like microcrystalline as conductive framework in defect-rich carbons (Fig. 12a, 12b). At low pyrolysis temperatures, massive heteroatom-defects are produced,providing active sites for K+storage, but resulting in many unstable sp3hybridization regions lack of electron transport capability (Fig. 12c). By adjusting the pyrolysis temperature, the C atoms are selectively rearranged, which in turn constructs ordered graphitelike microcrystalline conducting skeletons in disordered defects (Fig. 12d). As shown in Fig. 12e, the TEM image clearly shows the "Order in Disorder" of the carbon structure in the carbon material. The sp3defect region is surrounded by the sp2nanographene network, which achieves fast charge transfer kinetics while ensuring sufficient K+storage sites (Fig. 12g).This sp2-sp3hybridization region in CNS-1000 leads to superior rate capability (215 mAh g−1at 2 A g−1)(Fig. 12f) and long-term cyclic stability (maintain at 180.2 mAh g−1after 5 000 cycles). In addition, Song's group has achieved a series of hybrid carbons by a simple ball milling method[98-100]. Ball milling leads to the introduction of mass self-doping defects in the graphene flakes and edges, which serve as active sites for ion adsorption and storage. The defect structures are distributed in the conductive network formed by the sp2carbon in the sp2-sp3hybrid carbon. In conclusion, this sp2-sp3hybrid structure not only provides abundant energy storage active sites for the anode material, but also exhibits good charge transfer capability due to the interconnected sp2pathways surrounding the sp3structure.
Moreover, the sp hybrid carbon attracts considerable attention owing to the good electrical conductivity and affinity for metal atoms as compared with the sp2-sp3hybrid carbon[101]. In 2010, Li’s group[102]synthesized graphdiyne, a new carbon allotrope, by a chemical method for the first time. This new material possesses sp, sp2and sp3hybrid structures and is promising for potassium storage. Sun and co-workers[103]determined the adsorption sites and transport paths of storage K+in graphdiyne (GDY) by DFT calculation. It is found that K+can not only migrate within layers but also shuttle vertically between layers.The theoretical capacity of GDY for potassium storage (620 mAh g−1) is greatly increased compared to graphite (279 mAh g−1). In addition, the GDY interlayer spacing can be expanded fromca.0.37 to 0.41 nm by adjusting the annealing temperature to obtain superior rate performance (150 mAh g−1at 5 A g−1) and excellent cycling stability (over 90%after 2 000 cycles).
4.4 Composite structure
Composite materials, such as hard-soft carbon,graphite-soft carbon and graphite-hard carbon are promising for achieving the slope/plateau potassium storage. Wang and co-workers[63]synthesized the hard-soft carbon composite (Pi-700-P28) by carbonization of a mixture of polyimide and petroleum pitch at 700 °C (Fig. 13a). The XRD pattern in Fig. 13c exhibits that the Pi-700-P28 possesses good reversibility during charging/discharging. Moreover,ex-situRaman spectra (Fig. 13d, 13e) show a decrease inID/IGfrom 1.44 to 1.29 during the potassiation process, indicating that K+is absorbed onto the material defects.As shown in Fig. 13b, potassium storage in graphite region is restricted due to edge accumulation and local bending of the graphite layer during K+intercalation/deintercalation. Benefiting from the hybrid potassium storage behavior, the hard-soft carbon composite performs a high initial reversible capacity of 376.8 mAh g−1with an ICE of 71.04%. The (002)peak (Fig. 13g) andGpeak (Fig. 13h) of G-SC 3∶1 has fewer positional shifts than graphite after full potassiation, indicating that the introduction of soft carbon shows a significant positive effect on the structural stability of graphite. As a result, G-SC 3∶1 manifests the highest ICE of 67.3% whereas those of graphite and SC are only 54.1% and 57.8%, respectively (Fig. 13f). Meanwhile, compositing graphite with hard carbon has also been shown to improve the electrochemical performance of anode materials.Graphite-hard carbon composites were formed by coating disordered carbon nanosheets on graphite, resulting in flat discharge voltage platform and excellent cycle stability (215.7 mAh g−1after 1 000 cycles at 0.2 A g−1) and ICE (61.83%)[29]. Obviously, the potassium storage mechanism can be adjusted by combining different types of precursors to obtain a balanced performance of a carbon material with a high capacity, high ICE and good cycling stability.
5 Full device performance
The assembly of full device is particularly important in achieving the practical application of the anode material. Moreover, it is essential to guide the full device fabrication based on the structural and electrochemical relationships of the anode materials.Potassium storage devices can be divided into two types in full devices: potassium ion batteries (PIBs)and potassium ion hybrid capacitors (PIHCs). Here,we introduce the anode materials of the two potassium storage devices.
PIBs usually use carbonaceous materials (graphite, soft carbon, hard carbon, etc.) as the anode materials and K-containing compounds (Prussian blue analogs, layered metal oxides, polyanionic compounds,and organic crystals) as the cathode materials[104]. During charging, K+is extracted from the cathode into the electrolyte and migrates to the anode for intercalating.Correspondingly, K+inserted in the anode are extracted and reinserted into the cathode during discharge[105]. Lu's group[69]assembled a graphite/perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA) full cell using a concentrated electrolyte (KFSI: EMC, 1∶2.5,molar ratio) (Fig. 14a). Graphite/PTCDA delivers a cyclic stability of 92.9 % capacity retention after 50 cycles at 30 mA g−1(Fig. 14b) and a high-rate capability (from 80 mAh g−1at 10 mA g−1to 74 mAh g−1at 60 mA g−1) (Fig. 14c), based on the active materials in both anode and cathode. Similarly, Qin et al.[106]assembled a potassium ion full cell by matching the graphite anode with the potassium Prussian blue(KPB) cathode in a highly concentrated electrolyte(KFSI∶DME∶HFE, 1∶1.9∶0.95, molar ratio).The full cell shows a distinct voltage plateau in the voltage range of 2.0-4.0 V, achieving a high CE of 99.7% after 100 cycles. Xu et al.[49]reported a full cell coupling of the soft carbon (carbon nanofibers) anode and KPB cathode. The soft carbon/KPB full cell is tested in a voltage window of 2.0-4.2 V, showing an initial discharge capacity of 197 mAh g−1(based on the anode mass) and a 97% capacity retention(190 mAh g−1) after 30 cycles at 0.2 A g−1. Our group[10]assembled a hard carbon/KPB full cell using carbon nanosheets and KPB as the anode and cathode,respectively (Fig. 14d). The CE of the full cell remains above 96% after 20 cycles and the LED bulbs can be lit normally, indicating that the HC/KPB full cell works stably (Fig. 14e). Furthermore, replacing toxic metal compounds with graphite as cathode for potassium dual-ion batteries (PDIB)[107-110]has potential applications due to the fact that both of them are environmentally friendly and cost-effective carbon materials.
Potassium ion hybrid capacitors (PIHC) have attracted widespread attention because of the advantages of high energy density, high power and long cycle life compared to PIBs. Dual carbon PIHCs (DCPIHCs), in which both electrodes are composed of carbon materials, have been studied in activated carbon (AC) cathodes and various anodes (graphite, soft carbon, hard carbon, etc.). Graphite/AC PIHC was assembled with graphite as the anode for K+intercalation/deintercalation and AC as the cathode for K+adsorption/desorption. Passerini and co-workers[111]reported graphite/AC PIHC and sodium ion hybrid capacitor (SIHC) (Fig. 14f). The charge-discharge curves display a higher voltage plateau than conventional capacitors, resulting in higher energy density and power density (Fig. 14g). Fig. 14h shows the excellent cycling stability of graphite/AC PIHC and SIHC, which maintain 91% and 88% of initial capacity at 15 A g−1(based on graphite mass) after 5 000 cycles, respectively. Fan et al.[112]established a PIHC using a soft carbon as the anode and an AC as the cathode, which displays excellent energy density(120 Wh kg−1) and power density (599 W kg−1). To enhance the capacitive behavior of PIHC anode materials, more efforts are focused on the structural modification of soft carbon materials. Recently, Wu and co-workers[113]developed a novel accordion-like soft carbon structure with a hierarchical porous framework consisting of micro-, meso-, and macro-pores.Furthermore, the pseudocapacitance of the anode material is improved by electrodeposition to form nitrogen-doped graphene quantum dots. Ultimately, the PIHC is obtained by matching with a porous carbon(PC) cathode, achieving a superhigh energy and power density (171 Wh kg−1and 20 000 W kg−1).When a PIHC is designed, the hard carbon anode can provide high power density and superior cycling performance due to amorphous structures with large interlayer distances and high defect density. Cao et al.[44]assembled the PIHC using hard carbon as the anode and PC as the cathode, which manifests ultrahigh cycle performance (89.2% capacity retention after 6 000 cycles) and high energy/power density(120.2 Wh kg−1and 16 700 W kg−1).
In summary, the intercalation-dominated carbon materials can be designed as anode materials for potassium ion full cells, which is ascribed to the low and stable intercalation potential platform that can provide a high energy density and stable working conditions for potassium ion full cells. In contrast, carbon materials with surface-driven K+storage mechanism are more suitable for the development of anode materials for high power density potassium ion capacitors due to their faster ion transport and more active sites.
6 Conclusion and perspectives
At present, carbons are considered as important potassium storage materials due to their advantages of low cost, good electrical conductivity, controlled surface chemistry and structure. From the recent progress of various carbon materials in PIBs, it is found that the potassium storage capacity of the carbon materials is mainly related to K+transfer, electron transport and active sites. In this review, we introduced the effects of different active sites on potassium storage mechanisms. Graphite and graphite-like materials are dominated by K+interlayer storage with low potential plateaus. However, the serious volume expansion and kinetic hysteresis in graphite material result in poor cycle stability, capacity fading and low-rate performance. Soft carbon with a turbostratic structure can enhance K+adsorption at the edge of carbon sheets,which alleviates structural instability during the charging and discharging. In addition, soft carbon can be converted to graphite after annealing at temperatures higher than 2 500 °C, which is valuable for studying the relationship between carbon structure and electrochemical properties due to structure tuning by the annealing temperature. Hard carbon with abundant carbon defects and large SSA boosts the surface-driven K+adsorption, leading to high capacity and excellent rate performance. However, the low ICE and the sloping curve limit their practical applications. Therefore,it is essential to consider the trade-off between intercalation and surface-driven mechanisms when carbon structures are designed.
On this basis, we have systematically discussed the rational design strategies of carbon anodes such as heteroatom doping, pore generation and hybridization of sp, sp2and sp3. The introduction of pore structures in carbon materials can increase the surface area, accelerate the electrode/electrolyte contact, and shorten the diffusion distance of ions/electrons. Heteroatom doping can enhance the defective sites within the carbon material, thus providing more active sites for energy storage. It is found that the co-doping of different heteroatoms could improve the electrochemical properties of carbon anode materials by synergistic effects. Hybrid carbon materials possess excellent potassium storage performance through the combination of sp, sp2and sp3carbons. The composite structure can adjust the K+storage mechanism by combining different types of precursors, resulting in anode materials with balanced potassium storage properties.Moreover, the assembly of potassium storage full devices is reviewed according to the structure and electrochemical relationship of advanced anode materials.
Despite considerable progress has been made to date, the following challenges and opportunities may exist as the researches on carbon-based anodes progress.
(1) When advanced carbon anode materials are designed, the comprehensive properties of potassium storage should be considered. The research of electrochemical potassium storage in carbon materials is mainly in two directions: low potential and high capacity/high rate. The existing carbon anode materials have problems in balancing low potential potassium storage and high capacity/high rate performance, and further development is needed to optimize the carbon structure.
(2) Graphite has been attracting much attention as the most mature anode electrode for commercial batteries. However, the intercalating of large radius K+causes the collapse of the graphite structure during cycling. In order to improve the electrochemical properties of graphite in K+storage, many efforts have been made such as electrolyte development, electrolyte formulation optimization and morphology design of electrodes. Yet the cycle stability of the optimized graphite anode still does not meet the requirements for practical applications.
(3) For PIBs, the mechanisms of potassium storage in graphite and soft carbon have been well investigated. However, the complexity and uncertainty of the hard carbon structure result in controversy of the electrochemical mechanism, which requires further studies on the storage behavior of K+in hard carbon.In addition, the control of graphitized microdomains,defects and nanopores in hard carbon is critically important for potassium storage. Thus, it is urgent to understand the relevant mechanism driving the rational design of hard carbon.
(4) In the implementation of the whole device,the anode and cathode need to be matched thermodynamically and kinetically. Moreover, a clear understanding of the reaction mechanism for the electrodes is required to give full play to the high-performance potassium storage advantages of the electrode materials. And the mass of the electrode materials needs to be carefully calculated and matched.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (21975283), China Postdoctoral Science Foundation (2020M681762),State Key Laboratory of Chemistry and Utilization of Carbon-based Energy Resource (KFKT2021007), and CAS Key Laboratory of Carbon Materials (KLCMKFJJ2010).
