An ammonia-free denitration method: Direct reduction of NOx over activated carbon promoted by Cu-K bimetals
2022-05-30XIAORaoZHANGJunfengZHAOLingkui
XIAO Rao ,ZHANG Jun-feng,* ,ZHAO Ling-kui
(1. Department of Environmental Science and Engineering, College of Environment and Resources, Xiangtan University,Xiangtan 411105, China;2. Hunan Provincial Environmental Protection of Engineering Technology Center of Air Complex Pollution control (XTU),Xiangtan 411105, China)
Abstract: As ammonia slip becomes more serious with the traditional deNOx application, ammonia-free technologies have received more and more attention recently. Cu-K bimetal loaded activated carbon catalysts were prepared by equivalentvolume impregnation method for the direct reduction of NO and showed good NO reduction performance in a wide temperature range under temperature-programmed surface reactions (TPSRs) conditions in aerobic and anaerobic environments. The catalysts were characterized by BET, SEM, XRD, XPS, H2-TPR, Raman and FT-IR techniques and the NO reduction mechanism was analyzed. Experimental results show that the active functional groups formed on the surface of activated carbon are the important intermediate products and play a key role in the reduction reaction. The presence of O2 greatly promotes the formation of the intermediate, C(O) (Oxygen-containing functional groups on the carbon surface),leading to the increase reduction rate of NO. The bimetallic oxides catalysts are obviously effective to directly reduce NO.When the ratio of copper: potassium is 2∶1, the NO reduction efficiency is about 90% at 300 °C. The catalytic activity mainly depends on the redox cycle of CuO/Cu2O, and the potassium inhibits the agglomeration of copper on the surface of carbon materials and enhances the catalytic reactivity of Cu.
Key words: activated carbon;ammonia-free denitration;bimetal catalysis;Cu;K
Small and medium-size coal-fired boilers are major anthropogenic emissions sources of NOx, which still hold a large market share and are widely distributed in China[1,2]. Nitrogen oxides (NOx) leads to air pollution, acid rain and photochemical smog[3-5].Ammonia slip from the traditional deNOxsystem has been widely concerned in the past two decades, which is considered to be the main factor that causes frequent smog in a large area even after sulfur dioxide (SO2) and NOxare effectively controlled[6-8]. Therefore, the development of ammonia-free denitration technology is particularly urgent.
Selective catalytic reduction (SCR)[9]and selective non-catalytic reduction (SNCR)[10]are usually used for the NOx reduction when NH3is used as the reducing agent. However, there are some major problems with the SCR-NH3, such as high cost of construction and regeneration of the deactivated catalysts as well as NH3slip[11]. The problem of NH3slip also exists in the SNCR system[12,13]. Since 1970s and 1980s, the researches on NOxreduction at high temperatures by carbonaceous solids have been reported[14-19]. Carbon is considered as one of the promising materials for the removal of NO without ammonia. Over the past few years, activated carbon has attracted considerable attention due to its excellent advantages including excellent absorbability, low-temperature stability and high-temperature reduction[20,21]. The critical point is how to reduce the reaction temperature and improve the carbon utilization. In general, the different metal modification plays a huge influence on the selectivity and reducibility of carbon. Accordingly, some monometal oxides catalysts, such as alkali metals Ca, K, Cs,transition metals Cu, Fe, Ni Co, and noble metal Pt,etc, have been studied as catalysts loaded on activated carbon for removal of NO[22-26]. Alkali metals Cs, K can strongly enhance the C-NOxreaction without substantial carbon consumption by O2[27-29]. Ca as the active component can reduce the generation of CO2[30].In the NO reduction by C-based catalysts, transition metals can notably increase the N2selectivity[31-36].Different transition metals also have different performance in the catalytic reduction process. Less CO is released over Cu/Carbon in the process of deNOx. Cu/Carbon has higher selectivity to N2than Fe/Carbon[37]Ni/Carbon exhibits the highest N2selectivity for NOxreduction[38]. In addition, the transition metals differ in C-NOxreactions due to their different redox cycle capacity[27,39]. Feng et al.[40]studied the synergistic catalytic effect of alkali metal K and transition metal Cu on carbon reduction denitrification.A systematic investigation on the influence mechanism as well as a connection between bimetallic catalysis and NO conversion has rarely been reported so far.
In this study, the catalytic carbon reduction of NOx(CharR-NOx) was used to reduce the NOxin the flue gas. Carbon was used as a reducing agent and catalyst carrier modified by catalytic active components. The reaction mechanism was analyzed using temperatureprogrammed surface reactions (TPSRs). The catalytic effect of Cu-K bimetals on the reaction, along with the effect of oxygen in flue gas, was investigated to provide valuable research reference for the promotion of ammonia-free denitration technologies in industry.
1 Experimental
1.1 Material preparation
Shanghai Qiyue coconut-shell activated carbon(CSAC) was selected, ground, and screened to get 20-40 mesh particles. It was pretreated by boiling with deionized water to remove any water-soluble and volatile substances (such as ash and impurities) on the surface and the internal pores of the activated carbon.In addition, elemental analysis of the unmodified coconut-shell activated carbon was carried out using the Vario El Cube elemental analyzer. The result is shown in Table 1.

Table 1 Elemental analysis of coconut-shell activated carbon
The specific surface area and pore size of the activated carbon were calculated by the multi-point BET method and BJH method using the Quantachrome NOVA2200E automatic physical adsorption instrument. The result is shown in Table 2.

Table 2 Surface area and pore volume of CSAC obtained by BET (N2, 77 K)
The pretreated coconut-shell activated carbon(CSAC) was loaded with different types of metal oxide catalysts containing 10% MxO (metal oxide: CuO or K2O) using the equal-volume impregnation method with metal nitrate as the precursor. Using CuO as a preparation process sample, an appropriate amount of copper nitrate (Cu(NO3)2) was dissolved in water to prepare a salt solution, and the pH value was adjusted to 1-3 using nitric acid (HNO3). Then, the pretreated CSAC was added to the salt solution and sealed with plastic wrap. The mixture was subjected to ultrasonic treatment at 28 kHz for 30 min, soaked at room temperature for 12 h, and then placed in a blast-drying oven to dry at 90 °C for 12 h. Finally, Cu-CSAC was prepared by calcination at 400 °C for 2 h under the protection of N2atmosphere. In this study, copper and potassium bimetallic catalytic reducing agents with a total load of 10% were prepared and denoted asnCumK-CSAC, andn∶mwas the ratio of copper to potassium.
1.2 Characterization
The FT-IR spectra of catalysts were obtained by using ALPHA Fourier transform infrared spectrometer(Bruker Technologies, China) in the wavelength range of 400-4000 cm-1. Raman spectra of catalysts with different bimetallic components were obtained by using INVIA laser microscopy confocal Raman spectrometer(Renishaw, Britain) in the wavelength range of 50-3200 cm-1. An automatic D/MAX-2500/PC X-ray polycrystalline powder diffractometer (Rigaku, Japan)was used to obtain the XRD spectra of catalysts before and after the reaction by scanning with the Cu-Kα radiation source in the scanning range of 2θ=5°-90°.Zeiss Sigma 300 scanning electron microscope (Zeiss,Germany) was used to obtain the surface morphology images of catalysts before and after loading and reaction. Peak fitting and elemental analysis of catalysts before and after reaction were carried out by Thermo Scientific K-Alpha X-ray photoelectron spectroscopy (Thermo, America). The H2-TPR experiments were performed on AutoChem 2920 equipment (Micromeritics, America) to study the reduction behavior of active phases and metal species under different conditions.
1.3 Material activity measurements
The programmed temperature surface reductions(TPSRs) experiment of the materials for removal of NO was measured in a fixed bed quartz reactor (i.d.12 mm) at atmospheric pressure, as shown in Figure 1.The temperature range of the TPSRs experiment was 300-540 °C, and the heating rate was 3 °C/min. The simulated flue gas (SFG) components included 0.05%NO, 0-6% O2, and N2as the balanced gas.Subsequently, the SFG with different oxygen concentrations was mixed in the premixer to acquire the desired concentration. A gas hourly space velocity(GHSV) was 8000 h-1(the material mass was 2.0 g),which corresponded to a total flow rate of 600 mL/min during the experiments. The gas, including NO, O2, CO and CO2, was analyzed continuously with a C-600 multi-component flue gas analyzer (Seitron, France).

Figure 1 Schematic diagram of the CharR-NOx reaction unit
The reduction rate of NO was calculated as follows:
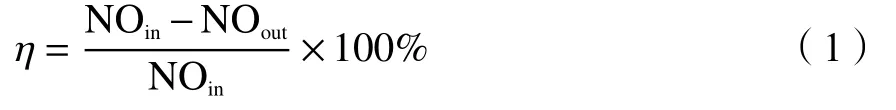
Carbon material consumption was defined as the mass of carbon material consumed in the process of programmed temperature surface reduction reaction.The carbon material generated the main oxidation product CO2and by-product CO after the CharR-NOxreaction. Therefore, the consumption of carbon material could be obtained by integrating the curves of products to calculate as the following formula.

where,Vm: the molar volume of the standard gas, 22.4 L/mol;MC: the molar mass of the carbon, 12;Q: the simulated smoke flow, 600 mL/min;t: the programmed heating time, 72 min.
2 Results and discussion
2.1 Reaction activity
2.1.1 Effect of inlet oxygen concentration
Figure 2 shows the denitration rate of the coconutshell activated carbon without metal loaded at different reaction temperatures and oxygen levels.
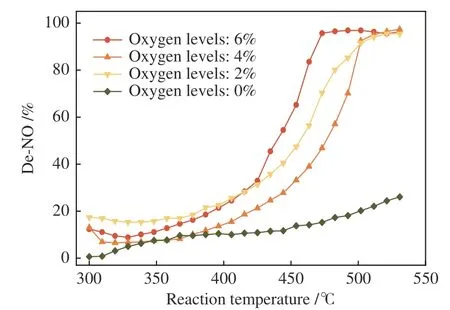
Figure 2 Effect of the inlet oxygen concentration on the CharR-NOx reaction
From Figure 2, it can be seen that the reduction rate of NO is higher when the reaction temperature is higher, and the denitration growth rate in an aerobic environment is significantly higher than that in an anaerobic environment. The overall denitration rate is less than 20% at low temperatures (300 -400 °C).Starting from 400 °C, the denitration rate by CSAC increases significantly in an aerobic environment. At 6% oxygen content, the denitration rate reaches 100%at 470 °C, and it is over 90% at 500 °C at 2% and 4%oxygen levels. In an anaerobic environment, the denitration rate is only about 20%, indicating that oxygen has an obvious promotion effect on CharR-NOxespecially at high temperatures. At the oxygen levels of 2% and 4%, the minimum temperature of 90%denitration (t90) exhibits no obvious deviation.However, the minimum temperature of 100%denitration (t100) at the 6% inlet oxygen content is significantly lower than those at 2% and 4% oxygen levels, indicating that an oxygen-rich environment is conducive to the downward shift of the CharR-NOxreaction temperature window.
2.1.2 Effect of bimetallic catalysis
Figure 3 shows the denitration effect of coconutshell activated carbon materials modified by 10%bimetallic copper and potassium with different loading proportions in aerobic and anaerobic environments. It also shows a comparison oft50(the minimum temperature at 50% denitration efficiency) andt90(the minimum temperature at 90% denitration efficiency)under different oxygen levels.
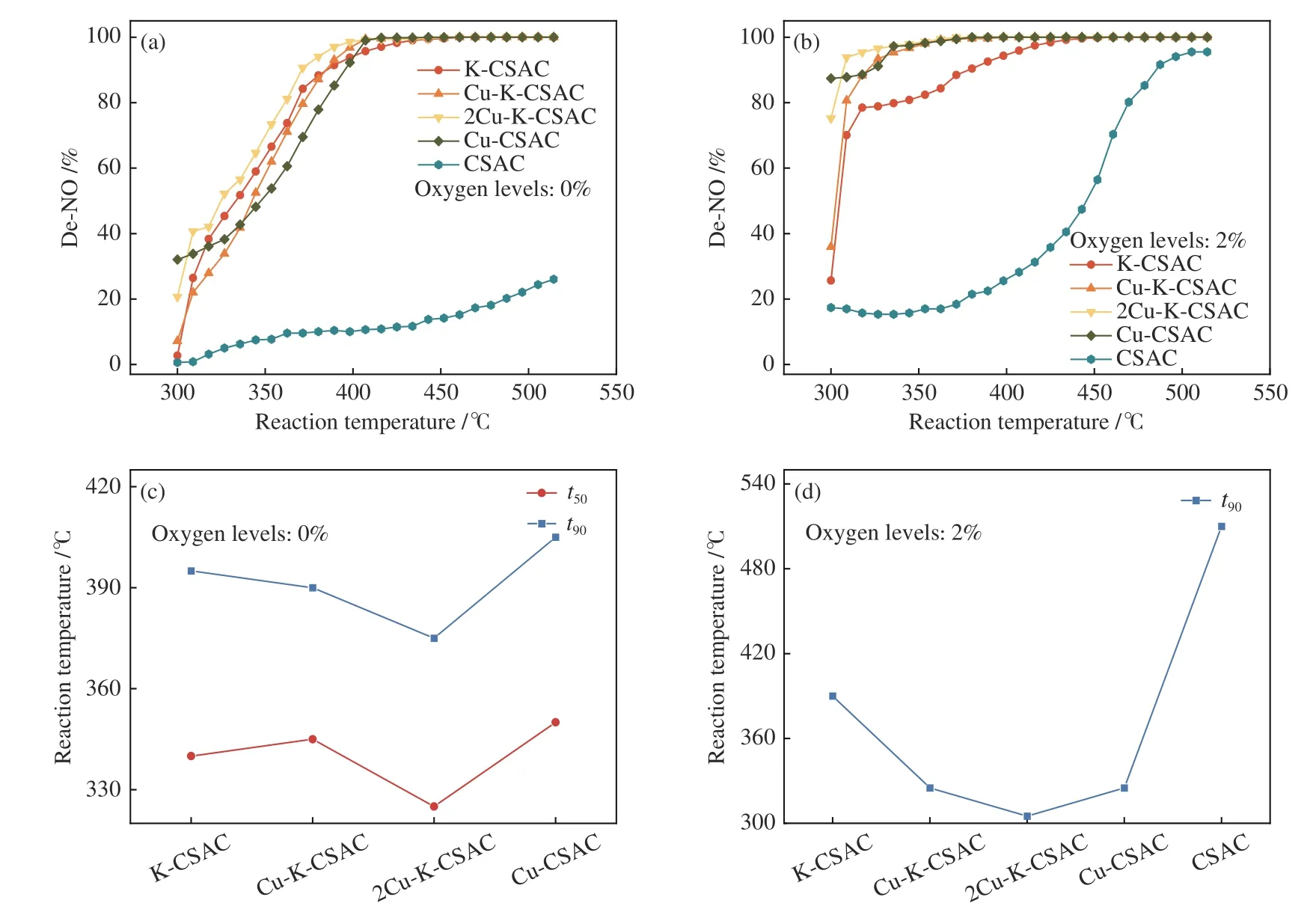
Figure 3 Effects of bimetallic catalysis on the reduction rate of the CharR-NOx reactions without oxygen ((a), (c)) and aerobic ((b),(d)) environments
As can be seen in Figure 3(a), in the absence of oxygen, irrespective of the transition metal copper,alkali metal potassium, or bimetallic synergy, the CharR-NOxreactivity of the modified carbon material is greatly improved. However, at about 300 °C, the synergistic effect of bimetals is not obvious, and the catalytic reduction effect of a single metal on NO is more obvious. Nevertheless, by comparing thet50andt90in Figure 3(c), especially the contrast line oft90, it can be seen that bimetallic synergy significantly reduces the temperature window of efficient denitration. Thet90values of 2Cu-K-CSAC, Cu-KCSAC, K-CSAC, and Cu-CSAC are 370, 390 , 395 and 405 °C, respectively. The best condition is copper∶potassium= 2∶1. In other words, 2Cu-K-CSAC can achieve a 90% NO reduction rate at the lowest temperature (370 °C).
It can also be seen from Figure 3(b) and 3(d) that the catalytic reduction of metals is quite obvious in an aerobic environment. Similar to the oxygen-free environment, by comparing thet90with that of unmodified coconut-shell activated carbon, it can be seen that the synergistic catalytic reduction of bimetals has a significant effect on reducing the reaction temperature window. Thet90values of 2Cu-K-CSAC,Cu-K-CSAC, K-CSAC, Cu-CSAC, and CSAC are 300, 325, 390, 325, and 510 °C, respectively. As copper∶potassium= 2∶1, 2Cu-K-CSAC can achieve a 90% NO reduction rate at 300 °C.
2.1.3 Carbon consumption
Figure 4 shows a comparison of the total carbon consumption of different metals modified carbon materials in the process of the TPSRs. As can be seen from Figure 4(a), in the absence of oxygen, the total carbon consumption is closely and positively correlated with the NO reduction efficiency of each material. In an anaerobic, the total carbon consumption is low (<18 mg). It can be inferred that under this reaction condition the carbon consumption is mainly by the oxidation-reduction of carbon and NO. As can be seen in Figure 4(b), the total carbon consumption increases significantly, and the main carbon loss comes from the highly active oxidation reaction between oxygen and carbon materials, which is the main side reaction in the CharR-NOxreaction process. In particular, the total carbon consumption increases significantly with the increase of the alkali metal potassium loading. This is probably because alkali metals have a more significant promotion effect on the carbon-oxygen reaction and a more obvious effect on CO2production.

Figure 4 Total carbon consumption of bimetallic catalytic carbon materials in the absence of oxygen (a) and oxygen (b)
It is difficult to meet the requirements of an oxygen-free environment during actual applications, so the materials that can still maintain low carbon consumptions in a low-oxygen environment become the object of further research.
2.2 Structural properties
2.2.1 FT-IR spectroscopy
Figure 5 shows the infrared spectrum of coconutshell activated carbon during each stage of the modification process. A characteristic peak is observed at 3450 cm-1, which is assigned to the stretching vibration absorption peak of hydroxyls (-OH) on the original carbon skeleton. The peaks at 1635 cm-1and 1060 cm-1correspond to the stretching vibration absorption peaks of ethylene linkages (C=C) and carbon-oxygen single bonds (C-O), respectively. They are all chemical bond structures on the original carbon skeleton, and it can be seen from the figures that the modification process has no specific effect on the types of C, H, and O functional groups, or the saturation of the carbon chain. In addition, after impregnation and before calcination, there is a sharp aliphatic nitro group(-NO2) characteristic absorption peak at 1386 cm-1for K-CSAC and Cu-CSAC, which disappears after calcination. It can be seen that the nitrate on the precursor can be completely decomposed after calcination at 400 °C, forming metal oxides on the surface of the carbon. The peak around 600 cm-1in the non-characteristic region can be attributed to the characteristic peak of the metal-oxygen bond (M-O). It is found that the C-O bond and the metal-oxygen bond(Cu-O) increase significantly after the impregnation of Cu-CSAC, indicating that the impregnation effect of copper nitrate is significantly higher than that of potassium nitrate. At 3780 cm-1, the double-frequency peak of hydroxys (-OH) appears in the calcinated coconut-shell activated carbon material, which indicates that the surface scattering of the calcinated carbon is enhanced and the surface flaws are increased.
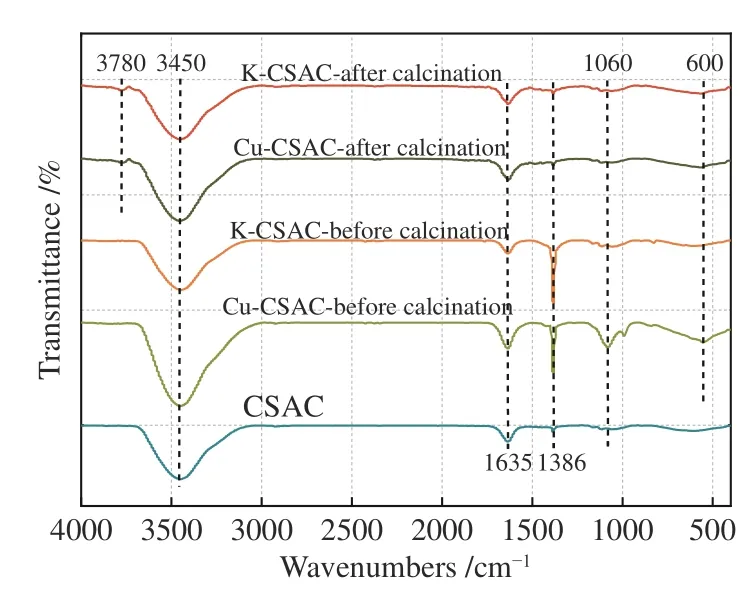
Figure 5 FT-IR spectra of the CSAC modification process
2.2.2 Raman spectroscopy
In Figure 6, the Raman spectra of bimetallic supported coconut-shell activated carbon materials are shown. The symmetric stretching vibration caused by thesp2hybridization of the carbon atom leads to the scattered D-peak near 1350 cm-1, which represents the occurrence of defects in the internal lattice of the carbon material and the degree of disorder of the material. The tensile vibration caused by thesp2hybridization of the carbon atom leads to the scattered G-peak near 1350 cm-1, which is the characteristic peak of the carbon material itself. It can be seen from the Raman spectra that the coconut-shell activated carbon has two peaks of the carbon material before and after modification. The load modification of Cu with a high atomic weight makes the D-peak shift of CSAC move to the lower band. With metal modification, the halfpeak width and peak area of CSAC increase, and the increase in the bimetallic supporting materials are more obvious, which is consistent with the phenomenon that the types of doping components increase.

Figure 6 Raman spectrogram of Cu-K bimetal supported CSAC
The intensity ratio of the D-peak to G-peak (R=ID/IG) is used to reflect the degree of disorder of the activated carbon materials. It is found that, the higher the number of active sites of the CharR-NOxreaction,the stronger the intensity of the D-peak, the higher the R-value, and the higher the degree of disorder of the materials. The R-value of the unmodified CSAC is 0.703. With the loading of metal elements, the Rvalues of the different metals modified CSAC increase,which is consistent with the phenomenon of the frequency-doubling peak in the FT-IR spectrum shown in Figure 5. The R-values of Cu-CSAC, 2Cu-K-CSAC,Cu-K-CSAC, and K-CSAC are 1.099, 1.158, 1.069,and 1.062, respectively. Among them, the R-value of 2Cu-K-CSAC exhibits the most obvious increase,which is consistent with the experimental phenomenon in Figure 3 whereby the 2Cu-K-CSAC exhibits optimal reactivity. At the 1614 cm-1Raman shift, 2Cu-K-CSAC exhibits a disorder-induced peak (D′-peak) with a weak signal, which also belongs to the characteristic band generated by the disorder between carbon atoms[41].Finally, it is worth mentioning that the G-peak of Cu-CSAC is split, forming a weak G--peak, which is related to the copper element loading capacity and its better performance in terms of carbon consumption.
2.2.3 SEM
The SEM micrographs of CSAC, 2Cu-K-CSAC,and Cu-CSAC before the CharR-NOxreaction are shown in Figure 7. It can be seen that the pore structure of the coconut-shell activated carbon is obvious and its surface is smooth. Significant macropores (around 500 nm) can be observed with a uniform pore size distribution, which promotes the loading of metal oxides on the surface. From Figures 7(a), 7(b) and 7(c),it can be seen that some holes on the surfaces of 2Cu-K-CSAC and Cu-CSAC collapse, and their surfaces become rough. With the pore size increase, the differences in the pore size distribution become larger.
High-magnification SEM micrographs (×50000)of 2Cu-K-CSAC and Cu-CSAC are shown in Figures 7(c) and 7(e). After the loading of CuO or CuO/K2O,the metal oxides (<100 nm) are mainly dispersed in the inner wall and in the vicinity of the macropores.CuO/Cu2O/K2O is mainly loaded with irregular spherical particles on the 2Cu-K-CSAC, and is relatively dispersed. The CuO/Cu2O on the Cu-CSAC is mainly loaded with irregular star-shaped particles,and its distribution is relatively dense. These indicate that the distribution of metal oxides on 2Cu-K-CSAC is more uniform, which further confirms the conclusion that the alkali metal potassium inhibits the aggregation of copper on the surface of the coconut-shell activated carbon to a certain extent, as shown in the XRD analysis.
The SEM micrographs of 2Cu-K-CSAC and Cu-CSAC after the CharR-NOxreaction are shown in Figure 8. The structures of the 2Cu-K-CSAC and Cu-CSAC coconut-shell activated carbon are unstable and crushed at high temperatures after participating in the CharR-NOxreaction. The crushing phenomenon of 2Cu-K-CSAC is more obvious, and the coconut-shell activated carbon matrix fracture and pore collapse are more severe, so the carbon loss of the material is more serious. This is consistent with the results of CharRNOxreactivity and the material carbon balance in Figure 3 and Figure 4.
The Prince later remarked that he thought Diana was a very jolly and attractive girl, full of fun, though Diana herself believed that he barely noticed me at all.
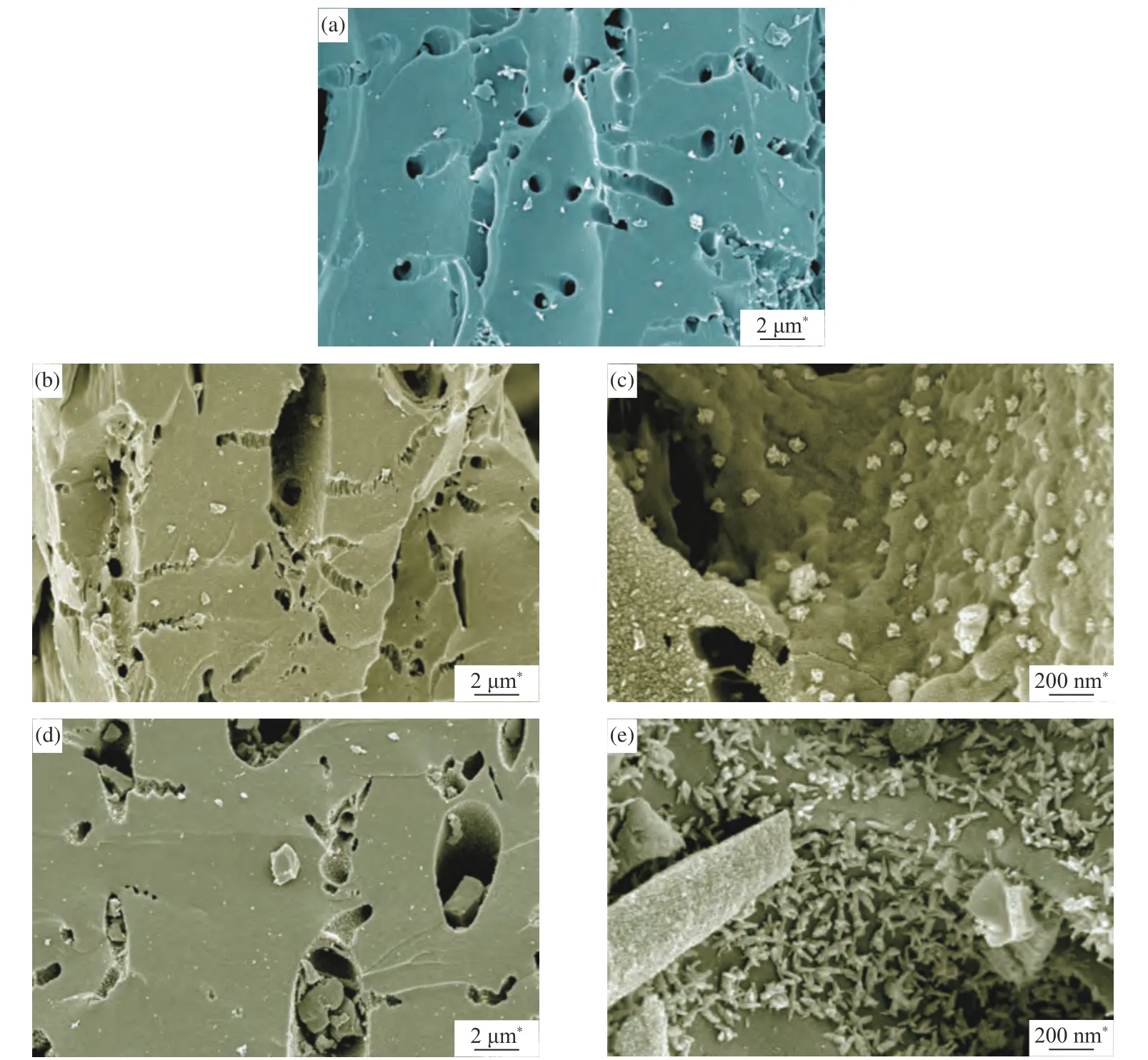
Figure 7 SEM images of CSAC (a), 2Cu-K-CSAC ((b), (c)), and Cu-CSAC ((d), (e))
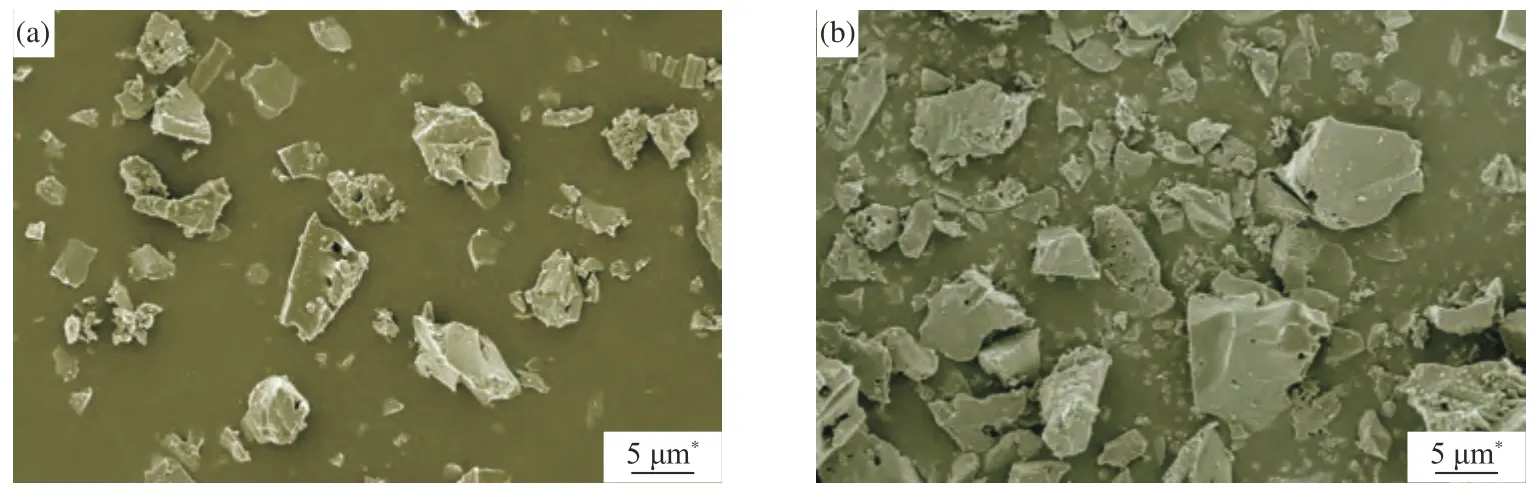
Figure 8 SEM images of 2Cu-K-CSAC (a) and Cu-CSAC (b) after participation in reactions
2.3 Redox properties
2.3.1 XRD
Figure 9 shows the XRD patterns of CSAC loaded with different metals. It can be seen from the figure that the broadened diffraction peaks of the graphitized structure exist at 2θ=24.6° for all the samples before and after the CharR-NOxreaction, and the diffraction peaks of carbon materials also exist at 2θ=43.9° and 51.2° for all the samples. Thus, the basic structure of the carbon material remains unchanged throughout the reaction process, and the broadened diffraction peaks indicate that it is an amorphous carbon structure, which is consistent with the results of Raman spectrum analysis in Figure 6.
Furthermore, Figure 9 shows that CuO and Cu2O are the main crystalline phases in the Cu-CSAC (a) and 2Cu-K-CSAC (b) samples. Unreacted modified coconut-shell activated carbons (Cu-CSAC-Before,2Cu-K-CSAC-Before) only have a few characteristic diffraction peaks of CuO/Cu2O with weak signals,indicating that the CuO/Cu2O exist on the surface of the coconut-shell activated carbon as an amorphous crystal with uniform loading and good dispersion.Further, the XRD signal of K-CSAC is weak, but also has very obvious characteristic diffraction peaks of K2O at diffraction angles of 29.7°, 31.5°, and 39.2°.

Figure 9 XRD patterns of CSAC loaded with different metals
Figure 10 shows the XRD patterns of Cu-CSAC(a) and 2Cu-K-CSAC (b). Interestingly, the CuO characteristic diffraction peaks detected in the bimetallic catalytic materials with the addition of metal potassium are much fewer than those detected in the copper mono-metal supported materials before and after the reaction. These results indicate that the participation of alkali metal potassium inhibits the aggregation of copper and improves the stability of catalytic active substances on the surface of coconutshell activated carbon to a certain extent, which is consistent with the results of SEM and the better reactivity of the bimetallic materials shown in Figure 3.
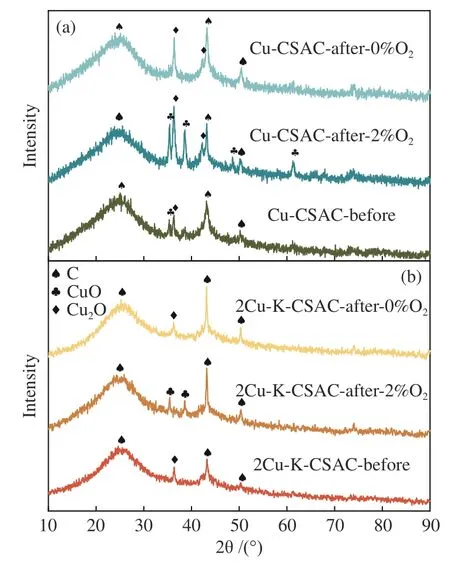
Figure 10 XRD patterns of Cu-CSAC (a) and 2Cu-K-CSAC(b) before and after the CharR-NOx reaction
2.3.2 H2-TPR
XRD analysis shows that the copper species in catalyst materials contain different valence states. H2-TPR experiments on the unloaded CSAC and the optimal active 2Cu-K-CSAC are performed to further explore the reducibility of copper-containing species as shown in Figure 11. It can be seen that the reduction activities of 2Cu-K-CSAC are significantly improved.There are two obvious low-temperature reduction peaks at 163 and 190 °C for 2Cu-K-CSAC, and its high-temperature reduction peak is lower than that of CSAC. A previous study[42]has shown that highly dispersed species with sufficient exposure to reducing gases can reduce the reduction temperature. Combined with XRD analysis results, the reduction peak at 163 °C can be determined as highly dispersed bulk CuO reduction, and the reduction peak at 190 °C can be attributed to Cu2O reduction to Cu. In addition, the H2-TPR results of K-CSAC show that K species can also effectively reduce the reduction temperature of carbon materials to 470 °C.
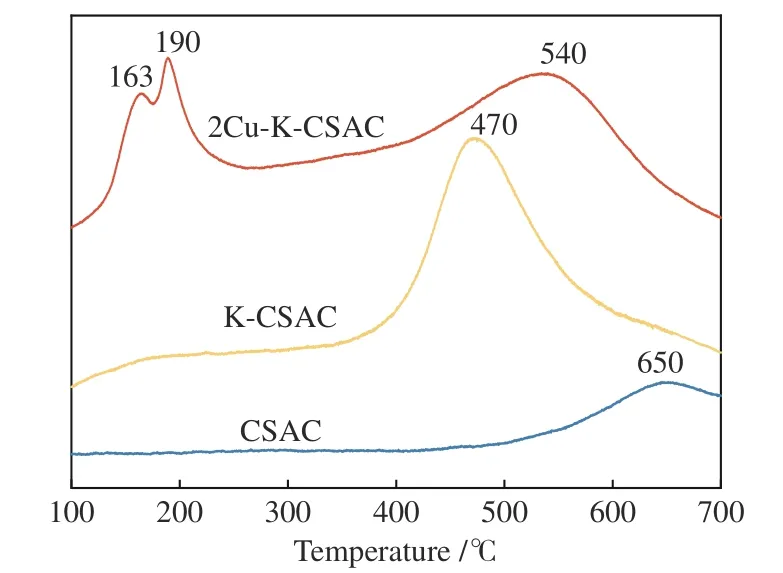
Figure 11 H2-TPR profiles of CSAC, K-CSAC and 2Cu-KCSAC before the CharR-NOx reaction
2.3.3 XPS
Figure 12 and Figure 13 show the XPS spectra of CSAC and 2Cu-K-CSAC before the CharR-NOxreaction. From the O 1sdiagram of the materials, it can be seen that the oxygen content increases and metaloxygen species appear after modification, which corresponds to the results of the XRD analysis. The higher the ratio of C-O to C=O, the stronger the reaction activity of the surface-adsorbed oxygen species, because the C -O bond energy is lower,allowing it to participate in the reaction more quickly,which is consistent with the results of an active reaction.
The surface composition and distribution of 2Cu-K-CSAC before and after the CharR-NOxreaction and the chemical states of each element are analyzed in Figure 13 and Figure 14. C is the primary original component of the activated carbon, and K is mainly distributed on the surface of the carbon in the form of K2O.
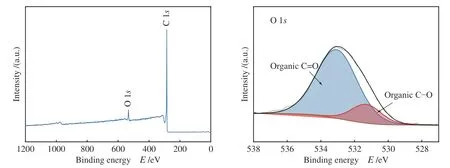
Figure 12 XPS of CSAC
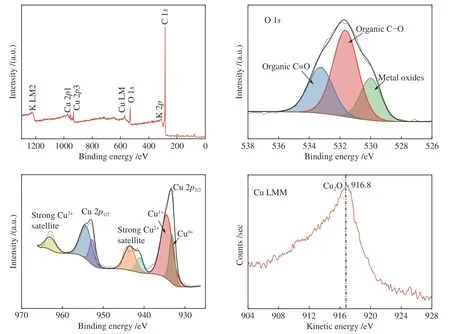
Figure 13 XPS of 2Cu-K-CSAC before the CharR-NOx reaction
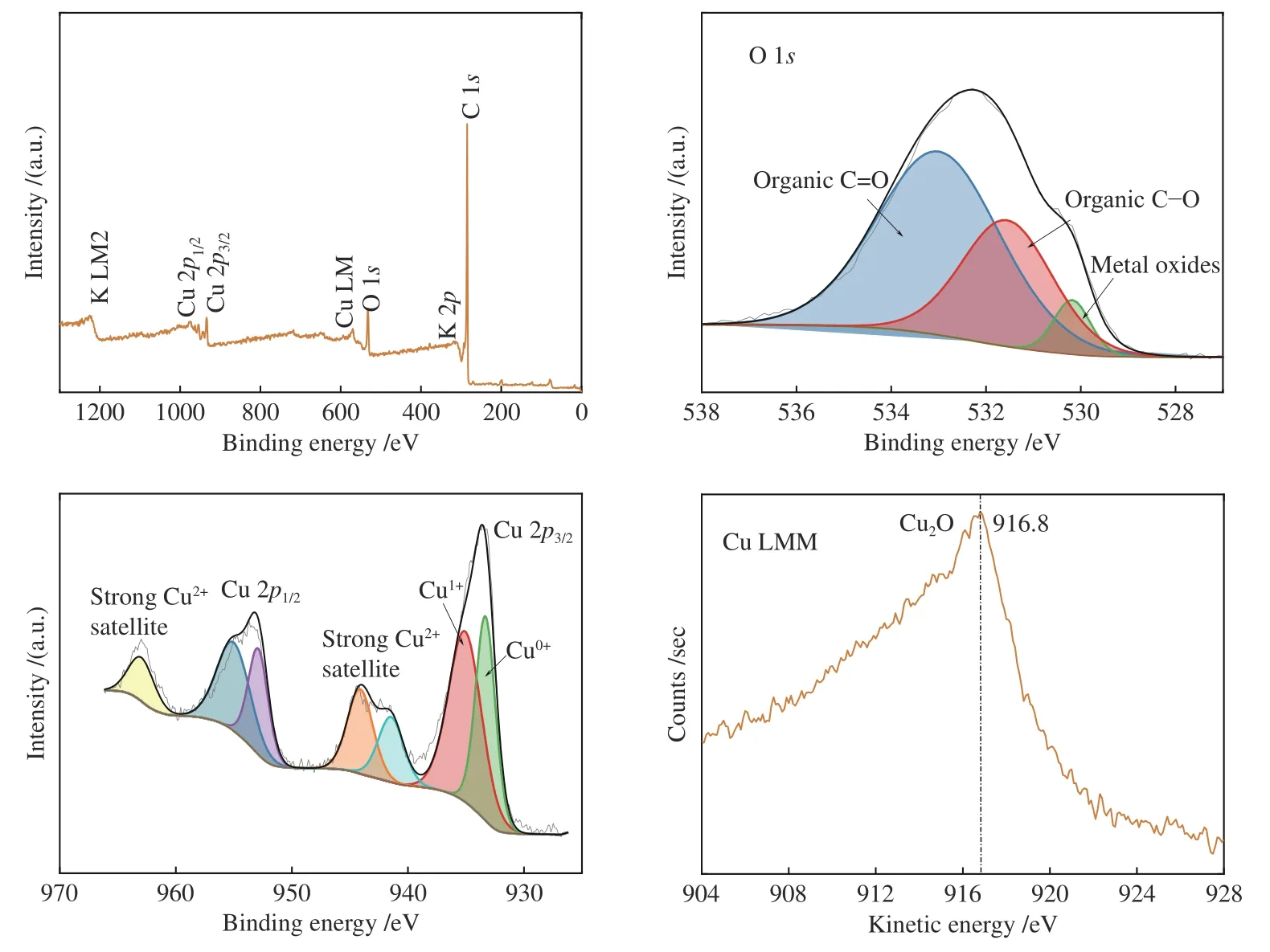
Figure 14 XPS of 2Cu-K-CSAC after the CharR-NOx reaction
From the Cu peaks, it can be seen that the binding energies of Cu 2p3/2are 933.18 and 934.48 eV, and the binding energies of Cu 2p1/2are 952.78 eV and 954.48 eV. Through the principal Cu LMM peak, the kinetic energy of Cu LMM is 916.8 eV. It can infer that the Cu2O crystal mainly exists on the surface. After the reduction reaction, the content of Cu(0) increases obviously, which indicates that both Cu2+and Cu+are reduced to Cu(0), which is consistent with the conclusions of XRD and TPR analysis. Cu2+has an observable collection of satellite features 943 eV.Therefore, it can also be inferred that a CuO crystal existed. The results above mentioned are consistent with the XRD results. The addition of Cu2+increases the concentration of free electrons and increases the electrical conductivity, enhancing the catalytic effect.
From the O peak spectra, the binding energies of O 1sare 530.00, 531.64, and 533.26 eV. These corresponds to O2-(metal-oxygen) at 530.00 eV, and the surface-adsorbed oxygen species C-O and C=O at 531.64 and 533.26 eV respectively. The results of the Raman spectra show that the material has structural defects. These defects increase the concentration of free electrons which is conducive to the generation of adsorbed oxygen. The adsorbed oxygen exists on the surface of carbon materials in the form of the C(O)functional group, which is an important intermediate product of the CharR-NOxreaction. It also actively participates in the redox cycle of CuO/Cu2O and catalyzes the reaction process. The change in the oxygen species before and after the reaction is not obvious, indicating that the service life of the material is considerably long.
According to the analysis in Table 3, the contents of Cu(II) and Cu(0) in 2Cu-K-CSAC increase before and after the reaction, while the content of Cu(I)decreases, proving that the oxidation and reduction of Cu species are carried out in the reaction process. The valence state moves slowly from the intermediate valence state to both ends, indicating that the redox cycle of the Cu species is stable. The change in Cu species on the surface of the carbon materials is analyzed using XPS curve fitting. The ratio of Cu(II) to Cu(I/0) increases as the reaction progress, which is consistent with the XRD results. Further, the reduction activity of carbon material is lower than that of oxygen and NOxoxidation, which can explain the relatively low loss of the 2Cu-K-CSAC carbon material, as shown in Figure 4.

Table 3 Cu 2p XPS curve-fitting analysis for 2Cu-K-CSAC
2.4 Mechanism discussion
From above results, the existence of an important intermediate product, the C(O) functional group, is found in the process of the CharR-NOxreaction[43,44].The oxidation capacity of O2on carbon materials is better than that of NO, and the reaction activation energy between oxygen and carbon is low. Based on the FT-IR results, along with the elemental analysis results, there are certain C(O) functional groups on the surface of the activated carbon material itself. In combination with the reaction mechanism diagram of NO and O2on the surface of the carbon materials(Figure 15), it can be seen that the C(O) functional group greatly promotes the reaction, and the presence of oxygen significantly increases the formation of C(O). In the process of carbon oxidation, an increased number of active sites C*are formed on the carbon surface, which promotes the reaction to continue.Moreover, the material’s structural defects lead to the formation of adsorbed oxygen on the surface.Therefore, in an aerobic environment, the reduction efficiency increases rapidly, and the temperature window of the CharR-NOxreaction moves down.
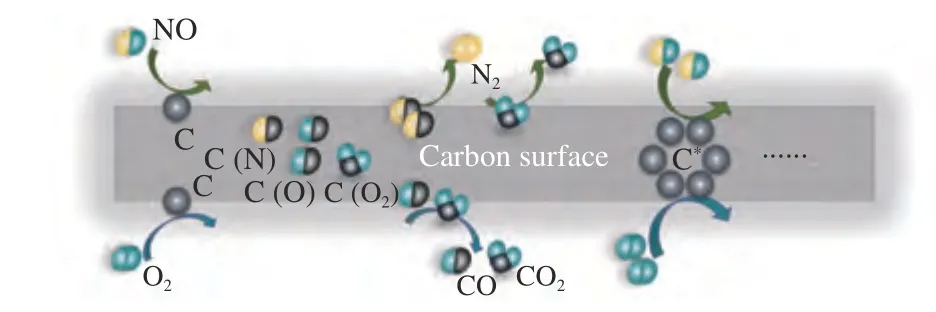
Figure 15 Proposed CharR-NOx reaction mechanism of NO and O2 on the carbon surface
Based on the XRD and XPS spectroscopic results,along with the earlier reports[27,39], the possible mechanism of bimetallic catalytic denitration by carbon is shown in Figure 16. The main function of K species is to make Cu species more evenly be loaded on the surface of the material. Similarly, from the XRD results, it can be seen that, after the reaction in an aerobic environment, the characteristic diffraction peaks of CuO increase and the signals become stronger, primarily owing to the presence of a CuO/Cu2O redox cycle, as shown in Figure 16. The redox cycle of CuO/Cu2O is considered to be the key mechanism of catalyst action in the CharR-NOxreaction[45]. In the presence of oxygen, the cycle moves towards CuO, such that more characteristic diffraction peaks of CuO appear on the surface of the product. In the absence of oxygen, only the characteristic diffraction peak of Cu2O can be detected. This catalytic mechanism can well explain the phenomenon that the CharR-NOxreaction activity of the material is better in an aerobic environment because the oxygen greatly promotes the redox cycle of the catalytic active component. O2and C simultaneously promote the redox cycle of CuO/Cu2O. The redox cycle of surface Cu species promotes the renewal of the surface functional groups of carbon materials, catalyzing the reaction between C and NOx.

Figure 16 REDOX cycle of CuO/Cu2O
3 Conclusions
A denitration agent without the addition of ammonia was prepared through incipient-wetness impregnation. The agent uses the reduction properties of the carbon material itself and the auxiliary catalytic effect of metals to achieve the efficient reduction of NO.
Oxygen has a good effect in terms of promoting the CharR-NOxreaction. At a lower oxygen content(2%), the reduction efficiency reaches a relatively high level. Therefore, the oxygen concentration should not be too high; otherwise, excessive amounts of carbon materials would be lost. The bimetallic catalytic reducing agent can significantly improve the reaction efficiency of CharR-NOx. The temperature window of the reaction is reduced, and the reduction rate of NO reaches 90% at 300 °C. On the one hand, the degree of disorder of the original carbon material structure is improved and the reactive oxygen species C -O increases during the loading process. On the other hand, the main catalytic group is the transition metal copper, CuO/Cu2O redox cycle is the key mechanism affecting the catalytic process, and alkali metal potassium can significantly inhibit the surface agglomeration of copper.
杂志排行
燃料化学学报的其它文章
- Catalytic hydrogenolysis of diphenyl ether over Ru supported on amorphous silicon-aluminum-TiO2
- 孪晶HZSM-5@Silicalite-1核壳结构催化剂的制备及甲苯甲醇烷基化性能研究
- Unraveling the role of Ni13 catalyst supported on ZrO2 for CH4 dehydrogenation:The d-band electron reservoir
- 钛副族金属氧化物催化合成气转化性能的研究
- 不同晶面Co基催化剂上CO活化行为研究
- 十氢萘选择性开环反应的研究进展
