ADAMTS7基因rs3825807位点单核苷酸多态性与子痫前期遗传易感性关系
2022-05-30楚静张璐汤潜刘世国詹瑛
楚静 张璐 汤潜 刘世国 詹瑛
[摘要] 目的 探讨含Ⅰ型血小板结合蛋白基序的解聚蛋白样金属蛋白酶7(ADAMTS7)基因rs3825807位点多态性与子痫前期(PE)遗传易感性的相关性。方法 使用TaqMan探针实时荧光PCR技术,对山东地区706例PE病人(病例组)以及926例正常孕妇(对照组)的外周血DNA进行扩增,比较两组孕妇ADAMTS7基因rs3825807位点基因型及等位基因频率。结果 病例组和对照组孕妇ADAMTS7基因rs3825807位点基因型及等位基因频率差异均无显著性(P>0.05);早发型、晚发型PE病人基因型及等位基因频率与对照组比较差异无统计学意义(P>0.05)。结论 ADAMTS7基因rs3825807位点多态性与山东地区汉族人群PE的遗传易感性没有相关性。
[关键词]先兆子痫;ADAMTS7蛋白质;RNA探针;实时聚合酶链反应;疾病遗传易感性
[中图分类号]R714.24;R363.25[文献标志码]A[文章编号]2096-5532(2022)03-0379-04
doi:10.11712/jms.2096-5532.2022.58.057
ASSOCIATION OF ADAMTS7 RS3825807 SINGLE NUCLEOTIDE POLYMORPHISM WITH GENETIC SUSCEPTIBILITY TO PRE-ECLAMPSIA
CHU Jing, ZHANG Lu, TANG Qian, LIU Shiguo, ZHAN Ying
(Department of Obstetrics and Gyneco-logy, The Affiliated Hospital of Qingdao University, Qingdao 266003, China)
[ABSTRACT] Objective To investigate the relationship between single nucleotide polymorphism (SNP) of a disintegrin and metalloproteinase with thrombospondin motifs 7 (ADAMTS7) locus rs3825807 and genetic susceptibility to pre-eclampsia (PE).Methods The peripheral blood DNA of 706 PE patients (patient group) and 926 healthy pregnant women (control group) in Shandong province were amplified by TaqMan probe real-time fluorescent quantitative PCR. The genotype and allele frequency of ADAMTS7 rs3825807 were compared between the two groups of pregnant women. Results There were no significant differences in the genotype and allele frequency of ADAMTS7 rs3825807 between PE patients and healthy pregnant women (P>0.05). There were no significant differences between patients with early and late onset PE and healthy pregnant women (P>0.05).Conclusion ADAMTS7 rs3825807 polymorphism is not associated with genetic susceptibility to PE in Han population living in Shandong province.
[KEY WORDS] pre-eclampsia; ADAMTS7 protein; RNA probes; real-time polymerase chain reaction; genetic predisposition to disease
子癇前期(PE)是一种高血压及炎症的妊娠疾病,主要表现为妊娠20周后孕妇出现血压增高、头痛恶心、蛋白尿等症状[1]。PE是导致孕产妇及围生儿病死率升高的主要原因之一[2-3]。然而,PE的发病机制至今尚未明确。有研究结果表明,PE与遗传、氧化应激、炎症反应失衡[4]、螺旋动脉异常重塑、胎盘缺陷及血管生成障碍等有关[5]。含Ⅰ型血小板结合蛋白基序的解聚蛋白样金属蛋白酶(ADAMTSs)是一个新定义的金属蛋白酶家族,共包括19个成员,其中多个成员在人胎盘中表达[6-7]。据文献报道,ADAMTS4和ADAMTS5参与胎盘的形成过程[8]。ADAMTS13可以促进滋养细胞发育,在PE中的表达降低[9]。而ADAMTS7在人体组织中广泛分布,主要参与动脉粥样硬化的发生发展[10]、血管基质的重塑以及骨分化[11]。有研究结果表明,ADAMTS7还能协调血管平滑肌细胞和内皮细胞的功能,从而促进新内膜的形成[12]。本课题组前期的研究结果显示,PE中ADAMTS7基因启动子甲基化水平较低[13],而这是否与基因的多态性有关目前还未可知。因此,本研究选择影响ADAMTS7成熟和平滑肌细胞迁移的rs3825807位点[14]进行检测,以进一步探讨ADAMTS7基因与PE遗传易感性的关系,为PE的早期诊断提供新的方向。现将结果报告如下。
1对象与方法
1.1研究对象
选取2018—2019年就诊于青岛大学附属医院、滨州医学院附属医院、聊城市人民医院、烟台毓璜顶医院及临沂市人民医院等山东省医院的孕妇1 632例作为研究对象,其中确诊PE病人706例(病例组),正常孕妇926例(对照组)。PE的诊断标准依据2018年国际妊娠期高血压研究学会(ISSHP)制订的《妊娠期高血压疾病:ISSHP分类、诊断和管理指南》[15],即妊娠20周以后出现血压升高伴或不伴蛋白尿。早发型PE即34周前发病者,晚发型PE即34周及以后发病者。排除标准:有慢性高血压、糖尿病、心脏病、肾病及肝病病史的孕妇,胎膜早破、前置胎盘、先兆流产、辅助生殖、双胎及巨大儿孕妇。收集所有研究对象的一般资料。
1.2样本采集及基因型检测
采集孕妇的空腹肘静脉血3 mL,置于EDTA抗凝管中,用2 mL EP管分装后置-80 ℃冰箱中保存备用。用血液基因组DNA提取试剂盒(北京天根公司)提取外周血基因组DNA。采用TaqMan探针实时荧光PCR技术对ADAMTS7基因rs3825807位点进行基因型检测。rs3825807位点上下游引物分别为5′-GTCCCCCTGTGAGGCAA-TTT-3′和5′-CCGTGGTACTCCACCATTTTG-3′。
PCR反应体系7 μL,包括2×Mix 3.5 μL、ddH2O 2.45 μL、20×SNP探针0.05 μL、DNA模板1 μL。应用SLAN-96S全自动医用PCR分析系统进行基因分型,PCR反应条件为:95 ℃、10 min,95 ℃、15 s,60 ℃、1 min,共40个循环。在每个周期60 ℃延伸过程中采集探针荧光强度。通过检测不同等位基因所标识的荧光信号,判读待测样本的基因型。
1.3统计学分析
采用SPSS 25.0软件进行数据的统计分析。采用Hardy-Weinberg遗传平衡检验检测群体基因遗传平衡,当P>0.05时,说明群体基因遗传平衡,样本具有良好的代表性。计量资料数据以x±s表示,两组比较采用t检验;基因型及等位基因频率以百分率表示,两组比较采用Pearson-χ2检验。P<0.05认为差异有统计学意义。
2结果
2.1两组一般资料的比较
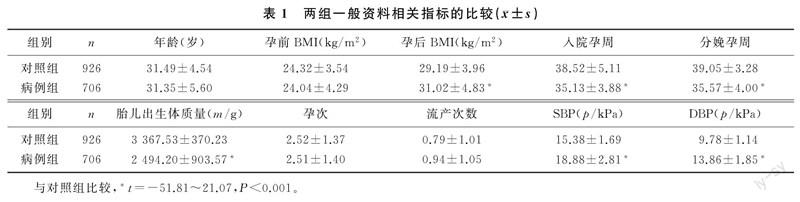
两组孕妇孕后体质量指数(BMI)、入院孕周、分娩孕周、收缩压(SBP)、舒张压(DBP)和胎儿出生体质量比较差异均有显著性(t=-51.81~21.07,P<0.001),而两组孕妇年龄、孕前BMI、孕次及流产次数比较则差异无显著性(P>0.05)。见表1。
2.2Hardy-Weinberg遗传平衡检验
Hardy-Weinberg的检验结果显示,ADAMTS7基因rs3825807位点在对照组和病例组均符合遗传平衡定律(χ2=0.362、0.722,P>0.05),表明本研究选取的样本具有较好的群体代表性。
2.3两组ADAMTS7基因rs3825807位点基因型及等位基因频率比较
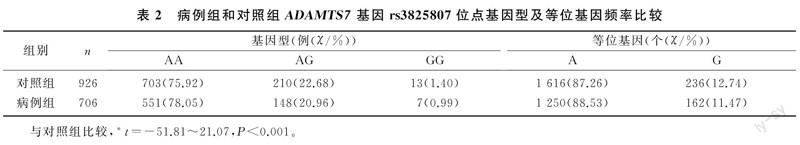
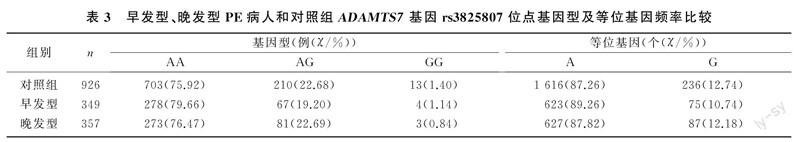
本研究病例组和对照组孕妇ADAMTS7基因rs3825807位点的3种基因型频率比较差异无显著性(χ2=1.329,P>0.05),等位基因频率也未见明显差异(χ2=1.207,P>0.05)。见表2。早发型、晚发型PE病人ADAMTS7基因rs3825807位点3种基因型及等位基因频率与对照组比较,差异均无统计学意义(χ2=0.146~2.000,P>0.05)。见表3。
3讨论
PE是一种妊娠常见的并发症,每年在全球范围内造成约7万名孕产妇死亡,其中大部分发生在低收入和中等收入国家[16]。而对PE发病机制的研究则可以帮助我们更好地诊治PE。
目前,有关PE发病机制常见的学说有如下几个。①氧化应激-血管内皮损伤学说。内皮一氧化氮合酶(e-NOS)可诱导一氧化氮(NO)的合成,NO可以使动脉床血管扩张。在PE中,氧化应激会增加脂质过氧化物的生成,导致内皮功能障碍[17]、e-NOS缺乏,可以导致胎盘床、肾血管系统和其他器官血管床的血管收缩,引起血压升高[18]。②免疫调节异常学说。胎盘、白细胞和肾足细胞中富含Toll样受体4(TLR4),研究表明PE的发生与胎盘和肾脏中TLR4的过表达有关[19]。TLR4的增加会导致炎症细胞因子的增加和胎盘/肾脏功能障碍[20]。此外,抗炎细胞因子的减少也会引起PE的发生[21]。③胎盘或滋养细胞缺血低氧学说。在正常妊娠中,胎盘的滋养细胞侵入子宫壁,用低阻力的血管系统取代高阻力的子宫螺旋动脉和小动脉。这种重塑在PE中存在缺陷,可引起胎盘缺血,进而导致可溶性fms样酪氨酸激酶1(sFlt-1)和可溶性内皮糖蛋白(sEng)的过量生产[22]。sFlt-1在血液中可以与血管内皮生长因子(VEGF)和胎盘生长因子(PLGF)结合,而高sFlt-1和低VEGF/PLGF参与了高血压的发生。同样,高sEng有助于高血压和蛋白尿的发展[23]。
ADAMTS7为金属基质蛋白酶家族的一员,基因位于15q25染色体上,其主要作用是降解细胞外基质,与多数心血管疾病的发生发展以及关节炎中软骨细胞外基質蛋白的降解密切相关[24-25]。还有研究指出,ADAMTS7的升高能引起孕妇流产[26]。ADAMTS7 rs3825807位点由腺嘌呤(A)至鸟嘌呤(G)的取代可导致ADAMTS7前结构域中丝氨酸至脯氨酸的取代[27],激活的ADAMTS7具有蛋白水解活性,会裂解软骨寡聚基质蛋白(COMP),影响ADAMTS7的成熟和平滑肌细胞的迁移[14,28]。此外,有研究表明,ADAMTS7 rs3825807位点上A等位基因与冠心病及缺血性脑卒中易感性相关[29]。本课题组前期研究表明,ADAMTS7是PE的重要调节因子,PE病人胎盘中ADAMTS7的启动子区甲基化程度降低,导致ADAMTS7在胎盘中的表达增加,进而抑制滋养细胞的迁移、侵袭等功能,导致母体螺旋动脉重塑不足和血流不足[13],最终导致胎盘缺血,引起PE。而本研究结果显示,病例组和对照组ADAMTS7基因rs3825807位点基因型和等位基因频率比较差异无显著性,早发型、晚发型PE病人基因型及等位基因频率与对照组比较差异亦无显著性。提示ADAMTS7基因rs3825807位点多态性与山东地区汉族人群PE发病可能不相关。
PE是一種很复杂的多基因遗传性疾病,受多个基因、多个位点的调控,且环境、饮食、生活方式等也会对其发生发展产生影响,故本研究结果仍需要通过扩大样本量进行更深入的研究确认。另外,对于ADAMTS7基因多态性与PE易感性关系的研究还需要选取多个位点进行研究,从而为PE的临床诊治及预防提供遗传学依据。
[参考文献]
[1]SILVA G B, GIERMAN L M, RAKNER J J, et al. Cholesterol crystals and NLRP3 mediated inflammation in the uterine wall decidua in normal and preeclamptic pregnancies[J]. Frontiers in Immunology, 2020,11:564712.
[2]RANA S, BURKE S D, KARUMANCHI S A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders[J]. American Journal of Obstetrics and Gynecology, 2020. doi:10.1016/j.ajog.2020.10.022.
[3]FISHEL BARTAL M, SIBAI B M. Eclampsia in the 21st cen-tury[J]. American Journal of Obstetrics and Gynecology,2020. doi:10.1016/j.ajog.2020.09.037.
[4]CHEN A P, ZHAO H F, WANG J L, et al. Haplotype analysis of candidate genes involved in inflammation and oxidative stress and the susceptibility to preeclampsia[J]. Journal of Immunology Research, 2020,2020:4683798.
[5]AHMADIAN E, RAHBAR SAADAT Y, HOSSEINIYAN KHATIBI S M, et al. Pre-Eclampsia: microbiota possibly playing a role[J]. Pharmacological Research, 2020,155:104692.
[6]KELWICK R, DESANLIS I, WHEELER G N, et al. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family[J]. Genome Biology, 2015,16:113.
[7]LI K, TAY F R, YIU C K Y. The past, present and future perspectives of matrix metalloproteinase inhibitors[J]. Pharmacology & Therapeutics, 2020,207:107465.
[8]NAMLI KALEM M, KALEM Z, Y?CE T, et al. ADAMTS 1, 4, 12, and 13 levels in maternal blood, cord blood, and placenta in preeclampsia[J]. Hypertension in Pregnancy, 2018,37(1):9-17.
[9]XIAO J, FENG Y, LI X Y, et al. Expression of ADAMTS13 in normal and abnormal placentae and its potential role in angiogenesis and placenta development[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2017,37(9):1748-1756.
[10]PU X Y, CHAN K, YANG W, et al. Effect of a coronary-heart-disease-associated variant of ADAMTS7 on endothelial cell angiogenesis[J]. Atherosclerosis, 2020,296:11-17.
[11]MEAD T J, APTE S S. ADAMTS proteins in human disorders[J]. Matrix Biology, 2018,71/72:225-239.
[12]KESSLER T, ZHANG L, LIU Z Y, et al. ADAMTS-7 inhi-bits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1[J]. Circulation, 2015,131(13):1191-1201.
[13]ZHANG L, ZHAO F, LI C, et al. Hypomethylation of DNA promoter upregulates ADAMTS7 and contributes to HTR-8/SVneo and JEG-3 cells abnormalities in pre-eclampsia[J]. Placenta, 2020,93:26-33.
[14]PEREIRA A, PALMA DOS REIS R, RODRIGUES R, et al. Association of ADAMTS7 gene polymorphism with cardiovascular survival in coronary artery disease[J]. Physiological Genomics, 2016,48(11):810-815.
[15]BROWN M, MAGEE L, KENNY L C, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice[J]. Pregnancy Hypertension, 2018,13:291-310.
[16]FIKADU K, G/MESKEL F, GETAHUN F, et al. Determinants of pre-eclampsia among pregnant women attending perinatal care in hospitals of the Omo district, Southern Ethiopia[J]. Journal of Clinical Hypertension (Greenwich, Conn), 2021,23(1):153-162.
[17]YEO S, DAVIDGE S T. Possible beneficial effect of exercise, by reducing oxidative stress, on the incidence of preeclampsia[J]. Journal of Womens Health & Gender-Based Medicine, 2001,10(10):983-989.
[18]CUBRO H, NATH K A, SUVAKOV S, et al. Mechanisms of vascular dysfunction in the interleukin-10-deficient murine model of preeclampsia indicate nitric oxide dysregulation[J]. Kidney International, 2021,99(3):646-656.
[19]CHEN W, QIAN L, WU F H, et al. Significance of Toll-like receptor 4 signaling in peripheral blood monocytes of pre-eclamptic patients[J]. Hypertension in Pregnancy, 2015,34(4):486-494.
[20]BERNARDI F C B, FELISBERTO F, VUOLO F, et al. Oxidative damage, inflammation, and Toll-like receptor 4 pathway are increased in preeclamptic patients: a case-control stu-dy[J]. Oxidative Medicine and Cellular Longevity, 2012, 2012:636419.
[21]DENNEY J M, NELSON E L, WADHWA P D, et al. Longitudinal modulation of immune system cytokine profile during pregnancy[J]. Cytokine, 2011,53(2):170-177.
[22]IVES C W, SINKEY R, RAJAPREYAR I, et al. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review[J]. Journal of the American College of Cardio-logy, 2020,76(14):1690-1702.
[23]EL-SAYED A A F. Preeclampsia: a review of the pathogenesis and possible management strategies based on its pathophysiological derangements[J]. Taiwanese Journal of Obstetrics & Gynecology, 2017,56(5):593-598.
[24]CHAN K, PU X Y, SANDESARA P, et al. Genetic variation at the ADAMTS7 locus is associated with reduced severity of coronary artery disease[J]. Journal of the American Heart Association, 2017,6(11):e006928.
[25]BAI X H, WANG D W, KONG L, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor[J]. Molecular and Cellular Biology, 2009,29(15):4201-4219.
[26]MU Y, ZHOU D N, YAN N N, et al. Upregulation of ADAMTS-7 and downregulation of COMP are associated with spontaneous abortion[J]. Molecular Medicine Reports, 2019,19(4):2620-2626.
[27]PU X Y, XIAO Q Z, KIECHL S, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a co-ronary-artery-disease-associated variant[J]. American Journal of Human Genetics, 2013,92(3):366-374.
[28]LI H W, SHEN M, GAO P Y, et al. Association between ADAMTS7 polymorphism and carotid artery plaque vulnerability[J]. Medicine, 2019,98(43):e17438.
[29]CHEN L F, HU W D, LI S N, et al. Genetic variants of ADAMTS7 confer risk for ischaemic stroke in the Chinese population[J]. Aging, 2019,11(16):6569-6583.
(本文編辑马伟平)