Preparation of the core-shell HMX@CS microparticles by biological excitation:Excellent hydrophobic-oleophilic properties and decreased impact sensitivity effectively
2022-05-24YueqiLiHengZhiPingYeXingqunZhngChngpingGuo
Yue-qi Li , Heng Zhi , Ping Ye , Xing-qun Zhng , Chng-ping Guo ,*
aSichuan Co-Innovation Center for New Energetic Materials, Southwest University of Science and Technology (SWUST), Mianyang, 621010, PR China
bAnalysis and Testing Center of Southwest University of Science and Technology (SWUST), Mianyang, 621010, PR China
Keywords:HMX Core-shell Sensitivity Hydrophobic-oleophilic Mechanical properties
ABSTRACT Inspired by the phenomenon of superhydrophobic plants and animals in nature, 1,3,5,7-tetranitro-1,3,5,7-tetrazocane (HMX)@copper stearate (CS) core-shell composites with similar properties was prepared.A rough shell layer on the surface of the HMX was observed by scanning electron microscopy(SEM),and a series of in-depth characterization confirmed the successful generation of CS and the coreshell structure of the samples.Differential scanning calorimeter (DSC) proves that the crystal transition temperature (204 °C) and high temperature decomposition exothermal temperature (284 °C) of HMX@CS is almost unchanged compared with pure HMX, which means HMX and CS have good compatibility.Then,the H50 of the samples also increased continuously(16.6 cm→33.7 cm)when the CS shell content increased from 1% to 5%, indicating that the CS shell has a certain buffering performance,and CS will absorb some heat and melt under the stimulation of impact due to its low melting point,which improved impact sensitivity of HMX effectively further.Moreover, HMX@CS has excellent hydrophobic and oleophilic performance, shows excellent wettability with lipid binder, and samples with appropriate CS shell content can continue to combustion stably after covering water.This waterproof and low sensitivity coating provides a new way for the development of multifunctional energetic materials.
1.Introduction
The research of energetic materials is an important field of military chemistry in the world.HMX (1,3,5,7-tetranitro-1,3,5,7-tetrazocane) belongs to ammonium nitrate explosives with high explosion performance [1,2].It has been widely used in solid fuel rocket propellent, nuclear weapons, PBXs (polymer bonded explosives), and other advanced ammunition [3-5].However, the modern battlefield environment not only requires ammunition to have greater damage power but also requires ammunition to have higher safety when exposed to external dangers[6,7].HMX still has many limitations in its high mechanical sensitivity and poor adhesive properties with polymer binders [8,9], considerable efforts have been devoted to the HMX with lower sensitivity and improved mechanical properties in the recent decades [10-13].The study of the insensitivity of explosives is helpful to improve the safety performance of explosives.Considering the technology and practical use,it is also necessary to have a forceful interaction between explosives and polymer binder [14].In PBXs, the interfacial interaction between the explosive crystal and binder determines its mechanical properties[15,16].The deformation and failure of PBXs are usually due to the weak adhesion between the explosive and binder [8,10], so the study of the mechanical properties of the explosives is also of great and positive significance in energetic materials.
Explosive desensitization technology mainly includes ultrafine[17], crystal control [18], eutectic [19], and coating technology,particle coatings are considered to be a common method to reduce explosive sensitivity,it can break through the limitation that cannot change the inherent molecules or components compared with other methods.The forming, mechanical and thermal properties can be adjusted by changing the material of the explosive coating.even make explosives have more diversified properties [20,21].As for the method of coating explosives, the physical and chemical properties of the coated materials are also diverse according to the different coating materials selected, so the methods adopted are also different, mainly including phase separation [22], chemical precipitation, and spray drying [23], etc.Over the past several decades,although remarkable progress has been made in decreasing sensitivity,insensitive explosives as the coating material can reduce the sensitivity of the original explosive and maintain the explosive performance,such as TATB[24,25]and TNT[26].F.Y.Gong[27]et al.studied and prepared the HMX/Ploydopamine (PDA) composites based on mussel inspiration, the phase transition of HMX is effectively inhibited and decreased the sensitivity of HMX after heating.Besides,PDA plays a similar function of double-sided adhesive that the adhesion between explosive and binder was greatly enhanced[9],PBXs tensile,compressive strength,strain and creep resistance were significantly improved [28].
The inspiration of bionic superhydrophobic surface comes from nature [29,30], there are many superhydrophobic phenomena in nature, among which the lotus leaf phenomenon is well known.After decades of research and development, superhydrophobic surfaces have been constructed on different materials for different purposes, such as self-cleaning properties, enhanced suspension properties, oil-water separation [30,31], etc.However, the idea of preparing a superhydrophobic surface has almost no application in energy-containing materials, and the key to preparing a superhydrophobic surface is to cover the surface of raw materials with a layer of materials with low surface energy and a rough micro-nano structure.Cheap metallic stearate has been used for creating a superhydrophobic surface by some researchers [32,33], and they also have an insensitivity effect for explosives[34].We developed a coating comprising copper stearate(CS)on the surface of HMX that decreased the sensitivity of HMX, CS will absorb some heat and melt under the stimulation of impact and friction due to its low melting point, further improving the safety performance of the composite.Meanwhile, the oil-wet property of the shell enables HMX@CS composite particles to have excellent wettability with the lipid binder,which is expected to improve the mechanical property of HMX-based PBX.Besides, the hydrophobic composite particles can maintain their stable explosion and combustion performance under various complex modern combat conditions in contact with water.
2.Experimental
2.1.Materials
HMX (1,3,5,7-tetranitro-1,3,5,7-tetrazocane) particles were obtained from our laboratory.Stearic Acid (SA) was purchased from Kelong chemical reagent plant (Chengdu, China), and Copper Acetate(CA)were purchased from Rgent chemicals Ltd(Tianjin,China),both with greater than 99% purity.
2.2.Synthesis of HMX@CS composites
The surface HMX coating of CS was carried out using in situ synthesis.HMX@CS (= 5%) core-shell composite particles were prepared in a typical experiment (Fig.1).0.23 g stearic acid was dissolved in 100 mL of anhydrous ethanol, then 5.0 g HMX was added to obtain the suspension under the condition of 500 rpm.0.11 g copper acetate was dissolved into 20 mL of anhydrous ethanol in another reactor,and then the anhydrous alcohol solution of copper acetate was slowly added dropwise to the suspension.Excess copper acetate was washed with ethanol during filtration and then dried in a vacuum at 45C for 12 h.The content of the shell was determined by changing the ratio of stearic acid to HMX,the added copper acetate has a mass of slightly more than the stearic acid and copper acetate molar ratio of 2:1, obtained the samples with different shell contents.The suspension without insitu ion exchange reaction was evaporated at 90C to prepare the HMX@SA composites.
2.3.Characterization
The morphologies of the HMX@CS micro-composites were investigated by scanning electron microscopy (SEM, Ultra-55, Carl Zeiss, Germany).Fourier transform infrared spectroscopy (FT-IR)spectra were recorded using KBr pellets for samples from 4000 cmto 400 cmon a Nicolet-5700 FI-IR spectrophotometer.Powder X-ray diffraction (XRD, X'Pert Pro, Panacco, Netherlands)with a monochromatized Cu Kα(λ=1.5418 Å)radiation was used to evaluate the crystal structures.X-ray photoelectron spectroscopy(XPS) was performed with an ESCALAB 250 electron spectrometer using Al Kirradiation.The samples were observed by differential scanning calorimeter (STA 449 F5 Jupiter, Netzsch, Germany) at a heating rate of 10C/min,in a temperature range of 30C-500C and the sample mass used was about 1.0 mg, thermogravimetry(TG) is also measured simultaneously.According to GJB-772A-97 explosives test method 601.2, the impact sensitivity test was carried out by using WM-1 type impact sensitivity instrument with a 5.0 kg drop weight.Each sample (35 mg) was tested 25 times to obtain an(Thevalue represents the drop height of 50%explosion probability).
3.Results and discussion
3.1.Morphology and structure characterization
The surface morphology of the raw HMX, SA@HMX, and CS@HMX was investigated using scanning electron microscopy(SEM).As shown in Fig.2a, the raw HMX was approximately 5-30 μm, which is mostly spherical or elliptical and has a smooth surface.To compare the morphological changes of the samples before and after the reaction with copper acetate, the composite product of HMX@SA was obtained by evaporation solvent.Fig.2b shows the SEM images of the HMX sample coated with stearic acid,which surface is slightly rougher than that of the raw HMX sample.The morphology and size of HMX@CS(Fig.2c-d)and HMX@SA are unchanged on the macro level.It can be seen that the surface of HMX becomes significantly rougher, almost complete the form CS shell formed except for a few cracks, and tiny protuberance distributed on its surface which improves the hydrophobic performance of HMX,it is similar to the bionics protrusion structure of a superhydrophobic surface, the water particles difficult entering the pits of the rough structure.
To further determine the composition of the composite samples,Fourier transform infrared(FT-IR)characterization was performed.The FT-IR spectra of HMX、HMX@SA and HMX@CS are shown in Fig.3, compared with HMX, the FT-IR spectra of the HMX@SA composite visibly exhibited new peaks at 1597 cm, 2849 cm,2918 cm, and 3415 cm, corresponding to the C=O stretching,-CHsymmetric stretching,-CHasymmetric stretching and-OH stretching of SA.And about 1415 cmrepresents the symmetric stretching of C-O, which is attributed to the low proportion of SA and absorption peak of HMX overlaps.CS(Fig.3d)is different from SA in the carboxylate end, Cureplace the hydrogen atom of carboxylic acid, resulting in the peaks at 3415 cmdisappeared,1597 cmabsorption peaks migrated and overlapped with HMX absorption peaks, then the peaks in 2849 cmand 2918 cmenhanced due to generate a longer -CH- chain [31,33].
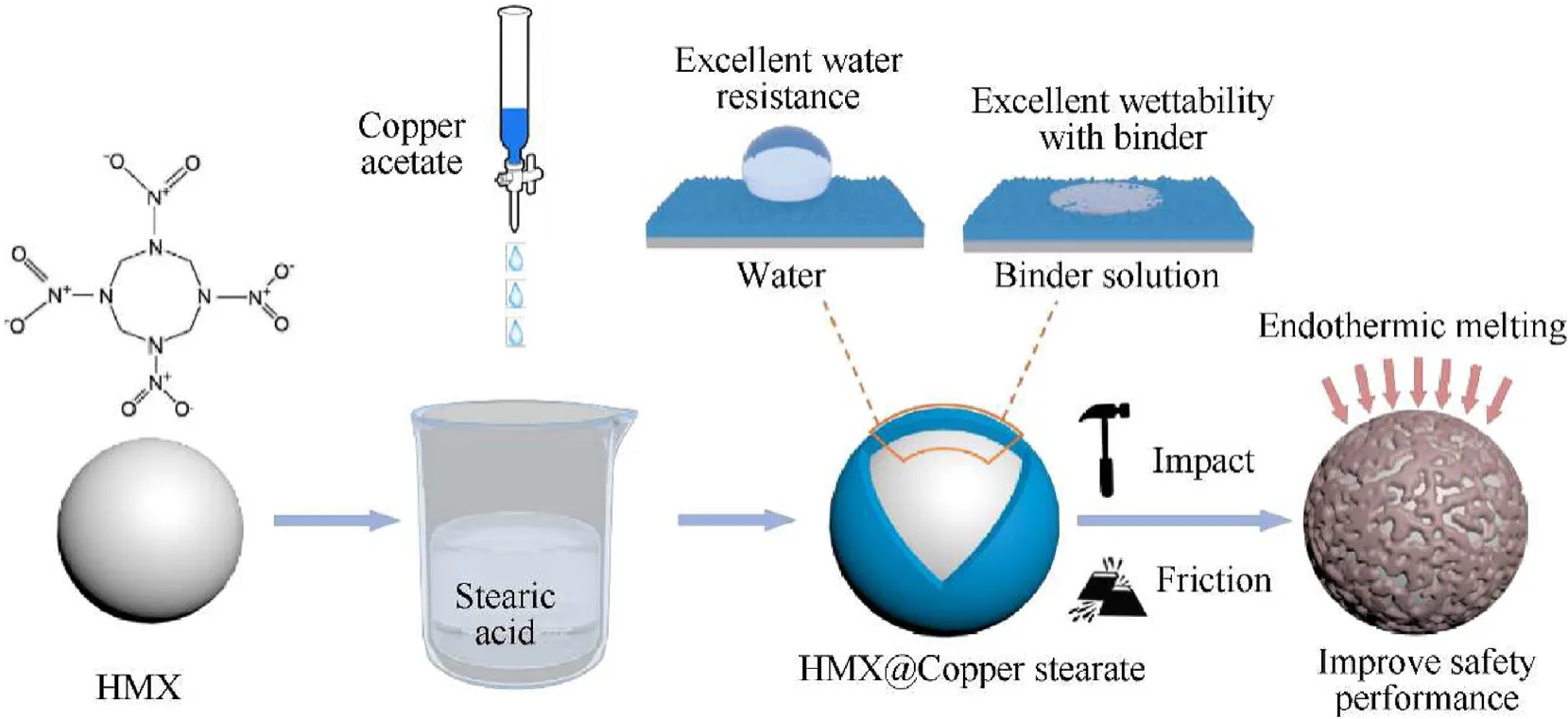
Fig.1.Schematic diagram showing the preparation of HMX@CS composites.
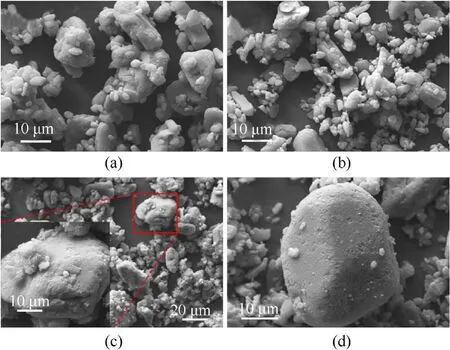
Fig.2.SEM images for (a) HMX, (b) HMX@SA (w = 5%) and (c) (d) HMX@CS (w = 5%).
To confirm the presence of CS and core-shell structure of HMX@CS, X-ray photoelectron spectroscopy (XPS) was used.The main elements detected on the surface of the sample included C,N,O,and Cu(Fig.4a),which demonstrated that Cuions replaced Hof the SA.Specifically, the peaks at 948.34 eV and 954.28 eV, corresponding to Cu 2pand Cu 2prespectively (Fig.4b), combined with infrared spectroscopy and SEM images, HMX@CS coreshell structure was generated.
3.2.Crystal structure characterization
The crystalline phases and composition of the product were identified by X-ray diffraction.Fig.5 shows the XRD patterns of raw HMX, HMX@SA, and HMX@CS, Fig.5a represents the XRD pattern of the HMX raw sample,which is the same as the standard β-HMX pattern[35],and has strong peaks at 14.60,16.04,20.54,23.02,26.19,27.16,29.65,32.94,37.28,41.19,and 50.68.Fig.5b and c respectively represent the HMX particles coated with SA and HMX particles coated with CS,all diffraction peaks are aligned with HMX,except for a slight difference in the intensity of the absorption peak,so proves the HMX crystal structure does not change.It is insufficient to change the crystal shape of HMX that the procedure of insitu ion exchange reacting to generate CS.
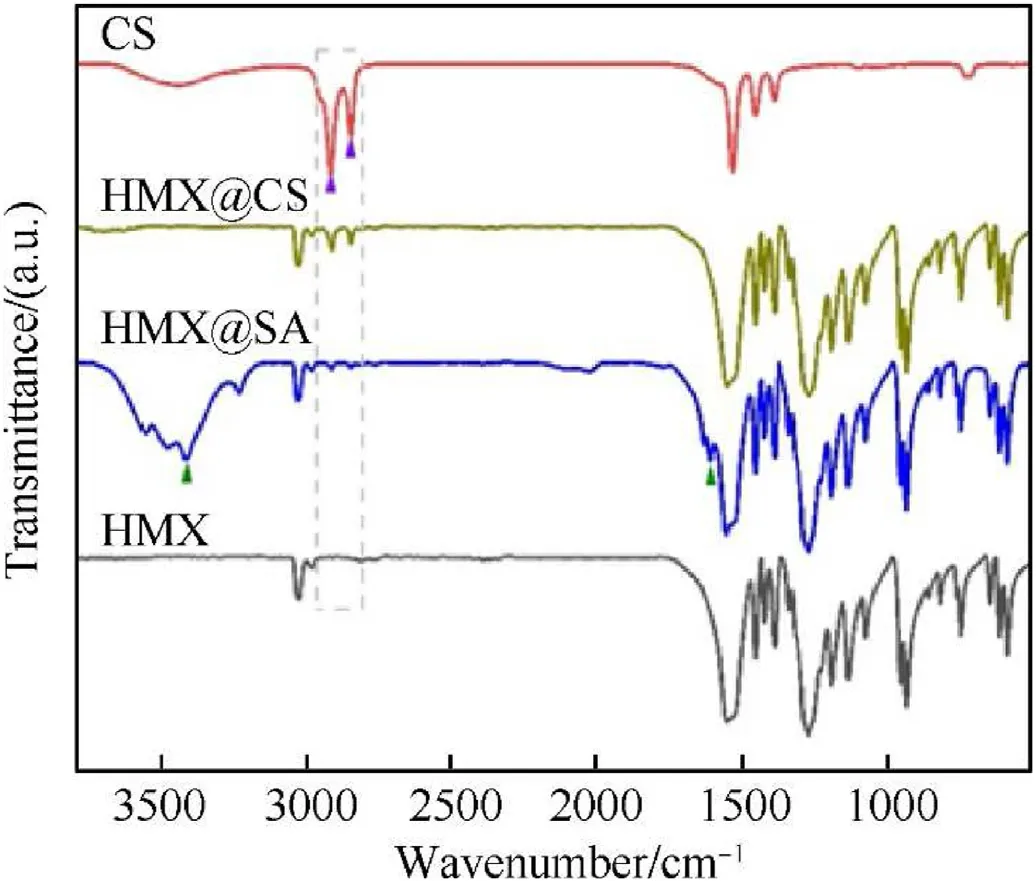
Fig.3.FT-IR spectra of (a)HMX, (b)HMX@SA, and (c)HMX@CS.
3.3.Thermal decomposition characteristic
Differential scanning calorimetry (DSC) and thermogravimetry(TG) were used to investigate the thermal decomposition performance of the prepared samples,the results are shown in Fig.6.The decomposition curve of pure HMX in the figure is divided into two parts, the absorption peak of crystal transformation at 203C and the exothermic peak of decomposition at 284C[18,34].Compared with pure HMX, the crystal transformation peak and the exothermic peak of the prepared samples did not offset.The area of the exothermic peak for samples decreased slightly and the sharpness also decreased, may be due to HMX is negative oxygen balanced explosive,and the molecular structure of CS is almost all C and H, so the addition of CS will have an impact on the thermal decomposition performance of HMX.However, the TG loss curves(Fig.6b) of the samples from 100C to 400C are almost overlapping, the higher the content of CS, the more the remaining products of the final thermal decomposition, but in general, the samples are almost completely decomposed.The differential thermogravimetry (DTG) curves (Fig.S1) except for the difference in the short period of the initial stage of thermal decomposition,the others are overlapped.DSC-TG and DTG curves prove that CS has little effect on the thermal decomposition performance of HMX,both substances have excellent compatibility.
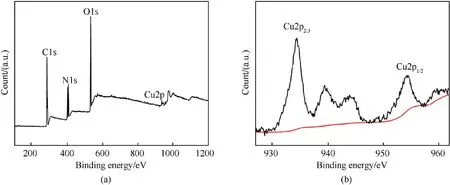
Fig.4.XPS spectra of (a) HMX@CS and (b) Cu 2p.
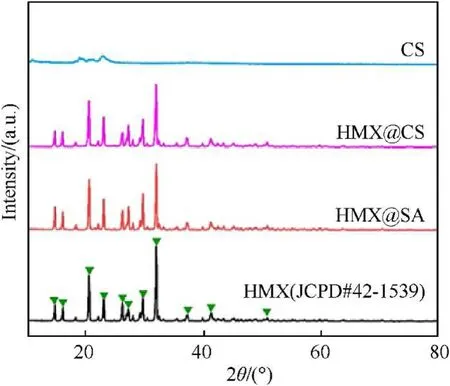
Fig.5.XRD patterns of (a) HMX, (b)HMX@SA and (c) HMX@CS
3.4.Sensitivity test
Fig.7a displays the results of impact sensitivity for pure and coated HMX samples.The sensitivity of HMX can be considerably reduced after coated with CS, and the drop in impact sensitivity more when the content of CS increased.Taking HMX@CS(=5%)core-shell composite as an example, theincreases to 33.7 cm compared with 16.5 cm of pure HMX, which significantly reduced its sensitivity to impact.When the shell content is the same, its desensitization effect is significantly better than that of insensitive explosives [24-26] and close to the desensitization effect of most polymers [20,21,36].It proves that the core-shell HMX@CS composite is less sensitive to mechanical stimuli due to its uniform and compact CS shell,which effectively acts as a buffer.Besides,the CS shell will absorb part of the heat when HMX receives impingement friction and other stimuli due to the low melting point (approximately 100C) of CS (Fig.7b), then the appropriate content of the insensitive shell reduces the possibility of hot spots under external stimulation and improves safety in practical applications performance.
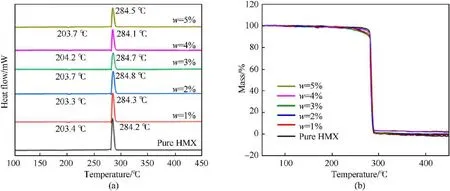
Fig.6.DSC-TG curves of HMX and HMX@CS (w = 1%, 2%, 3%,4%, 5%).
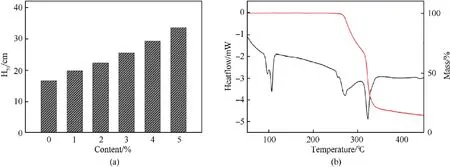
Fig.7.(a) The impact sensitivity (H50) of energetic samples with different CS content, (b)DSC-TG curves of copper stearate.
3.5.Surface wettability
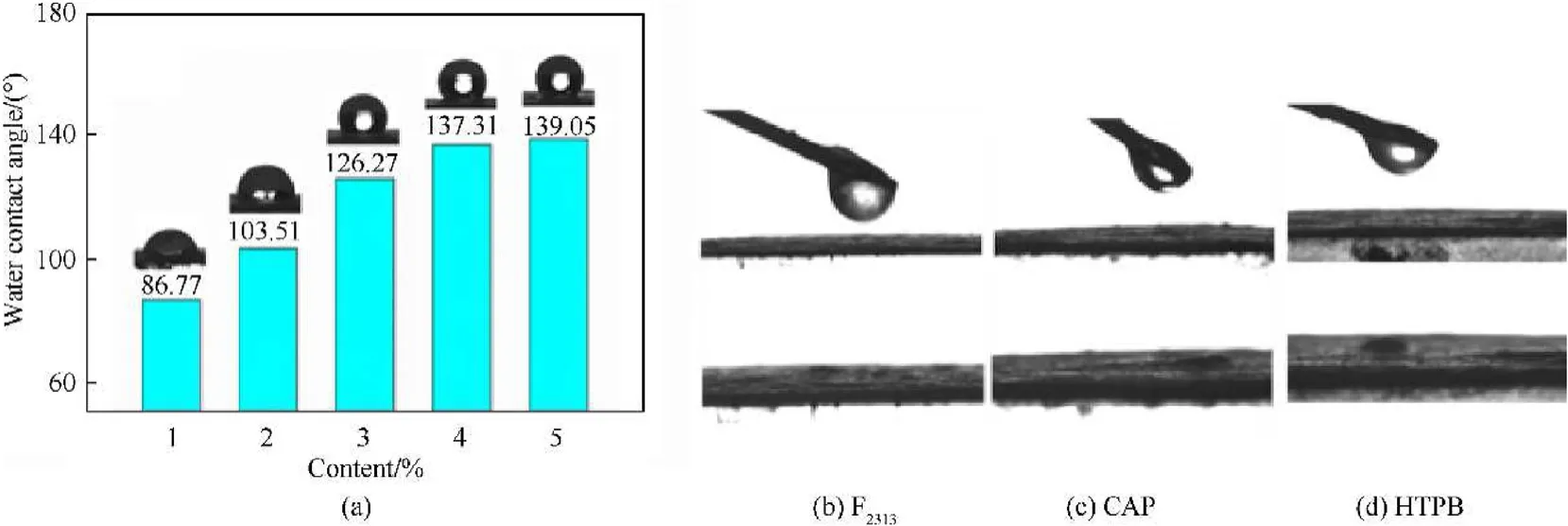
Fig.8.(a)Water contact angle of HMX with different CS(1%,2%,3%,4%,5%)content coated,and HMX@CS(w=5%)composites solutions contact angles for(b)F2313(c)GAP and(d)HTPB.
The interface interaction between explosive and binder is related to the mechanical properties of PBX.The static contact angle was used to characterize the interface wetting performance between HMX@CS and water, F, GAP, and HTPE three kinds of solutions.Fig.8a shows the contact Angle of HMX@CS to water,HMX@CS (= 1%) water contact Angle is 86.77, its contact angle to water also increases continuously with the constant increase of CS content, when CS content increases to 2%, 3%, 4% and 5%, the contact angle rise to 103.51,126.27,137.31, and 139.05respectively.Fig.8bcd shows the F, HTPE, and GAP three common binder kinds of solutions contact angle can be found that when the three kinds of binder solution drop on the surface of HMX@CS,the contact angle is zero,showed excellent wetting property,HMX@CS composites core-shell structure particles interact with the interface between the adhesive is strong and can close bond between powder and binder.The hydrophobic performance of HMX@CS particles is mainly attributed to the long carbon chain in the molecular structure of copper stearate,which makes HMX hard to be soaked by water and even can achieve combustion underwater.The oil-wet property enables HMX to interact with most lipid binders with a more powerful interface, which may improve the mechanical properties of HMX-based PBX.
To further understand the hydrophobic properties of HMX@CS,we made a simple combustion experiment (Fig.9 and Fig.S2), the HMX@CS samples (1 g) with different shell content are filled into the 5 cm long aluminum alloy groove,and the groove has a certain inclination angle.Then use a syringe to continuously drip water from the top of the inclined groove.When water is added dropwise at the beginning,water droplets will be formed on the sample due to the hydrophobic properties of the sample, with the continuous dripping of water,it will gradually flow into the end of the groove due to gravity instead of staying on the surface of the sample column.Then ignites the sample with an electric ignition device immediately, as shown in Fig.10, the sample of the 1% CS content fire extinguishing in 4 s maybe its hydrophobic effect relatively poor water permeated the samples within the column.Samples with a content of 2%, 3%, and 4% were able to burn steadily and continuously.Samples with 5%CS shell content cannot burn stably,and a black burning residue was observed in a section of the sample column that burned for a short time,the reason may be that CS low melting point and content is higher, the residue produced during combustion prevents the combustion from proceeding.
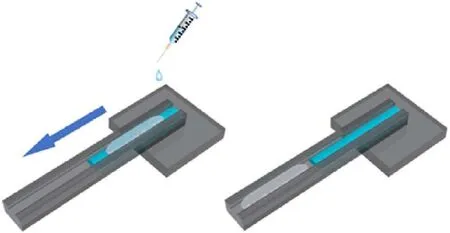
Fig.9.Schematic diagram of overlying water combustion experimental apparatus.

Fig.10.Images before and after added water and combustion process of HMX@CS (w = 1%, 2%, 3%, 4%, 5%).
4.Conclusions
In summary,we successfully prepared HMX@CS with core-shell structure by the simple in-situ ion-exchange reaction.This preparation method is efficient and low cost and doesn't change HMX crystal type during the process of preparation.In particular,a rough CS shell was created on the HMX surface, with excellent hydrophobic-oleophilic attributes, prepared samples with the appropriate CS shell content can be burned steadily and continuously after overlying water, lipophilic properties also let HMX@CS produce stronger interaction between lipid binder in the PBX.Moreover, the CS shell effectively reduces the mechanical sensitivity of HMX, and CS will absorb some heat and melt under the stimulation of impact and friction due to its low melting point which improved the performance of its safety further.HMX@CS can adapt to complex and changeable battlefield environments, and also provides strong support for the application and development of the multi-functional performance of explosives.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was financially supported by the National Natural Science Foundation of China (Grant NO.11702268) and Sichuan provincial key S&T Special Projects (Grant NO.19DZX0106).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.05.006.
杂志排行
Defence Technology的其它文章
- Defence Technology
- Roll angular rate extraction based on modified spline-kernelled chirplet transform
- Buckling of composite cylindrical shells with ovality and thickness variation subjected to hydrostatic pressure
- Quantitative prediction and ranking of the shock sensitivity of explosives via reactive molecular dynamics simulations
- An efficient light-to-heat conversion coupling photothermal effect and exothermic chemical reaction in Au NRs/V2C MXene membranes for high-performance laser ignition
- Hygrothermal effect on high-velocity impact resistance of woven composites
