High Linoleic Acid in the Food Supply Worldwide-What are the Consequences? (英文原文)
2022-06-01AndrewSINCLAIRDepartmentofNutritionDieteticsandFoodMonashUniversityNottingHillVictoria3168Australia
Andrew J. SINCLAIR(Department of Nutrition, Dietetics and Food, Monash University, Notting Hill, Victoria 3168, Australia)
Abstract: The macronutrient composition of food supply in China has altered dramatically in the past 70 years. Fat (oil) has increased more than 4.2-times while the carbohydrate content has declined by 34%.Vegetable oils are the major component of the fat intake and since these oils are rich in linoleic acid, there has been a significant rise in the consumption of this fatty acid (as much as a 4-fold rise). Linoleic acid has essential functions in the body in skin and as a precursor of prostaglandins and related compounds. The current intakes of linoleic acid are well in excess of the minimum requirements. In this review, the effects of a food supply rich in linoleic acid on pain in arthritis and headache, non-alcoholic fatty liver and neural function are explored, with emphasis on lipid mediators derived from linoleic acid and other long chain polyunsaturated fatty acids. The current world food systems have created an imbalance of dietary linoleic acid in relation to n-3 polyunsaturated fatty acids, and an imbalance in the lipid mediators derived from these polyunsaturated fatty acids which may be contributing to sub-optimal health status.
Key words: food supply; linoleic acid; polyunsaturated fatty acids; essential fatty acid; human health;experimental animals
1. INTRODUCTION
Over the past 70 years, the food supply in China has changed significantly. This is evident in any major city in China with abundant western supermarkets, western fast-food outlets and the type of foods on display in these supermarkets. These changes in the food offerings have introduced many highly processed foods which are rich in fat and simple sugars and are generally calorie dense but lacking the rich diversity of vitamins, minerals and fibre of whole foods[1].
The most striking changes in nutrient intakes in China over the past 60 years have been the decline in carbohydrate and increase in total fat intakes. Fat intakes have increased from 7.6 energy (en)% in 1952 to 32.0 en% in 2011 (4.2-times), while the carbohydrate contribution to the diet has declined from 83.0 en% in 1952 to 54.3 en% in 2011[2-3].Concern has been raised about these dramatic changes in macronutrients and many have queried whether they have contributed to the increasing incidence of overweight, obesity and type 2 diabetes in China in the past 30 years[2-3]. In this context, a recent randomised controlled study reported that a higher carbohydrate and lower fat diet was beneficially associated with a lower risk of excessive weight gain and increase in waist circumference,and a more favourable lipid profile[4].
In China the increased consumption of vegetable oils has contributed increasingly to the increased intake of fat. Since 1997, vegetable oils have contributed between 39.4% to 44.8% of the fat content of the diet[5]. A major consequence of increasing vegetable oils in the food supply is the increased intake of linoleic acid (LA), as these oils are invariably rich in LA. Recently available data over the period from 1997 to 2011 report that LA is the major PUFA in the Chinese diet with approximately 65% coming from vegetable oils. The LA intakes in males and females in 2011 were reported to be 19.8 g/day and 17.2 g/day, respectively[5].
Many countries, like China, also experience high intakes of LA as reported widely in the literature[6]. This article discusses and analyses the consequences for human health of a food supply worldwide with a high level of LA.
2. WHAT IS THE ROLE OF LINOLEIC ACID (LA) IN THE BODY
LA is an essential fatty acid (EFA) which was discovered in 1929 by Burr and Burr with studies in rats[7]. Rats fed on diets lacking fat grew less,developed scaly skin (paws and tail), tail necrosis,dandruff, increased water loss through the skin,were infertile and died[7]. These symptoms were cured by the addition to the diet of small amounts of vegetable oils containing LA. EFA deficiency was detected as a clinical problem in infants in the 1950’s (infant eczema) which responded to treatment with supplements of lard which contained small amounts of LA and arachidonic acid (AA)[8].EFA deficiency was detected in the late 1960’s and 1970’s in children and adults maintained on total parenteral nutrition[9-10]. The minimum requirements were estimated to be 2 en% for humans[8].
With the advent of gas chromatography in the late 1950’s, it was possible to separate fatty acids more easily than with the previously used spectroscopic methods. Holman’s group established that dietary polyunsaturated fatty acids (PUFA)strongly influenced the lipid composition of the animal. Feeding rats increasing doses of LA was associated with increased tissue levels of LA and AA, and feeding dietary alpha-linolenic (ALA) was associated with increases in long chain metabolites such as eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA)[9].They reported that LA and ALA were in competition for metabolism to the end products (AA and DHA,respectively). Their group proposed the preferred metabolic pathway of LA to AA, which subsequently has been established to involve delta 6 desaturation of LA (FADS2 enzyme) to 18:3n-6,followed by chain elongation to 20:3n-6 (Elongase 5) then delta 5 desaturation to 20:4n-6 (AA)(FADS1 enzyme)[11-12](Figure 1). These remarks need to be tempered by recent developments in the understanding of the importance in the genetic variation in the FADS gene cluster SNP’s in the synthesis of long chain (LC) n-6 and n-3 PUFA[13].
Linoleic acid is found in many foods. Once digested, absorbed and transported around the body,much of the LA is metabolised by beta-oxidation for production of energy. Most of the remaining LA is found in tissue lipids such as triacylglycerol,cholesterol esters and phospholipids. Adipose tissue is the major repository of LA, in the form of triacylglycerols. Some linoleic acid is metabolised in tissues such as liver to arachidonic acid (20:4n-6,AA) by the desaturation-chain elongation pathway.AA is also transported from the liver to other tissues where it is predominantly found in membrane phospholipids. LA and AA are precursors to specialised lipid mediators called eicosanoids and related compounds; these are produced locally in various tissues.
LA is the major PUFA in most tissues, except in the brain and retina[14-15](Table 1). Increasing intakes of LA increase tissue LA levels almost linearly while the tissue levels of AA reach a plateau[16]. In the brain and retina, the main PUFA are C20 and C22 PUFA such as AA and DHA[17]. It is known that LA is rapidly beta-oxidised in the brain before it can accumulate in membrane phospholipids[18]. Since the EFA and their metabolites were found in cell membrane phospholipids, the initial belief was that their main role in the body was involved with “fluidity’ of cell membranes.While this is still likely to be a role for PUFA, it is by no means the only role.
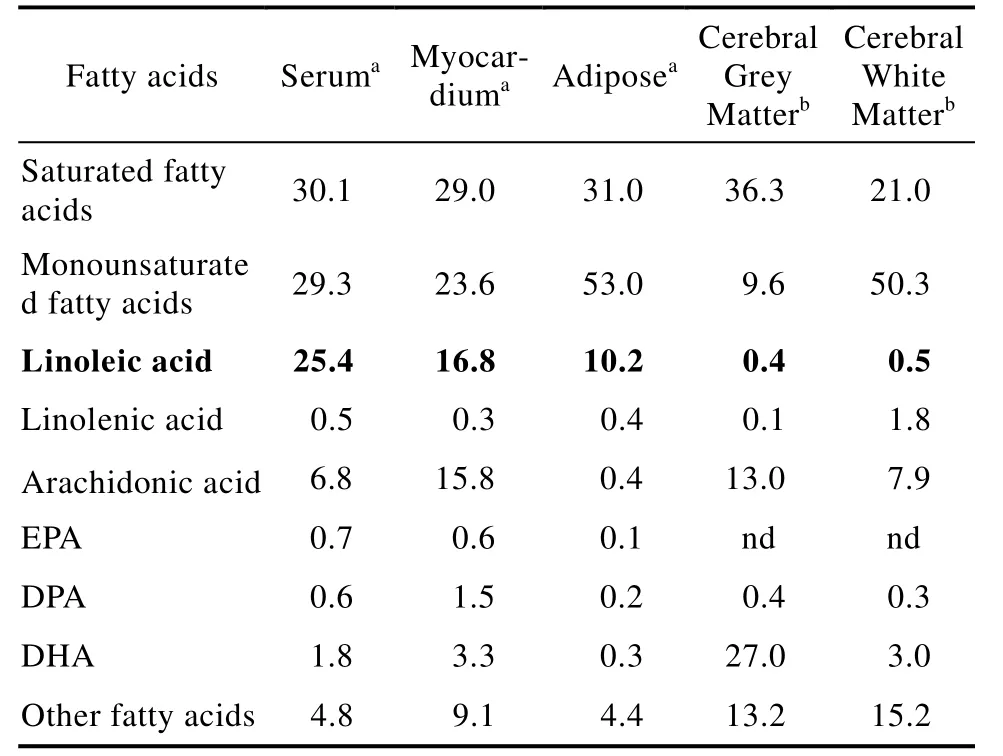
Table 1 Fatty acid proportions in human tissues(% of total fatty acids)
2.1 Precursors of oxygenated derivatives

Fig.1 Metabolic processing of linoleic acid (LA) from food to tissues
In the early 1960’s it became recognised that AA was the substrate for metabolism to oxygenated derivatives known as prostaglandins[19]. Since then,a broader range of compounds derived from AA has been identified and include metabolites derived from AA via the action of cyclooxygenase,lipoxygenases or cytochrome P450 and the class of compounds is now referred to as eicosanoids and related compounds[20]or specialised lipid mediators[21].The eicosanoids play a wide range of roles in normal and pathophysiology, including in inflammation.Some well-known functions include platelet aggregation by thromboxane A2 (TXA2), blood vessel constriction involving prostacyclin (PGI2),inflammation (PGE2, LTB4), and anti-inflammatory lipoxins (LXA4)[20-21]. More recently, it has become apparent that LA itself can be metabolised by 15-lipoxygenase to hydroxy-fatty acid metabolites such as 9- and 13-HODE and 9- and 13-oxoHODE[22-23]with a range of biological properties(Figure 1). Specialised pro-resolving lipid mediators are formed from the long chain n-3 PUFA such as EPA, DPA and DHA including resolvins, maresins and protectins. These compounds are involved in the resolution of inflammation[21].
2.2 Lowering plasma cholesterol levels
The food industry in USA developed a considerable interest in LA in the 1950’s and 60’s when it was established that vegetable oils rich in LA could lower blood cholesterol levels[24], in contrast to foods rich in saturated fat which raised cholesterol levels[25]. This finding was associated with the production of spreads (margarines) containing PUFA to be promoted in place of butter (rich in saturated fat). One unfortunate consequence was that manufacture of the fats for margarine involved partial hydrogenation of the vegetable oils to create fats with a suitable melting point. This process created trans-fatty acids which were subsequently identified as being harmful for health[25-26]. Since the diet of most people in China is not rich in saturated fat[5], unlike diets in many western countries, the relevance of LA for lowering plasma cholesterol in China is uncertain.
2.3 Role in skin and wound healing
Skin is a major barrier for preventing water loss as well as against thermal, mechanical and physical injury. In addition, skin reduces the harmful effects of UV radiation, is an immune organ and is involved in the synthesis of vitamin D from sunlight. Epidermal lipids are rich in ceramides, a type of sphingolipid, and these contain high proportions of LA. These ceramides play an important role in preventing trans-epidermal water loss[27]. In EFA-deficient rats, LA is replaced by oleic acid (a non-essential fatty acid) which does not adequately substitute for LA in this function.
Research by Ziboh and colleagues showed that there was active metabolism of LA and AA in skin by lipoxygenase enzymes[22]. AA was transformed into 15-HETE and LA to 13-HODE by 15-lipoxygenase. These mono-hydroxy fatty acids were incorporated in membrane PL, especially phosphatidylinositol and upon stimulation of induced inflammation resulted in diacylglycerols containing 13-HODE which they reported were associated with anti-inflammatory/ antiproliferative effects[22]. More recently, it has been shown that the skin barrier to water loss requires linoleate and two epidermal lipoxygenases: 12R-lipoxygenase and epidermal lipoxygenase 3. In a complex reaction involving LA, both lipoxygenases and a ceramide esterase, ceramide becomes bound covalently with protein to maintain the epidermal barrier function[28].
3. WHAT ARE THE CONSEQUENCES OF HIGH INTAKES OF LA?
Do we need more LA than dietary requirements?The minimum LA requirements to prevent EFA deficiency have been established at 2 en% based on curing the skin lesions[8], which amounts to approximately 4 g/day in a 2 000 kcal/day food intake. The estimated LA intakes from 13 countries were reported to range from 0.90 en% in the Philippines to 8.91 en% in USA[6]. Based on LA intakes in males and females in China reported by Shen et al[5], LA intakes in 2011 were between 7.5 en% to 7.8 en%. Therefore, China like many other nations has considerably higher LA intakes in comparison to the minimum requirement of 2 en%.Not only do many people throughout the world have high LA intakes, but they also have low ALA intakes and little or no DHA[6]. The n-3 PUFA intakes in China in 2011 for males were: ALA 2.0 g/d, EPA+DHA 26.9 mg/d and for females the values were ALA 1.7 g/d and EPA+DHA 24.3 mg/day[5].
As far as is known, there are no overt signs of LA excess, so to answer the question of whether there are consequences it is necessary to resort to probing at a physiological and molecular level.
3.1 Effects on tissue fatty acid levels
There are three major effects of increasing dietary LA. First, there is a rise in tissue LA levels;second, there is a rise in AA levels but to a lesser extent than for LA; third, there is a decrease in the proportion of LCn-3 PUFA[9,16,30]as shown in Figure 2. The tissue n-3 PUFA levels fall because of competition between LA and ALA for the FADS2 enzyme (Figure 1)[11-12]. Recently, it has been shown that increasing tissue LA levels by increasing diet LA is associated with increased levels of specialized lipid mediators derived from both LA and AA and reduced levels of n-3 derived lipid mediators[30-31].
In the following sections, the consequences of increased LA and metabolites and decreased long chain n-3 PUFA and metabolites will be considered.
3.2 Pain including chronic headache
3.2.1 Chronic arthritis
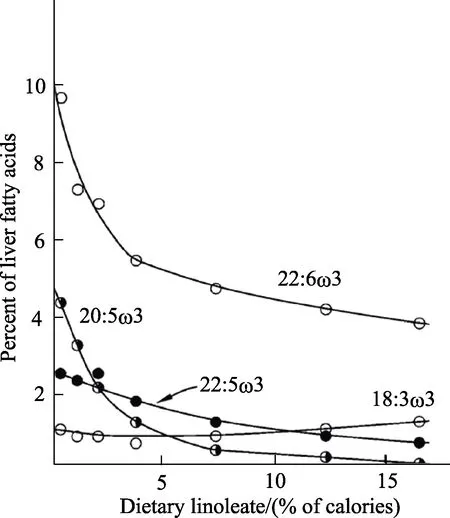
Fig.2 Effect of increasing levels of linoleic acid on the suppression of metabolites of alpha-linolenic acid (18:3n-3,present in diet at 1 en%) on liver fatty acid proportions[9]
Chronic arthritis is associated with significant pain. In the 1990’s, researchers speculated that this might be partly the result of an imbalance between tissue levels of AA and EPA as a result of diets being rich in LA and low in long chain n-3 PUFA,resulting in an over-exaggerated metabolism of AA to LTB4 (pro-inflammatory mediator)[32]. As a result,many trials have investigated the use of high doses of fish oils rich in n-3 PUFA in an attempt to minimize the impact of pro-inflammatory mediators derived from n-6 PUFA and increase the production of n-3 mediators capable of resolution of inflammation. A meta-analysis of these studies, in a total of 1 143 patients, found that 10 of 18 studies reported significant pain reduction with the use of 3~6 g/day of long chain n-3 PUFA[33]. The possible mechanisms at play were reviewed by James et al[34]including production of LTB5 from EPA (not pro-inflammatory), thus reducing the pool of inflammatory eicosanoids based on reduced LTB4 from AA and increased LTB5 from EPA.Furthermore, fish oil was capable of reducing the peptide mediators of inflammation (TNFa and IL-1B). Additionally, EPA, DPA and DHA are precursors of mediators with pro-resolving actions including maresins, resolvins and protectins[21].
3.2.2 Chronic headache
The other area which has seen considerable research progress relates to chronic headache pain.This type of pain has been defined as headaches lasting 4 hours or more for 15 days or more per month over at least 3 months, known to affect millions of people in USA[35]. This work started with the hypothesis that modern western diets enriched in LA had created an imbalance in mediators which detect painful stimuli (those derived from LA and AA) compared with mediators which oppose pain detection (from n-3 PUFA such as EPA and DHA). A study was conducted in 67 subjects with chronic headache in a randomised,single-blinded, parallel-group clinical nutrition intervention trial in which subjects were allocated to an intervention with high n-3 PUFA, low n-6 PUFA foods (H3L6), or a low n-6 PUFA foods (L6) for 12-weeks[35]. Subjects were provided with most of the foods required for the study and were counselled throughout the study. The median intakes of PUFA in the H3L6 group were 1 482 mg/d of EPA+DHA and 2.5 en% LA, compared with 76 mg/d EPA+DHA and 2.4 en% LA in the L6 group. These intakes were associated with increased levels of EPA+DHA and reduced LA in red blood cells in the H3L6 group, while in the L6 group the LA levels were reduced. It was found that the H3L6 groups had significant clinical improvements in headache hours/day, severe headache days and quality of life related to headaches in comparison with the baseline. The H3L6 group showed significant increases in anti-nociceptive mediators derived from EPA and DHA (18-HEPE and 17-HDHA) as well as decreases in hydroxy derivatives of LA and AA, which have putative pronociceptive properties.
In a second study, this research team investigated whether dietary interventions similar to those described above could reduce headache in subjects with migraines (2/3 with chronic migraine) in a three-arm, parallel group, randomised, modified double blind, controlled trial. The diets were high n-3 diet and usual LA (H3), high n-3 and low LA(H3L6) and control (typical US diet low n-3 and usual LA)[36]. The subjects were allocated to one of the three diets described above. At 16 weeks, the median intakes of n-3 PUFA in the H3 and H3L6 increased from less than 50 mg/2 000 kcal to 1 484 and 1 341 mg/2 000 kcal, respectively. The median intake of LA was 7.1 en% in the H3 group and 3.2 en% in the H3L6 group. In the control group the n-3 intake was 80 mg/2 000 kcal and LA 6.8 en%[37].Both intervention diets significantly reduced the frequency and severity of headaches, with indications of greater benefit for the H3L6 group compared with the H3 group. Of interest is that both interventions reduced the use of headache-related drugs. The red blood cell fatty acids changed as anticipated, with both groups showing significant increases in EPA and DHA and the H3L6 group showing decreased LA and AA. Both the intervention diets increased the anti-nociceptive bioactive mediators(including 17-HDHA) in circulation which suggested a lowered nociceptive state[36].
Studies in rats showed that increased doses of dietary LA dose-dependently increased the proportions of LA and AA in various tissues associated with idiopathic pain as well as increasing oxidised LA metabolites (pro-nociceptive lipid derivatives) in a dose-dependent manner in some tissues. In addition,these increased LA diets were consistently associated with tissue reductions in n-3 EPA and DHA and their n-3 monoepoxides (putative antinociceptive mediators)[30]. This study provided a foundation for further studies on reducing dietary LA in treating idiopathic pain.
Using a systems approach to link dietary linoleic acid with oxidised linoleic acid metabolites which might be associated with pain and itch,Ramsden et al[38]identified two novel linoleic acid metabolites. Firstly, they found that circulating 11-hydroxy-12,13-trans-epoxy-(9Z)-octadecenoate was correlated with clinical pain reduction. Second,they found that 9-keto-12,13-trans-epoxy-(10E)-octadecenoate was increased by 30-fold in skin from patients with psoriasis. These novel findings provided a link between linoleic acid metabolites and pain and itch.
The studies on arthritis and headache pain highlighted the association of these disorders with many current diets throughout the world which are rich in LA and depleted of n-3 PUFA[6]. This situation leads to an exaggerated metabolism of LA and AA to pro-inflammatory lipid mediators.Research studies have shown that pain reduction in both arthritis and chronic headache can be ameliorated by substantially increasing dietary long chain n-3 PUFA alone or by simultaneously reducing LA and increasing the long chain n-3 PUFA. Readers are referred to a recent review on mediators related to pain which emphasises pro-resolving lipid mediators[39].
3.3 Fatty Liver
The prevalence of non-alcoholic fatty liver disease (NAFLD) is rapidly increasing, in parallel with that of obesity and diabetes, ranging from 25%to 45% around the world[40]. NAFLD is characterized by hepatic steatosis and inflammation without excess alcohol consumption, and covers a range of chronic liver diseases from simple steatosis to nonalcoholic steatohepatitis, fibrosis and cirrhosis[41-42].A recent meta-analysis reported that long chain n-3PUFA alleviated NAFLD by improving lipid metabolism and inhibiting inflammation[43].
LA and its oxidation products have also been reported to be associated with NAFLD and alcoholic fatty liver. Increased levels of oxidised products of LA (9- and 13-HODEs and 9- and 13-oxoODEs)have been reported in non-alcoholic steatohepatitis,a severe form of NAFLD[44]. This study found that there was a significant correlation between LA oxidation products and markers of liver pathology such as inflammation, fibrosis and steatosis.
Following this report, attention turned to whether diets rich in LA might contribute to NAFLD[45]. In particular, whether the oxidised linoleic acid metabolites (OXLAMs) present in the diet might be a contributing factor. These OXLAMS occur as a result of thermal stress of the LA-rich vegetable oils[45]. In this study, mice were fed a diet enriched in OXLAMS however they did not find increased liver fat levels but reported higher fatty acid oxidation and lipid peroxidation in association with increased oxidative stress, mitochondrial dysfunction and increased liver cell death. Further work by this group indicated that a high LA diet in mice was associated with elevated levels of OXLAMS (9-HODE) which contributed to the worsening of effects of alcohol on ethanol-induced liver injury in mice[46].
3.4 Effects on neural and retinal function
3.4.1 Studies in animals
Safflower oil, peanut oil and corn oil are rich in LA and low in ALA, with ratios of [LA]:[ALA] of>50:1. Feeding animals with diets containing these oils as the only source of lipid results in n-3 PUFA deficiency, and results in significant depletions of long chain n-3 PUFA (in particular DHA) in tissues,especially in brain and retina[47]. Results from many studies consistently report that dietary n-3 deficiency results in changes in learning, coping with stress,behavioural changes, and responses in visual function, auditory function, and olfactory function.These changes have been ascribed to a deficiency of DHA in neural cell membranes where DHA is located. Sinclair[47]summarised the results of these studies and suggested the primary insult of neural DHA deficiency was on“the flexibility/compression of the membrane lipids which affects the optimal function of integral membrane proteins (receptors, voltage-gated ion channels and enzymes). As a consequence, this led to effects on second messenger systems, and subsequently affected neurotransmitter concentrations due to ‘weakened’ signals from the initiating receptors. Since there are more than 80 billion neurones and many times more synaptic connections between neurones, a very small loss of “efficiency”in signal due to altered properties of membrane proteins would be anticipated to result in meaningful changes in brain and visual function.One of the downstream effects that could also contribute to an impairment of neurotransmission could be due, in part, to sub-optimal brain energy metabolism (glucose entry into the brain), which is significantly reduced in omega 3 deficiency. It is thus concluded that DHA is an essential fatty acid for optimal neuronal function.”[47]
Is there more to be discovered here? The answer is yes, and recent data has highlighted how diets enriched in LA and low in ALA may lead to enhanced neural metabolism of AA to PGE2 and possibly other mediators. Begg et al[48]reported that mRNA levels for phospholipase A2, cyclooxygenase and PGE synthase were significantly elevated in the hypothalamus of elderly rats as well as significant elevations of hypothalamic levels of PGE2, suggesting an inflammatory response to the imbalance of membrane fatty acids in the hypothalamus (increased AA and other n-6 PUFA and decreased DHA). The n-3 deficiency was induced by diets enriched in LA and containing very low levels of ALA. This work was followed up by studies in mice on n-3 deficient or control diets examining the impact on learning using the Y-maze test, which involves spatialrecognition memory[49]. The n-3 deficient mice showed significantly fewer novel arm entries in the Y-maze test compared with the control (+ALA diet)animals. Of particular interest was that novel arm entries could be restored by feeding 3rdgeneration deficient mice either with a diet containing ALA or by the addition of a COX-inhibitor (naproxen at 0.07 mg/ml) to the drinking water. These data show that the cognitive impairment was not simply the result of a lack of DHA in the brain, but also involved mediator products of the AA-cyclooxygenase pathway. It is not known if COX inhibitors result in restoration of other aspects of neural function in animals deficient in n-3 PUFA.
3.4.2 Does this situation occur in humans?
Many people throughout the world have high LA intakes, low ALA intakes and little or no DHA[6].DHA in foods is essentially only available to those who choose to consume fish and other marine foods,or who can afford fish or DHA supplements. Many populations exist on vegetarian diets and increasingly people in western countries are choosing to be vegans[50]. Additionally, droughts, famines, and war totally disrupt food supplies with no ability of people in these situations to choose food based on its nutrient content. However, one constant is that LA is found in abundance in most foods throughout the world.
Neonatal infant brain fatty acids: Two studies in infants fed high LA-low-ALA infant formulas have reported decreased DHA proportions in the brain cortex compared with infants fed on breast milk (which contains DHA)[51-52]. However,nowadays, most infant formulas contain DHA so in these circumstances brain and retinal DHA levels should be supported adequately.
Weaning foods for infants: Diets low in ALA and DHA could impact on brain and retinal DHA levels if infants are fed low-ALA foods from birth and then weaned onto similarly low ALA and low DHA foods. Few weaning foods have been reported to contain adequate DHA contents[53-54]; thus the infants will be reliant on an adequate dietary ALA,sufficient stores of DHA in adipose tissue and foods containing DHA (fish and marine foods) which may or may not be available in different cultures.
Vegans and vegetarians: Vegan and vegetarian diets typically lack DHA, because this PUFA is found in fish, eggs and some meats. In addition,vegan and vegetarian diets typically have high[LA]:[ALA] ratios (>16:1). The fatty acids levels in vegan diets and tissues have been detailed in a recent review[50]. One of the conclusions of this review was that vegans should increase their ALA intake and decrease their LA intake to achieve a dietary [LA]:[ALA] of 4:1[50].
Low-income countries, famines and refugees:People in low-income countries do not have sufficient access to food supplies with adequate levels of either ALA or DHA, such as in dryland agricultural regions of the world. There have been few studies on the n-3 status of infants in such regions, although two reports on the composition of mother’s milk indicated relatively high LA levels and low DHA levels[55-57]. A recent study from Malawi on ready-to-use therapeutic food (RUTF) to treat severe acute malnutrition found that a RUTF which contained DHA and had a low linoleic acid level ([LA]∶[ALA] of 1.5∶1) used for 6 months resulted in significant cognitive improvements compared with the standard RUTF food which had an [LA]∶[ALA] of 11.6∶1[58]. Refugees face great uncertainty with access to food and would have few opportunities to choose foods based on nutrient content related to ALA or DHA.
4. CONCLUSIONS
Worldwide, the current intakes of LA are well in excess of the minimum requirements. In addition,the intakes of long chain n-3 PUFA are low in most countries. These two factors contribute to an imbalance of n-6 PUFA and n-3 PUFA in tissues.Since these PUFA are precursors of pro- and anti-inflammatory lipid mediators, there is an imbalance in these mediators in favour of inflammation. In addition, these lipid mediators are precursors of pronociceptive and antinociceptive mediators. Emerging evidence suggests this imbalance in pain-related mediators, including novel OXLAMS derived from LA, might be contributing to pain in both arthritis and chronic headache. This review also considers the imbalance of n-6 and n-3 PUFA in non-alcoholic fatty liver and neural function. The current world food systems have created an imbalance of dietary linoleic acid with n-3 PUFA which may be contributing to sub-optimal health status.
ACKNOWLEDGEMENTS
I acknowledge the people of the Kulin Nations,the Traditional Owners and Custodians of the lands on which I live and work. I pay my respect to Elders past, present and future.
