Research progress on recovering the components of spent Li-ion batteries
2022-06-13GAOShaojunLIUWeifengFUDongjuLIUXuguang
GAO Shao-jun, LIU Wei-feng,, FU Dong-ju, LIU Xu-guang
(1. Institute of New Carbon Materials, Taiyuan University of Technology, Taiyuan 030024, China;
2. College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China;
3. Research Institute of Tsinghua University in Shenzhen, Shenzhen 518057, China)
Abstract: With the recent rapid development of electric vehicles, the use and decommissioning of Li-ion batteries have increased,causing environmental pollution and the waste of valuable materials in spent batteries. Commercial Li-ion batteries are mostly composed of transition metal oxide or phosphate-based cathodes, graphite-based anodes, organic electrolytes containing harmful lithium salts, polymer separators, and plastic or metal shells. After the battery is retired, many precious metals and graphite have a high recycling value. We review the current status of research on recovering these components with an emphasis on the leaching and separation of cathode and anode materials, and electrolytes in these batteries. The problems encountered in the different methods are outlined in terms of recycling cost and secondary pollution. Future research trends are outlined for the commercial full recovery of spent Li-ion batteries.
Key words: Spent Li-ion battery;Graphite;Full composition;Recycling
1 Introduction
In recent years, the demand for diminishing traditional energy sources has imposed great impact on social development and human life. Therefore, changing the current energy consumption structure and developing new energy technologies have become the current research hotspots. Developing hig-quality green energy storage devices has become an urgent issue. Traditional lead-acid batteries, nickel-chromium batteries, alkaline batteries, and nickel-hydrogen batteries can no longer meet the needs of ever-increasing energy storage due to their limited energy density.The early rechargeable lithium-ion battery (LIB) model, which was constructed by Akira Yoshino in 1986[1,2], is different from the previous lithium batteries. The anode of LIBs is carbon material and the cathode is lithium cobaltate, both of which are of intercalation type. During the charging and discharging processes, lithium ions are repeatedly embedded and de-embedded between the cathode and anode. This rocking chair-like behavior earns LIBs the nickname“rocking chair battery”. In 1991, Japan’s Sony launched a LiCoO2/graphite anode system of LIBs,which in a real sense realized the industrialization and commercialization of LIBs, marking the LIBs into daily life. With the advantages of high energy density,high average output voltage (~3.6 V), high output power, low self-discharge, no memory effect, wide operating temperature range, and long service life[3-6],LIBs are now widely used in the 3C consumer electronics such as cell phones and electric vehicles.
With the increase in fuel prices and the guidance of policy orientations, the number of electric vehicles worldwide has continued to increase sharply. From Fig. 1[7]that shows the changes in the number of electric vehicles in various countries in recent years according to the statistics of relevant organizations, it is obvious that since 2016, the global electric vehicle inventory and electric vehicle registrations have been growing rapidly, especially in China and the United States. This also means that the demand for LIBs continues to increase. In 2019, the global production of LIBs is about 200 GWh, while in 2030 the global demand for LIBs increases almost tenfold to about 2 000 GWh[8]. LIBs need to be replaced or disposed of when their state of health (SOH) is depleted to 70%-80% of the initial capacity. Lithium iron phosphate battery can be charged 3 500 times before it decays to 80% of the limit of forced replacement. It is estimated that it can be used for more than 10 years. The ternary LIBs has been attenuated to the limit value after charged about 2 000 times, which can only be used for about 6 years. Therefore, in the next few years, a big challenge facing us would be the problem of processing a large number of spent LIBs[9,10]. According to the current installed capacity of LIBs, as of 2023, the scrap volume of lithium battery cathode materials in China will exceed 100 GWh for the first time[11]. It should be awared of the impact of a large number of spent LIBs on resource conservation and environmental protection, and how to realize the whole process, environmental protection, safety, and high-value disposal of spent LIBs.
LIBs are composed of the anode, cathode, electrolyte, separator, and shell. The cathode consists of active material and polymer binder, which are loaded on an aluminum foil. The active material includes lithium transition metal oxides (LiCoO2, LiNiO2,LiMn2O4, LiNixCoyMnzO2(LNCM)), phosphates,silicates, borates and sulfates, etc. The anode is also composed of active material and polymer binder,which are coated on a copper foil. Graphite is basically used as the anode material for commercial LIBs,while the binder is typically polyvinylidene fluoride(PVDF). The electrolytes are mostly organic solutions, such as lithium hexafluorophosphate (LiPF6),diethyl carbonate (DEC), ethylene carbonate (EC), dimethyl carbonate (DMC), and methyl ethyl carbonate(EMC). The separator is a polymer material, and the shell is usually made by stainless steel or plastic.
The heavy metals as well as organic electrolytes in spent LIBs are toxic and prone to cause serious environmental pollution (such as dust contamination[12]),heavy metal contamination, fluorine contamination,and organic contamination if spent LIBs are disposed of with simple or inappropriate disposal methods (e.g.,landfills)[13]. On the other hand, studies have shown that decommissioned LIBs contain on average 4.0%Co, 2.4% Ni, 8.8% Mn, 1.4% Li, 4.3% Al, 5.7% Cu,and 22.4% Fe[14,15], among which the content of Li and Co is much higher than that in ores and thus of high potential value with relatively high prices and high recovery value. Therefore, from the perspective of environmental protection and resource conservation, the recycling of spent LIBs is necessary and urgent.
In this article, the composition, structure, and working principle of LIBs and the recovery progress of spent LIBs are reviewed. The recycling of spent LIBs is combed in detail, including pretreatment technology, advanced treatment of cathode materials(leaching, separation and purification of valuable metals), advanced recovery of anode materials (recovery of graphite and residual lithium), and recovery of electrolytes. A series of prospects for the future development direction are put forward.
2 Working principle and structure of LIBs
LIBs can be categorized into the cylindrical shape, coin shape, square shape, and soft bag shape according to their shape and specification needs.When LIBs work, driven by electrical and chemical signals, Li+shuttles between the anode and cathode,causing the mutual conversion of electrical energy and chemical energy, and realizing energy storage and release. In this process, the anode and cathode materials are the main reaction sites for Li+, i.e., the cathode material often carries the original lithium source,while the anode material is waiting for it. During the charging and discharging processes, the freely moving Li+is the active component of the reaction. The electrolyte provides a medium for the movement of Li+, and a high-quality electrolyte generally needs to have good conductivity, high fluidity, and high pressure resistance. The separator is used to avoid self-discharge or even short-circuit, so that the entire electrochemical process is stable and continuous. Meanwhile, the separator must selectively allow Li+to shuttle freely in order to successfully complete a series of electrode reactions. Therefore, it is an ion conductor and an electronic insulator. The external packaging shell is a protective device for the inner battery, which not only isolates internal and external contact, but also resists a part of external squeeze, collision, puncture, and other physical damage.
2.1 Working principle of LIBs
The work of LIBs mainly relies on the conversion between chemical and electrical energy, which is achieved between the anode and cathode through the migration of lithium ions in the electrolyte and the transmission of electrons in the external circuit[16]. The specific working principle is shown in Fig. 2, taking the common LiCoO2‖ polymer electrolyte ‖ graphite system as an example. During charging, lithium ions are extracted from the crystal lattice of the cathode material LiCoO2and embedded in the anode material graphite through the electrolyte. At the same time,electrons flow from the cathode to the anode along the external circuit. The cathode potential gradually rises,the anode potential gradually decreases, and the battery potential becomes higher. During the discharge process, lithium ions and electrons move in opposite directions. Lithium ions are extracted from graphite,pass through the electrolyte, and are embedded in Li-CoO2lattice. Electrons flow from the anode to the cathode along the external circuit, generating electric current and converting chemical energy into electrical energy[17].
The chemical reaction during charging and discharging of LIBs can be expressed as follows:
Charging:
In the formula, M is Ni, Co, Mn, Fe or several metal composites.
2.2 Composition and structure of LIBs
The components and the weight distribution of each part of LIBs are shown in Fig. 3[18]. The known high-performance LIBs cathode materials can be divided into three categories according to the crystal structure: the polyanionic, spinel and layered. In addition to the classic LiNiO2and LiCoO2cathode materials, some derivatives of LiMO2cathode materials have been developed, i.e., Ni, Mn and Al are used to replace some of the cobalt in LiCoO2cathode materials to form new ternary cathode materials. The spinel structure is usually denoted by AB2O4, mainly represented by LiMn2O4and the materials modified through element doping and surface modification. The polyanionic cathode material is a three-dimensional frame structure composed of transition metal and polyanion(XO4)n−, and common polyanionic cathode materials include phosphates, silicates, borates, and sulfates,etc[19]. The current usage of major electric vehicles is shown in Table 1[20]. The two types of batteries commonly used in commercial LIBs are those with ternary layered cathode materials and lithium iron phosphate cathode materials.
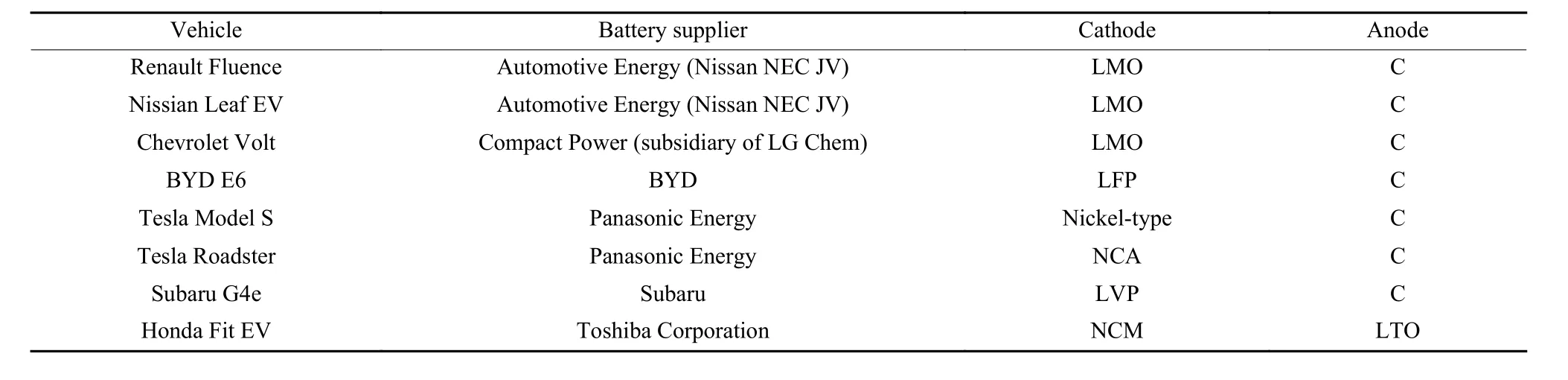
Table 1 Usage of various electric vehicle cathode and anode materials.
As can be seen from Table 1, the anode materials of current commercialized LIBs are basically graphite. Graphite has abundant reserves, low cost,mature processing technology, and has excellent cycle stability when used as an anode material, which makes it the most widely used negative electrode material at present[21]. However, with the widespread applications of LIBs, the performance requirements of LIBs are increasing, and the energy density of graphite anodes gradually appears to be limiting. Therefore,many anode materials with higher energy density have been proposed. According to the electrochemical reaction mechanism, the current anode materials are mainly divided into three categories, namely, embedded, alloy, and conversion materials[22,23]. Embedded anode materials are mainly referred to as carbon materials, especially graphite. Conversion anode materials mainly include metal oxides, phosphides, sulfides,etc. Alloyed anode materials mainly include Si, Zn,Sn, Ge, P and Sb[24]. However, most of the latter two classes of materials have not been commercialized.Therefore, most of current commercial anode materials are still graphite, as shown in Table 1.
The electrolytes of LIBs are mostly organic solvents containing lithium salts, and currently used lithium salts include LiClO4, LiBF4, LiAsF6, LiPF6,LiCF3SO3, Li [N(CF3SO2)2], etc[25]. Organic solvents are usually EC, DMC, propylene carbonate (PC), and dimethyl sulfoxide (DMSO) with high dielectric constant and low viscosity. The separator is usually a multilayer polymer such as microporous polypropylene (PP) or polyethylene (PE). The LIB shell is mainly made of iron, aluminum, and flexible packaging aluminum-plastic film.
3 Recycling of spent LIBs
Spent LIBs contain a large amount of rare metals and precious metals, and their unreasonable disposal not only seriously pollutes the environment, but also causes a great waste of resources. The cathode materials contain a large amount of cobalt, nickel, manganese, and other toxic heavy metals. Especially, the heavy metal cobalt is highly permeable and can easily enter the inner layer of the skin, resulting in polycythemia, and is likely to cause lung and gastrointestinal lesions, which is carcinogenic[26]. In addition, the lithium salt, organic solvent, and organic separator contained in the electrolyte are prone to serious pollution. LiPF6contained in the electrolyte is highly corrosive and will decompose to produce highly toxic HF when it meets water and produce P2O5when it burns,which will pollute the environment. The organic solvents are highly volatile and difficult to degrade in nature, which will easily pollute the atmosphere, soil and water resources. In Table 2, the hazardous components and their hazards in the main components of LIBs are summarized[27-29].
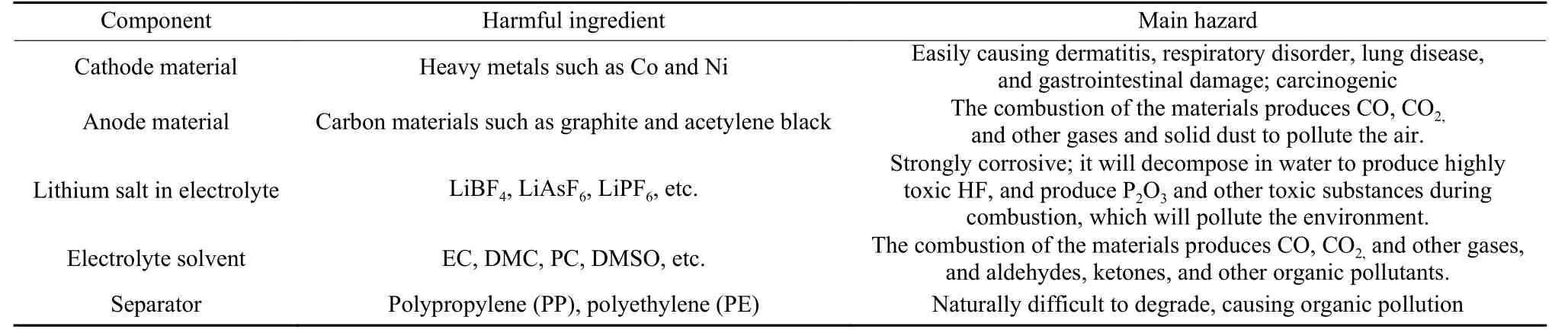
Table 2 Harmful components in the main components of LIBs and their hazards.
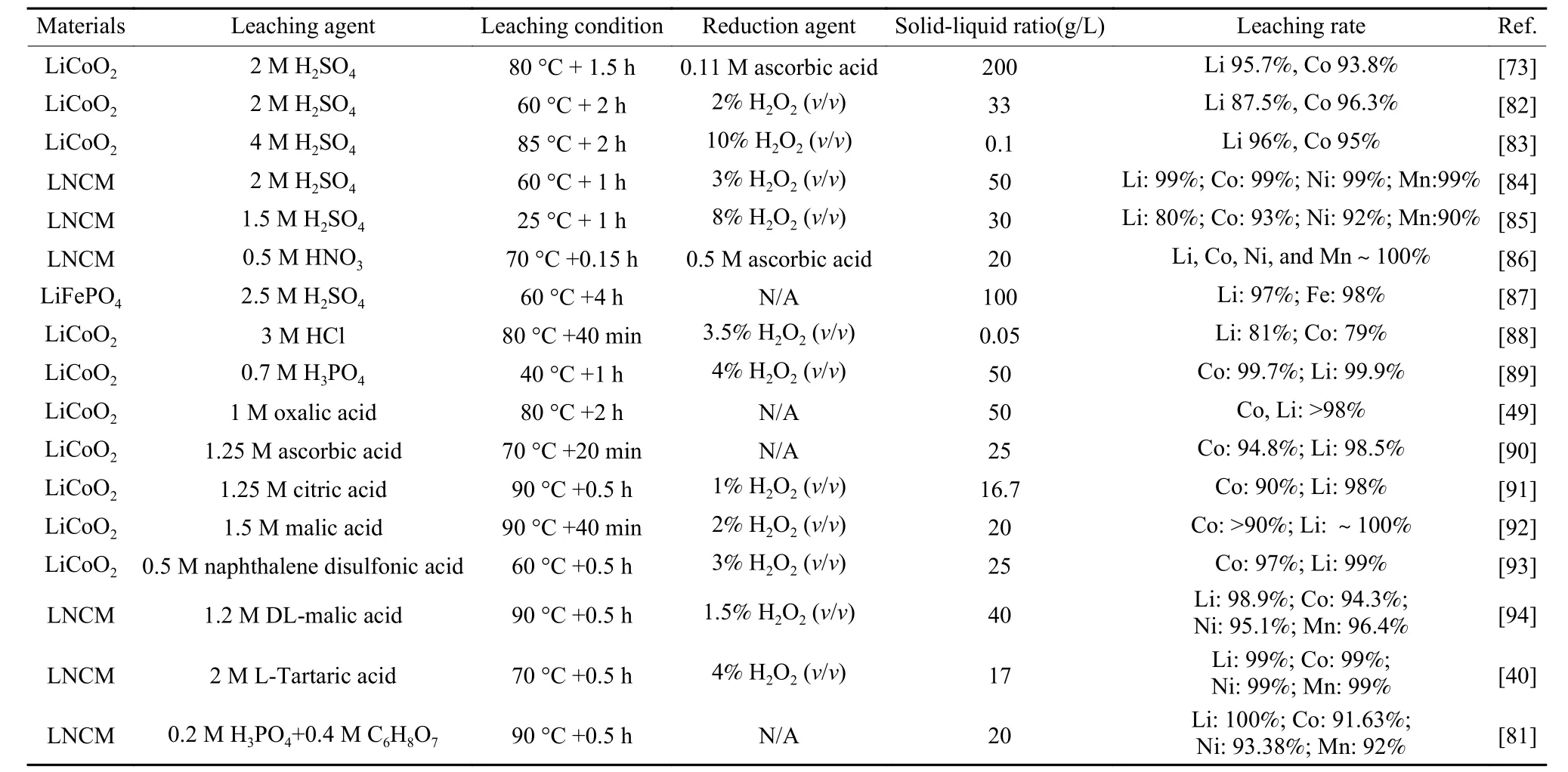
Table 3 Summary of the research status of leaching spent LIB cathode materials with acids as the leaching agents.
Research shows (Fig. 4(a)) that on average, LIBs of waste electric vehicles contain 4.0% Co, 2.4% Ni,8.8% Mn, 1.4% Li, 4.3% Al, 5.7% Cu and 22.4%Fe[30]. Especially, the content of Li and Co is much higher than that in ores, thus showing high potential value with relatively high prices and high recovery value. According to the United States Geological Survey 2019 (USGS), in 2018, the mineral resources of cobalt, nickel, manganese, lithium, and copper in China accounted for 1.16%, 3.15%, 7.11%, 7.14% and 3.13% of the global reserves, respectively[31]. And the contradiction between the supply and demand of the metals required for LIBs production has become more and more prominent in China. Cobalt metal, in particular, is not only one of the most important raw materials for the production of cathode materials for LIBs,but also an indispensable used in various industries. In 2018, global cobalt mineral resources were about 6.9 million tons, with a production capacity of about 140 000 tons, and China's production capacity was only 3 100 tons[32]. As the largest consumer of cobalt in the world, China is extremely dependent on foreign imports for cobalt resources, and about 80% of cobalt is used in the LIBs industry[33]. In addition, China is also heavily dependent on imports for lithium consumption. China consumes about 50% of the world's annual lithium production, but produces only 7% of the global lithium each year[34]. With the rapid development of China's electric vehicle industry in recent years, the demand for battery raw materials such as lithium, cobalt, nickel, and manganese will continue to increase, further exacerbating the gap between the supply and demand for these metals. The contents of lithium, cobalt, nickel, and manganese in spent LIBs are much higher than that of general ores. Therefore,recycling of spent LIBs can effectively alleviate the shortage of these metal resources.
Research on the recycling of LIBs has been ongoing since the introduction of LIBs by Sony in the 1990s. In 1995, with the funding from the French Environment Agency, Recupyl established the first global research pilot on the recycling of LIBs[35]. Since then, commercial and research groups have focused on increasing recycling capacity through the use of more efficient recycling processes. Currently, the companies on researching the recycling of used LIBs are located worldwide (Fig. 4(b))[25]. At the same time,China has issued a series of policies for the recycling of spent LIBs. In 2016, the Ministry of Ecology and Environment's “Technical Policy for Pollution Prevention and Control of Waste Batteries” and the General Office of the National Development and Reform Commission's “Producer Responsibility Extension System Implementation Plan” were successively introduced. In 2018, China promulgated the “Interim Measures for the Administration of Recycling and Utilization of Power Batteries for New Energy Vehicles”, which provides an overall policy orientation for the development of my country's battery recycling industry.
In the face of the increasing number of spent LIBs with great potential value, traditional centralized landfill and direct incineration processes not only cause great waste of resources, but also lead to serious pollution of land, air, and water resources. The current recycling process of LIBs is to disassemble spent batteries into different parts according to their compositions, and then separate and recover the components and reuse them to produce useful materials or batteries. The flow chart of recycling of spent LIBs is shown in Fig. 5.
3.1 Pre-discharge treatment of spent LIBs
The residual voltage of spent LIBs is usually around 4 V. Therefore, on the basis of safety considerations, waste batteries first need to be deeply pre-discharged to prevent their short circuit from causing spontaneous combustion or explosion. The current discharging methods are mainly physical and chemical ones.
The physical method mainly includes short-circuit discharge, low-temperature discharge, and thermal discharge. The short-circuit method is to connect a resistor to both ends of LIBs and use the process of short-circuiting the battery to release heat to consume power. This method is widely used in small batteries, and the discharge speed is fast. Conductive powders such as graphite and copper have also been used to discharge used LIBs, and the results show that the mixing of spent LIBs and graphite powder can allow the short circuit of anode and cathode. Ku et al.[36]used conductive metal powders and graphite to discharge spent batteries. Although this method requires much less discharge time, it generates a lot of heat and the temperature of the battery cells rises rapidly,which may cause explosive hazard. Low-temperature discharge involves freezing used LIBs in an ultra-low temperature environment to deactivate them. Some researchers[37,38]used liquid nitrogen to create a lowtemperature environment where used LIBs are placed at −198 °C to crystallize the electrolyte and the LIBs become non-conductive, thus temporarily deactivating the used LIBs, which has the same effect as discharge. The low temperature cooling makes lithium inert and eliminates the risk of fire caused by oxidation of elemental lithium. This method is suitable for the treatment of large capacity batteries and capable of treating a large number of used LIBs simultaneously.Thermal discharge[39]is generally achieved by thermal deactivation. Accurec process uses vacuum heat recovery (VTR) to discharge spent LIBs. In this process,organic components (electrolyte, plastic, and adhesive) are removed by pyrolysis. Thermal discharge has the advantages of safety and low pollution, and is expected to realize industrialization.
The chemical method involves immersing spent LIBs in salt-containing electrolyte solutions such as NaCl and Na2SO4to deeply release the residual charge until the voltage is less than 2.0 V[36,40-42]. Discharging using salt solutions is not only more efficient but also safer as it can absorb a large amount of heat generated during battery discharge[43]. Zhang et al.[44]showed that waste batteries could be completely discharged within 24 h in 5% NaCl solution. Other studies have shown that the addition of ascorbic acid accelerate the discharge process. Song et al.[45]used manganese sulfate electrolyte as the discharge system to study the discharge efficiency of batteries under different external conditions. The experimental results showed that under the conditions of electrolyte concentration of 0.8 mol·L−1, temperature of 80 °C,pH of 2.78, and ascorbic acid concentration of 2 g·L−1,the battery voltage can be reduced to 0.54 V after 8 h of discharge. However, ascorbic acid is expensive and not available for use in industry. Acidic and alkaline solutions have also been proved to be useful for the discharge of spent LIBs. However, due to the corrosivity of acid-base solution, the battery shell is easy to be damaged, resulting in electrolyte leakage, and consequently environmental pollution. Therefore, during discharging with acid-base solutions, attention should be paid to control the concentration to avoid damage to spent battery.
3.2 Disassembly of spent LIBs and separation of each component
Since LIBs have a complex structure and consist of many components, it is usually necessary to disassemble and separate them to remove the shell first,and then to enrich the valuable components. After the deep discharge of spent LIBs, manual disassembly or mechanical crushing is generally used to separate the components of the battery. Manual disassembly is mainly used in laboratory research, while mechanical separation is commonly used in industry to separate the battery into the cathode, anode, separator, and plastic or metal shell.
Mechanical crushing method refers to the use of mechanical force to crush and remove the shell of LIBs, and a combination of gravity sorting and electromagnetic sorting processes to separate the battery components for the purpose of resource recovery.Zhang et al.[46]crushed and screened the cathode and anode pieces of used LIBs, and found that different components would be enriched in different particle sizes, with aluminum foil, copper foil, plastic and diaphragm enriched in 1.4 mm particle size products,while the cathode and anode active materials concentrated in 0.2 mm particle size products. Bi et al.[47]used a combination of electrical sorting and magnetic flotation to achieve a separation efficiency to more than 85% for copper foil shreds and aluminum foil shreds in battery crushing products. Yu et al.[39]used a Falcon centrifugal separator to separate LiCoO2and graphite, and experimentally demonstrated that with a water counter pressure of 0.025 MPa and a rotational speed of 50.00 Hz, the best separation effect can be obtained. After mechanical processing, the density and size of LiCoO2particles are larger than those of graphite particles. As shown in Fig. 6(a), the high density particles are deposited into the centrifuge separation tank through the bed and form the bottom flow, while the low density particles are subjected to less centrifugal force, thus achieving the sorting effect.
Though the separation between different components of spent LIBs can be achieved by mechanical crushing and sorting, the separation effect is poor as a result of the complexity of the battery components,which interfere with each other during the mechanical treatment. Other technical means are still needed to achieve the separation between the cathode and anode active materials and the collector fluid.
High temperature pyrolysis mainly removes organic impurities such as the binder, conductive carbon and electrolyte from the enriched components. At high temperature, the organic matter decomposes, resulting in the disappearance of the binding force between binder and active materials. In the process of high temperature pyrolysis, the binder PVDF decomposes at about 400 °C, while graphite is oxidized at 500-600 °C. Vacuum heat treatment or reduction heat treatment not only burns off the organic binder, but also reduces the high-valent transition metal ions in the cathode material, which facilitates the leaching of valuable metals in the subsequent recovery process.Yao et al.[48]pyrolyzed the battery fragments at 600 °C for 2 h, followed by cooling to room temperature, and the cathode active material was easily separated from the metal aluminum foil. The conventional heat treatment method is simple to operate. However, this method releases fluorine-containing gas from organic binder PVDF and produces carbon monoxide from conductive carbon, which do harm to the environment and human beings. Therefore, it is not suitable for industrialization. Sun et al.[49]proposed a novel vacuum pyrolysis method. Under 600 °C and 1.0 kPa atmospheric pressure, vacuum pyrolysis of battery fragments for 30 min realized the complete stripping of the active material from Al foil, of which the main components are LiCoO2and cobalt oxide (CoO). Vacuum pyrolysis can not only effectively separate the active substances, but also recover the organic binder and electrolyte. Therefore, it is expected to separate and recover the whole components of the battery in an environment-friendly situation. However, the operating conditions are relatively complex, and great effort is still needed to realize industrial applications. In addition, vacuum pyrolysis requires high equipment requirements and processing costs.
For the anode material, the adhesive PVDF exists between the copper foil of LIB anode and the active material. Heat treatment is adopted to place the anode of used LIBs in a certain high temperature interval to allow the adhesive to volatilize or decompose,so that the copper foil collector fluid can be separated from the graphite powder of the anode active material.
Hanisch et al.[50]dried the electrode material at 150 °C for 12 h and calcined it in a muffle furnace at 500 °C for 15 min, and the final experimental results showed that the recovery of the active material of the latter was 99.5%, which was significantly higher than that of the former (80%). Chen et al.[51]heated the spent LIBs at 300 °C for 60 min to remove PVDF, separated the cathode active material and aluminum foil by crushing and sieving, and then calcined in a muffle furnace at 550 °C for 30 min. In the TG-DSC curve shown in Fig. 6(b), there is a significant weight loss around 520 °C, which indicates the rapid decomposition of PVDF and carbon.
The dissolution method to separate impurities is based on the principle of similar phase solubility, using a more polar organic solvent to dissolve the binder PVDF in the battery, usually N-methylpyrrolidone(NMP), N-dimethylacetamide (DMAC), N-dimethylformamide (DMF), and dimethyl sulfoxide(DMSO)[52]. Li et al.[53]immersed the cathode material at 100 °C for 1 h, easily peeling off the cathode active material from the aluminum foil. Yang et al.[54]used ultrasound-assisted NMP dissolution, immersed the cathode material in NMP, and sonicated it for 3 min at room temperature to separate the cathode active material from the aluminum collector, with a separation efficiency of 99%. The results showed that ultrasonic treatment was helpful to improve the separation efficiency of cathode active materials. The method of ultrasonic assisted solvent dissolution separation of positive active material and aluminum foil is feasible, but there are still some problems such as incomplete removal of residual impurities, which need further treatment for complete separation. Therefore,this method is still far from the practical large-scale application. Studies have shown that ionic liquids(ILs) can significantly improve the separation efficiency. Zeng et al.[55]studied the dissolution of PVDF at 180 °C and 300 r min−1by using ionic liquids. The dissolution rate of PVDF reached 99%. The addition of ILs is conducive to improving the separation efficiency. However, ILs are usually expensive and difficult to recover, which makes them difficult to be applied in industry. Wang et al.[56]used deep eutectic solvents (DESs) instead of ordinary solvents to separate active substances and achieved good results, which may be green solvents for potential industrial applications (Fig. 6(c)).
For the anode material, the solvent dissolution method works by mixing the crushed anode material with the corresponding organic solvent. The graphite is separated from the copper foil by weakening the binder force between the graphite material and the copper foil collector through the interaction between the organic solvent and the binder. Yang et al.[57]immersed the LIB anode material in sulfuric acid. The graphite was separated from the copper foil naturally after the sulfuric acid solution was leached out for 5 min, but there was still some residual graphite on the copper foil. Contestabile et al.[58]put the anode material in N-methylpyrrolidone (NMP) at 100 °C for 1 h, which weakened the bonding force between graphite and copper foil. The graphite and copper foil were separated, the active material was recovered by NMP, and the copper foil could be reused.
3.3 In-depth recovery and treatment of cathode materials
After a series of pretreatment, the cathode material generally needs to go through the recovery process by the fire method or wet method to separate and purify valuable metals, finally obtaining precious metals that can be reused.
3.3.1 Pyrometallurgical recycling
There are mainly two ways to recover and treat spent LIBs by pyrometallurgical recycling: (1) high temperature melting method: under the action of high temperature (generally higher than 1 000 °C), the organic matter in the battery is burned and removed, the electrode material is melted, and Co, Ni, Mn, Cu, and other alloys are recycled[59,60]. (2) High-temperature reduction method: in a high-temperature reducing atmosphere, the cathode active material of the battery is directly reduced from oxide to metal for recycling[61-64]. At present, fire recovery has been used to recover spent LIBs in commerce. For example,Pyro-smelting process to process used LIBs into natural ore. Pyrometallurgical recycling and treatment of spent LIBs has the advantages of high efficiency and simple process, which is convenient for industrial application. However, the high-temperature treatment consumes a lot of energy and easily produces toxic gases, requiring additional equipment to purify and process the flue gas.
3.3.2 Hydrometallurgical metal reclamation
Hydrometallurgical metal reclamation is the most promising and widely studied recovery method. The recovery process includes leaching (acid leaching, alkaline leaching and biological leaching) and separation and purification of valuable metals (solvent extraction, chemical precipitation, electrochemical deposition, ion imprinting, and ion sieving, etc).
(1) Leaching
Leaching is a process of converting valuable metals from the pretreated cathode active materials into metal ions in solution, which is very important for the hydrometallurgical process of cathode materials.Leaching is mainly divided into acid leaching, alkali leaching and biological leaching. In addition, ultrasonic, mechanochemical reduction, and extreme conditions are applied to improve the leaching efficiency.
(I) Acid leaching
Acid leaching refers to the dissolution of valuable metal oxides to form ionic state under acidic conditions provided mainly by inorganic or organic acids,and a combination of inorganic acids and organic acids. Table 3 shows some research results on the leaching of spent LIBs cathode materials by acids as leaching agents. At the beginning of the research stage, inorganic acids with strong acidity (H2SO4[65],HCl[66], HNO3[67]) are mainly used to leach spent LIBs.As the cathode active materials of LIBs, LiMO2(LiMn2O4, LiCoO2, LiCoxMnyNizO2) have a relatively stable structure, their reactions with acids are not complete enough, resulting in low leaching rates.Therefore, reducing agents (H2O2[68]glucose[69],NaHSO3[70], and ascorbic acid[71]) are usually added during the leaching process to reduce the insoluble ions in the battery to easily soluble ions, destroy the structure of the cathode active materials LiMO2, and promote the leaching of valuable metals[72]. Peng et al.[73]studied the leaching of valuable metals in waste LIBs with the addition of H2SO4and reducing agent ascorbic acid. Under the conditions of 80 °C, 90 min,2 mol·L−1H2SO4, 0.11 mol·L−1reducing agent ascorbic acid concentration, and a solid-liquid ratio of 200 g·L−1, the leaching rates of Li and Co reached 95.7% and 93.8%, respectively. The combined action of inorganic acid and reducing agent promoted the leaching rate of valuable species in spent LIBs to more than 90%. In order to improve the efficiency of acid leaching, researchers have developed a combined leaching method of reduction roasting and hydrometallurgy. Liu et al.[74]first mixed the waste electrode material with 10% coke and calcined at 650 °C for 30 min, then leached the calcined product with water to obtain a Li-rich solution, and finally obtained Li2CO3by evaporation and crystallization. The filter residue from which Li was filtered out was acidleached with H2SO4(no reducing agent added) to obtain a solution containing divalent metal ions. The leaching rates of Li, Ni, Co and Mn were 93.67%,93.33%, 98.08%, 98.68%, respectively. This method reduces the cost of reducing agent, and the metal solution can be used to prepare the ternary precursor for recycling. However, excessive chemicals eventually become pollutants that need further treatment. Liu et al.[75]used LiFePO4as a reducing agent to remove Fe and P by induced crystallization. The introduction of FePO4·2H2O seed crystals and the use of spent LiFePO4achieved reductant-free and close to stoichiometric acid recovery of spent LIBs (Fig. 7(a)).The recovery efficiency of key metals such as Ni, Co,Mn, and Li reaches 96%, and the leachate can be further used for the preparation of ore hydroxide precursors and lithium carbonate. Inorganic acids usually produce toxic gases such as Cl2, SO2and NOxduring the leaching process. The spent liquid of inorganic acids has strong acidity, which is prone to cause secondary pollution. In addition, the inorganic acids are likely to corrode the leaching equipment.
Organic acids have gradually become research hotspots because of their mild acidity, easy degradation, relatively strong acidity, and no secondary pollution. The commonly used organic acids are acetic acid[35], oxalic acid[76], citric acid[77], formic acid[78],D, L-malic acid, lactic acid[79], and tartaric acid. In Fig. 7(b)[78]the effect of inorganic and organic acid concentrations on the leaching rate of spent LIBs is compared. Organic acids yield higher metal leaching rates than inorganic acids, and the organic ores of the same concentration, or the same leaching rates at lower concentration. Sun et al.[49]studied the leaching and separation of Co and Li using oxalic acid as both leaching and precipitating agent. Under the conditions of 1.0 mol·L−1oxalic acid, 80 °C, 120 min, and a solid-to-liquid ratio of 50 g·L−1, more than 98% of the valuable metals were leached. As shown in Fig. 7(c),the reaction efficiency of LiCoO2increases significantly with the increase of the oxalic acid concentration, indicating that increasing the amount of oxalate is indeed beneficial to the reaction efficiency and cobalt precipitation. The strong precipitation effect of oxalate on Co results in the addition of reducing agent H2O2does not have much effect on the metal leaching rate. Yu et al.[80]leached the cathode active material of spent LIBs with citric acid leaching agent without solvent separation pretreatment. By determining the dosage of citric acid and hydrogen peroxide reducing agent, reaction temperature, leaching time, and other conditions, a high leaching rate of 99% was finally obtained. The analysis shows that citrate has strong chelation with Co2+and Li+, which improves the leaching efficiency. Although organic acid leaching has many advantages over inorganic acid leaching, its high cost, low leaching efficiency, and small leaching capacity per unit volume limit its large-scale industrial application.
Either pure inorganic or organic acid leaching shows its drawbacks or limitations. In order to combine the advantages of the two systems, the mixture of organic ands inorganic acids is adopted to obtain higher leaching rates and lower contamination. Zhuang et al.[81]proposed a novel hydrometallurgical process using phosphoric acid (leaching agent) and citric acid(leaching and reducing agent) as a mixed acid to leach LiNi0.5Co0.2Mn0.3O2cathode material. Under the leaching conditions of 0.2 mol·L−1H3PO4, 0.4 mol·L−1C6H8O7, 30 min, 90 °C, and a solid-liquid ratio of 20 g·L−1, the leaching rates of Li, Ni, Co and Mn were 100%, 93.38%, 91.63% and 92.00%, respectively.
(II) Alkaline leaching
In wet recovery there exists not only acidic system leaching, the use of alkaline system can also achieve the leaching effect to recover metal elements.The general acid leaching method exhibits poor selectivity for different metals (Li, Ni, Co, Mn as well as Fe, Cu), which makes the separation and purification of metals from the leachate complicated and often leads to excessive wastewater discharge. The use of an alkaline system is expected to achieve selective leaching of Co and Ni, thus reducing the difficulty of subsequent metal separation. Since the cathode is composed of active materials and aluminum foil, Al as an amphoteric metal can be dissolved either in acid or in alkali at the same time, while the valuable metals such as Co, Ni, and Mn in the active materials are insoluble in alkali solutions, alkali leaching is thus often used to selectively remove Al[95]. Ferreira et al.[96]leached the cathode with 15% NaOH solution, and the removal rate of Al reached 58%. Chen et al.[83]leached the active material with 5% NaOH solution after pretreatment of the electrode material. The results showed that Al was soluble by 99.9%. Wang et al.[97]carried out reduction roasting with aluminum foil as a reducing agent, and then leached the valuable metals with the NaOH leaching agent. The leaching rates of lithium and aluminum reached 93.67%and 95.59%, respectively.
Studies have shown that ammonia is an ideal leaching agent for selective leaching of Cu, Ni, and Co. In the appropriate pH range, the reaction process is shown in the following formulas. Ku et al.[36]used ammonia as a leaching agent, added pH buffer ammonium carbonate and reducing agent ammonium sulfite to leach the ternary cathode material of spent LIBs. The leaching efficiency of Co, Ni, and Li reached more than 98.6%. Zheng et al.[98]used ammonia ammonium sulfate as the leaching solution and sodium sulfite as the reducing agent, the leaching rates of Ni, Co, and Li all exceeded 98.6%. Zhang et al.[99]first leached a large amount of Li by acid, then leached Cu, Co, and Ni by adding ammonia oxide,and the leaching rate exceeded 95%. Chen et al.[51]proposed a heat treatment-ammonia leaching process to treat waste LIBs. First, the cathode active powder was calcined in air atmosphere at 300 °C and 550 °C,and ammonia leaching was performed with the(NH4)2SO4-(NH4)2SO3system. The leaching rates of Ni, Li, Mn and Co reached 98%, 98%, 92% and 81%,respectively. In addition, it is found that the metal ions precipitate into different salts at different ammonia concentrations, so the selective leaching of different ions can be realized by controlling ammonia concentration.
(III) Bioleaching
Microbial leaching is a mineral biological oxidation process assisted by microorganisms. In this process, insoluble metal oxides are converted into watersoluble metal sulfates, which can realize the leaching of waste battery materials. Its performance depends mainly on the ability of microorganisms to convert insoluble solid compounds into soluble and extractable forms[12]. Compared with inorganic and organic acid leaching, bioleaching has the advantages of environmental friendliness, low cost, and low requirements for industrial applications.
Mishra et al.[100]culturedAcidithiobacillus ferrooxidansin a medium containing elemental sulfur and iron to produce sulfuric acid, and then leached valuable metals from the cathode material of spent LIBs. Xin et al.[101]compared the leaching performance of three biological leaching systems of acidophilic sulfur oxidizing bacteria (SOB), iron oxidizing bacteria (IOB), and mixed bacterial system (MS-MC) on electrode materials. The pure SOB system leached 98% of Li, while the MS-MC system leached 95% of Li and 96% of Mn from LiMnO2. Through pH adjustment, the MS-MC system leached over 95% of the metals from LiNixCoyMn1−x−yO2. The leaching mechanism is mainly due to the contact between the H2SO4produced by the cells and the cells which give the biological leaching performance. The dissolution and leaching of Co, Ni, and Mn are mainly due to the combined effect of Fe2+reduction and acid dissolution (Fig. 8(a)).
The leaching process by bacteria requires a lower and less variable pH, which greatly increases the cost of leaching. If the fungus can survive in a wide pH range and has high leaching rates for metals, it will re-
place bacterial leaching. Biswal et al.[102]compared the bioleaching rates of Co and Li in spent LIBs by Aspergillus niger strains MM1 and SG1 and acid Thiobacillus 80191. As shown in Fig. 8 (b), fungal leaching has a higher leaching rate for metals than bacterial leaching and acid leaching. The leaching rates of Co and Li by fungi reach 82% and 100%,while those by bacteria are only 22% and 66%, respectively. Fungi can be used to leach large amounts of precious metals from spent LIBs by catalyzing the production of acids under certain conditions. Fan et al.[103]used Aspergillus niger to produce glucose oxidase that can oxidize glucose. Shown in Fig. 8(c) is that glucose oxidase catalyzes the oxidation ofb-dglucose to gluconic acid using molecular oxygen as an electron acceptor, which produces hydrogen peroxide in the reaction, resulting in gluconic acid from the leaching agent. At the same time, this strategy showed a good leaching efficiency. The leaching rate of precious metals such as Co, Li, Mn, and Ni in spent LIBs all exceeds 95% (Fig. 8 (d)). The biggest advantage of fungal bioleaching is environmental friendliness. So it is suitable for large-scale recovery of valuable metals in spent LIBs.
Compared with traditional methods, bioleaching is more environmentally friendly with mild reaction process and low energy consumption. However, the low leaching efficiency and low solid-to-liquid ratio are the Achilles heel for the practical applications of bioleaching process. Therefore, although the bioleaching method has significant advantages in energy saving, it is still a long way to realize its actual industrial applications.
(IV) Enhanced leaching
Whether it is acid leaching or alkaline leaching,higher temperature and longer leaching time are usually required. Ultrasound, appropriate pretreatment and even extreme conditions can improve the leaching efficiency.
Ultrasound is a commonly used auxiliary method to strengthen the leaching process. Experiments have shown that ultrasound can increase the leaching rate of valuable metals in different materials[104,105]. In the leaching process, on one hand, ultrasound can promote continuous contact and mutual dispersion between solid and liquid. On the other hand, ultrasound can release a large amount of energy on the solid-liquid interface. Both of these factors help improve the leaching efficiency. Li et al.[53]studied ultrasonicassisted leaching of cobalt and lithium from positive electrode active materials. The results showed that under 90 W ultrasonic power, the recovery rates of Co and Li in the citric acid-hydrogen peroxide leaching system reached 96.13% and 98.4%, respectively. The mechanism of ultrasonic cavitation on the leaching process is illustrated in Fig. 9. The unique cavitation effect of ultrasound is the main reason for improving the leaching efficiency.
Studies have shown that proper pretreatment has a significant effect on improving the leaching effi-
ciency of cathode materials. Guan et al.[66]reported that in a dilute acid solution, a new type of mechanochemical reduction process can extract more than 77%of Li, 91% of Co, 100% of Mn, and 99% of Ni from actual spent cathode materials. The Li leaching rate is significantly improved after the material undergoes milled pretreatment. This is mainly due to the reduction of particle size, the increase of specific surface area, and the change of particle crystal structure due to mechanical activation, thereby increasing the leaching rate. However, for the efficient extraction of Co,in addition to the above reasons, it is more important that the oxidation state of Co is changed from Co (III)to Co (II) as a result of mechanochemical reduction.
Other researchers used some extreme conditions to increase the leaching rate. For example, Liu et al.[106]studied the co-processing of LiCoO2powder with spent LIBs and waste polyvinyl chloride (PVC)in subcritical water. In the process of subcritical coprocessing, PVC was dechlorinated to produce hydrochloric acid, so that the leaching rate of Co in LiCoO2crystals reached more than 95%, and the leaching rate of Li was close to 98%.
In summary, the use of a suitable leaching system can achieve effective leaching and recovery of metal elements in the cathode materials of spent LIBs.And the form of the recovered product is a mixed solution of multiple elements such as Li, Ni, Co, and Mn. In the subsequent processing steps, metal salt compounds can be extracted from the leachate by selective separation and used as industrial raw materials.
(2) Separation and purification of valuable metals
After the active material is leached, most of the valuable metals such as Li, Co, Ni, Mn, Cu, Al, and Fe enter the leaching solution. In order to separate valuable metals from complex solutions, a large number of methods have been proposed, such as solvent extraction, chemical precipitation, and electrochemical deposition, as well as ion imprinting and ion sieving.
(I) Solvent extraction
Solvent extraction is a liquid-liquid separation process that uses extractants to separate metals from leaching solutions. The current extractants are di-(2-ethylhexyl) phosphoric acid (D2EHPA)[107], trioctylamine (TOA), acorga M5640[108], 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester (PC-88A)[109],bis-(2, 4, 4-trimethylpentyl) phosphonic acid (Cyanex 272)[110]etc. Chen et al.[111]used Co-loading D2EHPA(Co-D2EHPA) to extract Mn from the Mn, Co, and lithium-containing leaching solution, and the extraction rate of Mn reached more than 99%. Compared with single-solvent extraction, in the extraction process, two or more extractants are commonly used to mix and synergize extraction to improve the selectivity of metals. Compared with the single extractant system, the mixed extraction with two or more extractants is often used to improve the selectivity of the metal in the extraction process. Wang et al.[112]used extractants D2EHPA and PC-88A to recover Co from the LIB leaching solution mainly containing Co, Ni,Mn, and Cu by a two-step extraction method. First,D2EHPA extractant was used to remove Mn and Cu at pH values of 2.7 and 2.6, respectively, and then PC-88A further extracted the leaching solution at a pH value of 4.25 to effectively separate Co and Ni. Finally, cobalt ions were separated with oxalic acid to obtain CoC2O4. Hong et al.[113]used different extractants such as PC-88A, Cyanex272, and D2EHPA to carry out solvent extraction and recovery of Co, Ni,and Mn from spent NCM cathode materials. They investigated the extraction characteristics of Co, Ni, and Mn, and determined the solution in the separation process. Under the optimal ratio of each metal ion, each metal was effectively separated, and the recovery rate of Co reached 99%. Solvent extraction has the advantages of low energy consumption, good separation effect, and simple operating conditions. However, the extractant is expensive, which increases the processing cost of the recycling industry.
(II) Chemical precipitation
Chemical precipitation is to use a precipitation agent to precipitate the valuable metals in the leachate. For example, Contestabile et al.[114]bubbled CO2gas to convert dissolved lithium into lithium carbonate precipitation. Zhang et al.[115]used a saturated sodium carbonate solution to precipitate lithium carbonate. The precipitation process was carried out at a temperature close to 100 °C, because the solubility of lithium carbonate in aqueous solutions is inversely proportional to temperature. About 80% of the precipitated lithium was recovered. The chemical precipitation method has the advantages of low cost and low energy consumption, but its application may be subject to the difficulties of metal separation and recovery in complex solutions.
(III) Electrochemical deposition
Electrochemical deposition is an effective method to recover pure metals or metal hydroxides from the leachate. Freitas et al.[116]recovered cobalt from waste LIBs by electrodeposition. During this process,pure cobalt was formed on the surface of the electrode. When the pH value was 5.4, the charging efficiency was the highest, reaching 96.9%. Lupi et al.[117]used constant current and constant potential electrowinning technology to recover nickel from spent LiCoxNi1−xO2cathodes. At the appropriate conditions,the deposition efficiency of nickel in the electrolyte reached 87%, and the nickel content in the mother liquor was less than 1×10−4. Electrochemical deposition has the characteristics of simple operation and high purification efficiency, but its energy consumption is high.
(IV) Ion imprinting and ion sieving
The traditional separation and purification methods mentioned above have their own pros and cons.Therefore, there are still many challenges that hinder the direct and selective separation of single metals from the leaching solution of spent LIBs. Adsorptive separation of materials has a good application prospect because of their simple process, economy, and environmental protection. However, in spent LIBs, it is difficult to separate and recover valuable ions, because conventional adsorbents can not realize the specific identification and separation of various metal ions. In order to improve the specific recognition ability of adsorption materials, surface ion imprinted materials(IIMs) with selective adsorption ability can be synthesized by surface ion imprinting technology.Surface ion imprinting (IIT) can selectively identify and separate metal ions[118]. Ion imprinting material is to construct imprinted sites on the surface of separation materials, which has been used to separate lithium ions from salt lake brine[119-121]. Crown ethers(CE), especially 12-Crown-4, have been widely used in IIMs in recent years. Because of their selectivity towards lithium ions, they are usually designed as functional monomers for IIMs[122-124]. Therefore, the integration of 12-Crown-4 into IIMs is expected to realize the selective separation and recovery of lithium from the leachate of waste LIBs. Li et al.[125]prepared a new ion imprinted membrane (SP-IIM) (Fig. 10(a)) by combining graft polymerization and chemical modification, using polydopamine (PDA) oxidized by sodium periodate as the interface adhesive layer, and used it for the selective recovery of Li+from spent LIBs.The sheet-like surface of the SP-PDA layer provides a uniform surface layer for 12C4 loading, which endows the SP-IIM with very high adsorption capacity for Li+. In a 2×10−4Li+solution, the adsorption capacity of SP-IIM towards Li+reached 42.58 mg·g−1after 180 min at pH 7.0 (Fig. 10(b)). In the presence of competing metal ions, the selective separation factors of Li+/Mn2+, Li+/Co2+and Li+/Ni2+were 6.71, 5.84 and 3.03, respectively (Fig. 10(c)). It provides a new way to selectively recover Li+from the leachate of spent LIBs. Therefore, the use of ion-imprinted adsorption and separation materials is expected to achieve the selective separation and extraction of various valuable metals in spent LIBs.
At the same time, ion sieve has also been proved to be feasible for the selective separation and extraction of various valuable metals from spent LIB leaching solutions. Wang et al.[126]used ammonia medium to selectively extract Li, Ni and Co from pretreated waste lithium powder. Subsequently, a manganesetype lithium ion sieve was used as the adsorbent to effectively separate lithium from the leaching solution containing Co2+, Ni2+, Li+and NH4+.
Since there are many types of cathode materials for lithium-ion batteries, the current recycling system is not perfect, and not all recycling processes are economically feasible. At present, pyrometallurgical recycling is mainly suitable for batteries with high cobalt and nickel contents, such as lithium cobalt oxide and ternary cathode material batteries. On the other hand, hydrometallurgical metal reclamation can be applied to different types of cathode materials compared to pyrometallurgical recycling. However, due to the low material value inherent in lithium iron phosphatetype cathode materials, neither of the above two recycling processes can recover valuable products from such cathode materials. For the recovery of lithium iron phosphate cathode materials, the direct recovery method is generally adopted, which can maximize the recovery value. Direct recycling aims to use the materials that still have usable value in waste cathode materials to synthesize them into new materials. It has few processing steps and has a low impact on the environment. It is the only recycling method that can produce significant value from lithium iron phosphate battery.
3.4 In-depth recycling of anode materials
3.4.1 Recovery of negative electrode graphite
Graphite powder is an important non-metallic mineral resource, which can be used in many applications such as lubricants, batteries, refractory materials,thermal conductive materials, and corrosion-resistant appliances. According to reports, the demand for high-grade graphite is growing at a rate of 10%-12%per year. Therefore, it is necessary for anode graphite to be recovered in waste LIBs.
In the pyrotechnic recovery process, there is not much description about the recovery of negative electrode graphite. Under normal circumstances, researchers believe that in the process of pyrotechnical recycling, the anode graphite has been burnt out, and the recovery emphasis is on the precious metals in the cathode material.
At present, the deep recovery of anode graphite mostly adopts wet recovery. Yang et al.[127]first collected the waste graphite through two-stage calcination, and then simply acid leached and purified the collected graphite under the conditions of 1.5 mol·L−1HCl, 60 min, and solid-to-liquid ratio 100 g·L−1. By controlling the pH to 7 and then to 9, they stepwise removed 99.9% of aluminum and 99.9% of copper from the leachate, and then added sodium carbonate to the leachate to precipitate high-purity (>99%) Li2CO3. Ma et al.[128]pulverized and sieved the electrode material,and used 5 mol·L−1H2SO4and 35% (mass fraction)H2O2for acid leaching at room temperature, and filtered to obtain a graphite filter cake. The leached graphite mixed with NaOH powder was sintered at 500 °C for 40 min to remove most of the impurities,washed with deionized water, and dried. Finally, refined graphite was obtained. The specific discharge capacity of the recovered graphite at a rate of 0.1 C was 377.3 mAh·g−1, still showing good electrochemical performance. Graphite can be recovered by combing calcination with acid leaching to obtain a product with a high purity, but the process is complicated and the efficiency is low.
3.4.2 Recovery of residual lithium in anode
At present, the research on the recycling of spent LIBs is mostly concentrated on the cathode materials,and the research on the anode materials is relatively rare. Previous reports introduced the separation and recovery method of graphite and copper foil in the anode materials, but there still exists lithium in the anode materials. It is deposited on graphite electrodes during the first charging process by electrolyte reduction on the surface of graphite electrode as insoluble lithium salts and in SEI film. It is also inserted into the mesopores of the graphite material during the operation of LIBs. According to statistics, the anode material contain up to 31 mg·g−1of lithium. For the recovery of lithium in the anode material, the main method is leaching. Guo et al.[129]scraped the negative electrode active material directly from the copper foil current collector, and then calcined it at 500 °C for 1 h to remove the organic binder, and then used hydrochloric acid as the leaching agent to leach lithium ions at appropriate leaching conditions, reaching a leaching rate of reach 99%. This method has a high leaching rate, but polluting gases are generated during the recovery process, and the inorganic acid used is highly corrosive to the equipment. Liu et al.[130]used biodegradable citric acid to leach lithium in the anode active material of spent LIBs. From the scraped graphite directly from the copper foil collector, the leaching rate of the lithium ion can be as high as 99.5% by controlling the leaching conditions with citric acid.
3.5 Electrolyte recovery
In most cases, the pyro and wet treatment processes of LIBs do not deal with the electrolyte. In order to obtain the greatest benefit from spent LIBs, it is necessary to use an economical and environmentally friendly means to recycle all components of the battery as much as possible. The electrolyte is the most valuable component besides the cathode materials, so it should be recycled from the perspective of profit maximization. McLaughlin[131]used liquid nitrogen to cool the lithium battery at −195.6 °C to reduce the reactivity of the active materials in the battery, crushed the battery at this temperature, and injected LiOH into the powder material. The solution causes the hazardous electrolyte to react to produce a stable lithium salt solution. After further concentration and purification of the lithium salt solution, commercial LiOH or Li2CO3can be obtained. This method is controllable,efficient, and safe, but it does not solve the problem of fluoride recovery. Current studies have shown that vacuum pyrolysis and extraction methods are more effective in the treatment of electrolytes.
Sun et al.[49]used vacuum pyrolysis technology to separate organic binder and electrolyte in spent LIBs.The separated cathode material was heated to 600 °C at a heating rate of 10 °C·min−1, kept warm and evaporated under vacuum for 30 min, while keeping the system pressure below 1.0 kPa, and then collected the pyrolysis product in a cold trap at −10 °C. Fourier transform infrared spectroscopy analysis found that the collected pyrolysis products were mainly fluorocarbon organic compounds, which avoids environmental pollution and resource waste caused by the emission of fluorides.
Extraction process is also feasible. It uses organic solvents or supercritical fluids as extractants to collect electrolyte in battery materials. Qiu et al.[132]freezed disassembled electrode sheet and separator until the electrolyte did no longer flow, and then pulverized the electrode sheet and separator, then put the pulverized materials into an organic solvent to soak,and finally obtained solid components and filtrate after centrifugal separation. The initial filtrate was distilled under reduced pressure to obtain the organic solvent and fine filtrate. The fine filtrate was made into an electrolyte product after adjusting the components. Studies have shown that CO2in the supercritical state has the effect of extracting the electrolyte.Steven[133]put waste LIBs in a supercritical CO2reactor, to dissolve the electrolyte in supercritical CO2,then separated CO2and battery from the reactor, and finally precipitated the electrolyte by restoring supercritical CO2to atmospheric pressure. Mu et al.[134]used CO2supercritical extraction of the electrolyte in the waste battery to extract organic solvents, lithium salts, and additives, with a recovery rate of more than 90%. Liu et al.[135]studied the extraction behavior of the components during supercritical CO2extraction of electrolyte, and found that higher pressure increased the total extraction rate, which was attributed to the high polarity of supercritical CO2. However, the extraction rate of EC is opposite to the pressure, so the polarity plays a more important role than the density of the supercritical CO2. Under optimum process conditions, the overall highest recovery rate was 88.71%±0.87% (mass fraction). At the same time, it is recommended non-polar carbonate should be extracted with non-polar or weakly polar extraction medium, and polar carbonate should be extracted with polar solvent, or a medium-polar co-solvent should be added to the non-polar solvent.
4 Summary and outlook
With the increasing applications of LIBs in the field of consumer electronics and electric vehicles, a large number of spent LIBs have been produced in recent years. With the blowout decommissioning of power batteries, the realization of efficient and clean recycling is not only a practical guarantee for the safe supply of resources, but also a strategic demand for the construction of ecological civilization. In order to address the potential pressure of spent LIBs on the environment and prevent the waste of resources, an enormous amount of recycling studies have been carried out at home and abroad. This article focuses on the working principle and components of LIBs and the recovery of all components in spent LIBs. The recycling of spent LIBs must first undergo pretreatment processes such as discharge, disassembly and separation of various components, and then focus on recovery of cathode and anode materials, and the electrolyte.
However, looking at the current recycling process of spent LIBs and the status quo of the industry,there are still many unresolved problems that require researchers to continue their efforts, which are summarized as follows.
(1) The each recycling method of spent LIBs is not universal. At present, various electrode materials are constantly emerging, but the recycling methods are basically for a certain material or a certain type of material, which brings difficulties to industrial recycling. We need a more flexible and general process to recycle different types of spent LIBs.
(2) In the process of deep recovery and treatment of cathode materials, the leaching system mostly uses acid-base system, which has high energy consumption and great harm. However, the cost of biological leaching is high, and the practical applicability is not high. Further research on the leaching system should be carried out to find a mild and practical leaching method.
(3) Research on the selective separation and purification of various metals is still too lacking. At present, ion sieving and ion imprinting have been proved to be promising for the selective separation of valuable metals in spent LIBs. Future researchers can conduct detailed research in this area.
(4) There is little research on the recovery of anode materials and electrolytes, especially the recovery of residual lithium in anode materials. A series of systematic recovery paths should be designed for anode materials and electrolytes to realize the recovery and reuse of the whole battery.
(5) Some studies have shown that due to the recycling mode and transportation cost, the recycling of spent LIBs may not be economically feasible, or it may not significantly reduce the environmental impact than the initial manufacturing process. Therefore,the government needs more policy support and innovation of recycling methods to increase the economic and environmental feasibility of recycling LIBs.
(6) Carbon active materials can be used to modify traditional electrode materials for prolonging battery life, such as graphene, carbon nanotubes and carbon nanospheres[136,137]. In addition, higher performance battery types can be developed, such as sodium ion battery, potassium ion battery and lithium sulfur battery. The generation of waste batteries is reduced by improving battery performance to prolong battery life. Therefore, in the future, we can develop new battery materials with more economy and high performance or new batteries with higher performance.
Acknowledgements
All authors acknowledge the financial support by the National Natural Science Foundation of China(51972221, 51603142, 51902222), Key R&D Program of Shanxi Province (International Cooperation,201903D421077), Key Program of Yinchuan Science and Technology Bureau (2021ZD08), the Sustainable Development Project of the Science and Technology Innovation Commission of Shenzhen(KCXFZ20201221173214040), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2019L0255, 2020L0097).
