An efficient light-to-heat conversion coupling photothermal effect and exothermic chemical reaction in Au NRs/V2C MXene membranes for high-performance laser ignition
2022-05-24BoYangPengfeiTangChunjiaoLiuRuiLiXiaodongLiJinChenZhiqiangQiaoHongpingZhangGuangchengYang
Bo Yang , Peng-fei Tang , Chun-jiao Liu , Rui Li ,c, Xiao-dong Li ,*, Jin Chen ,Zhi-qiang Qiao , Hong-ping Zhang , Guang-cheng Yang ,*
aSchool of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang, Sichuan, 621010, China
bInstitute of Chemical Materials, China Academy of Engineering Physics, Mianyang, Sichuan, 621900, China
cSchool of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094, PR China
Keywords:V2C MXene Light-to-heat conversion Exothermic chemical reaction Plasmonic Au nanorods High temperature pulse Laser ignition
ABSTRACT MXene, a new type of two-dimensional materials, have been demonstrated as one of the best photothermal materials owing to their strong light-matter interaction and high photothermal conversion efficiency in recent years.Herein, we report the intriguing light-to-heat conversion property of vanadium carbide (V2C) MXene under irradiation of millisecond laser pulse.Unlike the typical photothermal materials, the V2C MXene not only converts the incident laser energy to heat by the physical photothermal effect, but also triggers the exothermic oxidation of the V2C MXene.The oxidation could be greatly promoted with addition of plasmonic Au nanorods(Au NRs)for light absorption enhancement.Owing to the unique light-to-heat conversion property, the Au NRs/V2C MXene membrane could serve as high temperature pulse (HTP) generators that is proposed for numerous applications with high demand for immediacy.As a proof-of concept application, Au NRs/V2C MXene membrane was applied for laser ignition of the high energy density materials, such as 2,4,6,8,10,12-(hexanitrohexaaza)cyclododecane(HNIW or CL-20).An improved ignition performance, in terms of lowered laser threshold, is achieved as compared to the state-of-the-art light-to-heat conversion materials.
1.Introduction
The light-to-heat conversion has been widely used in tumor treatment [1], water evaporation [2], laser-induced ignition [3],antibacterial [4] and other aspects.Currently, two major types of materials have been developed for light-to-heat energy conversion.One type of the materials convert the light energy to heat energy via the physical photothermal effect[5-8]and other type generate heat by a physical/chemical coupled process [9,10].The latter type of materials convert the absorbed light to heat through various physical process, such as plasma heating, the thermal vibration of molecules and the generation and relaxation of electron holes[11-13], leading to a drastic temperature rise.Such rapid temperature increase provides initial energy for exothermic oxidation of the materials themselves.Due to accumulation of two exothermic processes, namely the photothermal conversion of light energy to heat and thermally activated release of stored chemical energy,the physical/chemical coupled process is more efficient for light-toheat energy conversion, especially for producing the hightemperature pulse (HTP) under irradiation of millisecond laser pulses [10].Such drastic temperature rise is essential in applications with high-demand for immediacy, including the circuit breaker[14],laser welding[15],laser cutting[16],and laser ignition[10,17].Graphene oxide is a typical photothermal material capable of generating HTP by the physical/chemical coupled process owing to its combined advantages of relatively high photothermal effect and rapid exothermic deoxygenating reaction once it is triggered by heat [9,18-20].However, GO is not the best material for HTP generation by the physical/chemical coupled process due to its nature drawbacks, such as 1) the much lower heat release from deoxygenating reaction of GO than most of the energetic materials limits the achievable maximum peak temperature[17,21];and 2)the low safety since its thermal stability can be significantly affect by the inorganic by-products from GO synthesis (primarily potassium salts) [9].
In searching better materials for HTP generation by physical/chemical coupled process, MXenes offer more promising opportunity due to their unique properties.MXene,a new member of 2D nanomaterials family,is usually isolated via selectively etching the aluminum layer from its MAX phase parent in aqueous HF solution[22-24].With incorporation of carbon atoms in lattice of the out layer of transition metal atoms,the band structures of MXenes can be regulated in a wide range,so that it has properties from metals to semiconductors to insulators [23,25].Even better, the TiCMXene is demonstrated to convert the absorbed light into heat with a perfect internal photothermal conversion efficiency of 100%[26].Combined with their broadband strong light absorption, the MXene has been widely investigated in photothermal applications,such as photothermal therapy [27], solar energy [28], all-optical modulator [29], and sea water desalination [30].So far TiCTis the most widely studied MXene.It possesses high light absorption,perfect internal photothermal conversion efficiency (~100%) [26]and high heat energy release (5-7 kJ/g) [10], rendering it a promising candidate materials for photothermal applications,especially for HTP generation by the physical/chemical coupled process.However,the main heat release of oxidation of TiCMXene is from two reactions,one is oxidation of TiCTinto TiOand carbon,the other is oxidation of the carbon to CO[31,32].Since the carbon cannot rapidly release their enormous chemical energy through oxidation,the HTP performance of TiCTMXene is limited due to the incomplete chemical energy release under the irradiation of laser pulse in a short time.Moreover,the formation of Ti-Ti bonds at higher reaction temperature also prevents the chemical energy release of TiCTMXene [10].Therefore, the TiCTMXene has a low proportion of available chemical energy in the rapid HTP generation process.This discovery inspires us to look into other MXene with higher reactivity.
VC MXene has excellent photothermal conversion efficiency[33]comparable to that of TiCbut higher than other stat-of-theart photothermal materials, such as MoC [34], NbC [35], Au nanovesicles [36], CuSnanocrystals [37], carbon nanodots [33],etc.The enthalpy change(ΔH)of oxidation of VC is comparable to that of the TiCMXene.Moreover,the lower carbon content in VC than that in TiCfacilitate the formation of smaller and thermodynamically more unstable carbon products with higher reaction activity owing to steric hindrance of the metal atoms,and thus is expected to achieve more complete chemical energy release under the laser pulse irradiation.What minor defect in something otherwise perfect was that the light absorption peak of MXene locates in the visible light region, and has poor absorption in the near-infrared region (808 nm, 1064 nm), the wavelength range most commonly used for photothermal based applications[38].To address this issue,surface plasmon resonance(SPR)effect induced by noble nanometals (especially Au and Ag nanomaterial) is introduced to enhance the optical absorption of the MXenes[10,39-41].Among them, Au nanorods (Au NRs) has received special attention due to its unique optical properties [42].Studies have shown that the longitudinal plasmon absorption band(LPAB)of metal NRs reduces the geometric symmetry and enriches its plasma effect [43], which renders regulating the light absorption peak to the desired wavelength range [44].These results demonstrate the feasibility of enhancing the light absorption of MXenes by introducing Au NRs with desired SPR frequency.
Herein, we report efficient light-to-heat conversion of Au NRs/VC MXene membranes for HTP generation with increased peak temperature.As shown in Fig.1, self-supporting VC MXene membranes modified with Au NRs were fabricated for the HTP generation.It is found that the Au NRs could greatly enhance the light absorption of the VC MXene membrane,resulting in a drastic rise in temperature by the physical photothermal effect.Furthermore, an exothermic oxidation reaction can be triggered once the temperature exceeds the oxidation temperature of VC MXene.The physical photothermal effect as well as exothermic oxidation of VC MXene,including break of C-V bonds and oxidation of V atoms and the resultant carbons, contributes the main heat to the improvement of HTP.
2.Experiment
2.1.Materials
The aqueous suspension of VC MXene (5 mg/mL) was bought from Beike 2D Materials Co., Ltd.(Beijing, China) [45].Au NRs(0.1 mg/mL, cetyltrimethylammonium bromide (CTAB)-modified,Length-diameter ratio: 6.5, IR absorption peak: about 1040 nm)were purchased from XF Nano Material Technology Co., Ltd.(Nanjing, China).
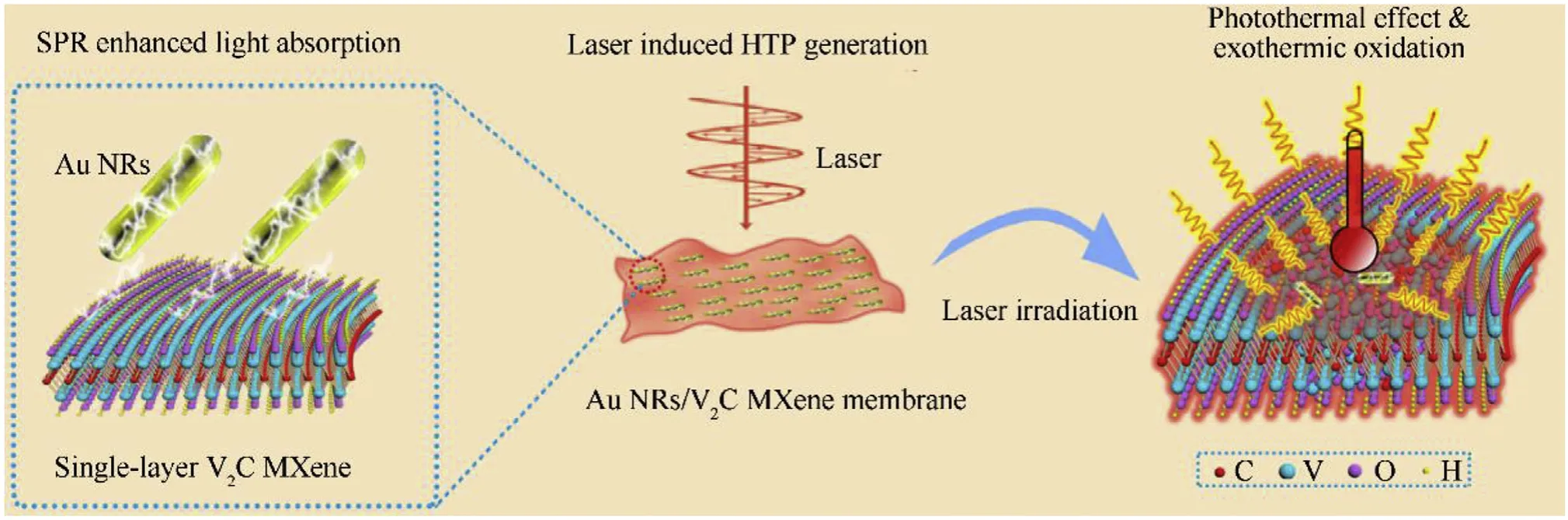
Fig.1.Schematic illustration of the light-to-heat conversion coupling photothermal effect and chemical energy release in Au NRs/V2C MXene membranes for laser induced HTP generation.
2.2.Fabrication of V2C MXene and Au NRs/V2C MXene membranes
CTAB was removed from Au NRs solution by centrifugation and redistributed in a small amount of ultrapure water,then the Au NRs suspension was added dropwise to a vial containing suspension of VC MXene (4 mL) under magnetic stirring for about 10 min at room temperature(protect the solution from light during magnetic stirring).Both Au NRs/VC MXene and VC MXene membrane was fabricated by freeze-drying wetted membranes formed from vacuum filtration of 4 mL VC MXene suspension with or without Au NRs.
2.3.Characterization
Transmission electron microscopy (TECNAI G2 F20, FEI, USA),field emission scanning electron microscopy (Carl Zeiss, MERLIN Compact,Germany)and atomic force microscopy(Dimension ICON,Bruker AXS, Germany) were used for material characterization.Thermogravimetry/differentia scanning calorimetry (TG/DSC,STA449F5 Jupiter, NETZSCH, Germany) and Fourier transform infrared spectroscopy (FTIR, Tensor, Bruker, Germany) were performed to investigate the thermal characteristic and the gaseous products released during pyrolysis of VC MXene and TiCMXene.The samples were heated from room temperature to 800C at 10C/min in Oatmosphere with a gas of 60 sccm.A METASH UV-8000S UV-visible-NIR spectrophotometer (Shanghai Metash Instruments Co., Ltd., China) were used for light absorption measurement in the range of 300-1100 nm.The bonding states of the materials were analyzed using an X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Fisher Scientific, UK).
2.4.HTP and laser ignition test
The HTP generation of the VC MXene based membranes is tested according to the previous work [10,17].Briefly, a 1064 nm continuous laser (MFSC-10, Maxphotonics) equipped with a mechanical shutter was used for HTP and laser ignition test.The laser beam spot size was fixed at 4 mm.The laser induced transient temperature of the samples was captured by a DIAS DPE10MF infrared-thermometers.The actual emissivity of VC MXene and Au NRs/VC MXene was determined to be 0.4, which was applied to compensate the thermometer.The emissivity was determined according to the previous work[10].
2,4,6,8,10,12-(hexanitrohexaaza)cyclododecane (CL-20) powder with pure ε phase was used for laser ignition tests [17].The CL-20 powder has an average particle size of about 1.13 μm.Prior to the laser ignition tests, the CL-20 particles were dried in an electric thermostatic drying oven at 60C for 2 h to remove the moisture.Then 20 mg dried CL-20 was filled into a shallow pit(φ7×1 mm)in the center of an aluminum plate and pressed to form a pellet with 7 mm in diameter and 1 mm in thickness.VC MXene and Au NRs/VC MXene membranes were cut into small pieces that have size 0.5 cm × 0.5 cm.The thickness of the membranes is ~5 μm.The membrane is pressed on the upper surface of CL-20 pellet to form a tight contact.The laser ignition was tested by a Photron VEO410L camera.A homemade Schlieren system and a Photron VEO410L camera were used to record the Schlieren images.The ignition thresholds, defined as the statistical energy of the incident laser that triggers 50% of successful ignitions [17], were measured by changing the duration of the laser pulse at fixed laser intensity of 31.8 and 79.6 W/cm.
3.Results and discussion
TEM analysis shows that the VC MXene has a hole/particle-free layered structure,Fig.2a.AFM image also confirms the 2D structure and thickness of the VC MXene.As shown in inset of Fig.2a, the heights of the regions marked by lines 1 and 2 are 3.5 and 3.9 nm,corresponding to ~2 stacked layers.The oxidation of VC was investigated by TG/DSC-FTIR, where two exothermic peaks accompanying with an increase in mass is observed between 150 and 260C(Fig.2b).Since negligible COand CO gas were released in this temperature range,the exotherm should mainly come from the oxidation of V atoms.The three exothermic peaks between 260 and 360C that accompanied with a decrease in mass and obvious COand CO release should be attributed to oxidation of the carbon products.With the temperature increase to 400C, a sharp exothermic peak was observed.Since an increase in mass and massive COand CO gas releases were also observed in this temperature range,the sharp exothermic peak should be attributed to further oxidation of VOto VOand carbon products to COand CO.The endothermic peak at 680C should belongs to the decomposition of VOto VO.
In a sharp comparison and as expected,the oxidation of TiCTMXene occurred at a higher temperature from 200 to 450C.It is clear that the exotherm lower than 420C should be mainly contributed by the oxidation of Ti atoms, as confirmed by an obvious mass increase and a small amount of CO and COrelease in the temperature range of 200-420C,Fig.2c.The oxidation of the carbon products occurs mainly at temperature>420C,indicating the carbon products of TiCTare more stable than that of VC Fig.S2.The relatively low oxidation temperature of the VC MXene,especially the low-temperature oxidation of the resultant carbon products, indicates a high activity of the VC MXene, which is essential for rapid and complete chemical energy release in laser induced HTP generation.Moreover,the Δof oxidation of VC is as high as 5.4 kJ/g(Fig.2d),comparable or even higher than the typical energetic materials.
Au NRs can strongly absorb and scatter incident light,especially at the resonant wavelength[55,56].The resonant wavelength of the Au NRs can be regulated from visible to near-infrared range by adjusting their aspect ratios [55-57].To achieve a high light absorption, Au NRs with desired resonant wavelength were chosen.Fig.3a shows the morphology and structure of the Au NRs.The average length and width of Au NRs are 65 nm and 10 nm,respectively, corresponding to an aspect ratio of 6.5 (inset in Fig.3a).Well resolved lattice fringes with lattice constants of 0.203 nm is observed in the high-resolution TEM image(Fig.3b)of a single Au NR,corresponding to the(2 0 0)facets of the FCC gold.The Au NRs displayed a localized surface plasmon resonance(LSPR)at 980 nm (Fig.3c), which is close to the wavelength of the commercially available laser for photothermal based applications(such as 808 nm and 1064 nm).The unique 2D structure favors formation of the VC MXene membrane with a parallelly ordered,and layered structure Fig.S1a, which endows the material with flexible and freestanding property.As verified by the SEM analysis from top-view(Fig.S1b),the VC MXene membrane also possesses a smooth and particle-free surface, indicating no apparent oxidation was occurred during the membrane preparation process.With the addition of Au NRs,no visible changes can be observed from the cross-sectional survey of the membrane (Fig.3d), while from topview SEM analysis, Au NRs can be observed embedding in the VC MXene membrane (Fig.3e).
As expected, the addition of Au NRs can greatly enhance the light absorption capacity of VC by the SPR effect.As shown in Fig.3f, the VC MXene membrane has two obvious absorption peaks at 560 nm and 920 nm.The absorbance of Au NRs/VC membranes increase rapidly with the ratio of Au NRs, reaching a highest value at 7.5% Au NRs addition.It is noteworthy that the characteristic absorption peak of the VC MXene in the NIR range has a red shift from 920 to 975 nm with increase of the ratio of Au NRs.This red shift of the absorption peak should be derived from the absorption contribution of the LPAB of Au NRs,which locates at 980 nm.Moreover, the absorption of light over the entire wavelength range has increased, which provides convenience for selecting the right laser wavelength to suit the needs of different applications, such as the 1064 nm for laser ignitions.

Fig.2.(a) TEM images of V2C MXene.Inset shows the AFM image of V2C MXene.TG/DSC-FTIR curves of (b) V2C MXene and (c) Ti3C2 MXene.Measurement was performed in O2 atmosphere at a scan rate of 10 °C/min.(d)Comparison of ΔH values of V2C MXene to typical energetic materials,including Al/CuO[46-48],Al/Fe2O3[47,49],Al/MoO3[47,48],TNT[50,51], RDX [51,52], HMX [51,52], [Zn2(ATZ)2(TZ)2]n [53], [Cu2(to)(dns)(H2O)]n [54], and Ti3C2 MXene [10].
To characterize the HTP generation performance of VC and Au NRs/VC MXene, the laser-induced temperature was monitored.The experimental setup is illustrated in Fig.4a,where a pulsed laser(1064 nm, 4 mm spot size) vertically irradiates the samples.The resulting temperature evolution during the irradiation of the laser pule was monitored by an infrared thermometer.As shown in Fig.4b,both VC MXene and Au NRs/VC MXene membranes show rapid temperature rise and decay.For more clarity, the peak temperatures () as a function of laser irradiance are also shown in Fig.4c.Theof the two membranes increased rapidly with the irradiance increasing from 7.90 to 31.8 W/cm,followed by a slight rise with further increase the irradiance.The Au NRs/VC MXene membrane generates much higherthan the VC MXene membrane.The Au NRs enhanced light absorption should be the source of theincrease in Au NRs/VC MXene membrane,which not only contribute to the physical photothermal effect of the Au NRs/VC MXene membrane but also promote a more complete release of the chemical energy of the VC MXene.
To understand the HTP generation mechanism of the VC based membranes, the morphological and structural changes of the VC MXene upon laser irradiation were characterized by TEM and XPS analysis.As confirmed by TEM,the pristine Au NRs/VC MXene has a typical layered structure Fig.S3a, consisting of few-layered VC MXenes with layer spacing about 0.252 nm (Fig.S3b).After irradiated with a 23.9 W/cmlaser pulse (Fig.S3c, d), the layered structure becomes blurred, and many VOnanoparticles were formed (Fig.S3c).VC is identifiable in the HRTEM image by its(110) crystal plane distances of 0.325 nm (JCPDS NO.81-2392),Fig.S3d.With the laser intensity increased to 47.8 and 79.6 W/cm,the layered structure completely disappeared, instead, VOnanoparticles with increased size and decreased lattice distance were observed(Fig.S3e),indicating formation of VOnanoparticles with more perfect lattice structures due to the increased HTP (Fig.S3eh).The morphology evolution of VC over laser intensity reveals that the oxidation of the VC MXene starts at low laser irradiance of 23.9 W/cm.In addition,it is clearly that the exothermic oxidation of the VC MXene could be triggered and tuned by the photothermal effect itself, a desired feature for generating high performance HTP by the physical/chemical coupled process.
XPS gives further insights into the chemical composition and structure change of the VC MXene based membranes upon laser irradiation.For the pristine VC MXene membranes, the highresolution V 2p spectrum could be deconvoluted into five components:V-C,Vθp,VpVθp 1/2 and Vp 1/2 at 513.2,514.1,516.4, 520.8, and 523.9 eV, respectively, where 0 <θ < 4 (Fig.5a).The V-C and Vbonds are quantitatively confirmed to be 7.61%and 81.38%, respectively, in agreement with the XPS results collected from freshly prepared VC MXene [58,59].After laser irradiation of the VC MXene membrane(47.8 W/cm),the ratio of V-C in V 2p component decrease sharply to 1.72%,while the ratio of Vbonds increase to 63.83% (Table S1).The residual of V-C and Vθ+ bond indicates that the oxidation of the VC MXene is not complete.A similar change was also observed from the O 1s and C 1s spectrum(Fig.5b and c).The C-V and V-C-O bonds,accounting for 5.62%of the total C 1s and 78.98%of the total O 1s spectrum of the pristine VC MXene, deceases to 0.7% and 39.63% after laser irradiation.The oxidation of the V atoms during laser irradiation could be confirmed by sharp increase of the O-V bonds from 16.20% to 56.42% in O 1s spectrum.
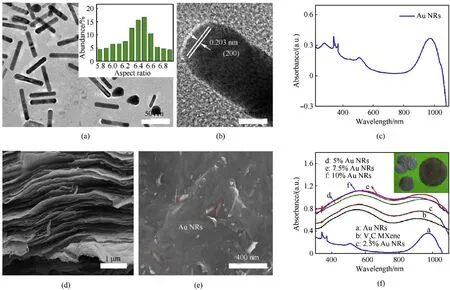
Fig.3.(a)TEM images of Au NRs.Inset shows the aspect-ratio distribution of Au NRs.(b)The HRTEM image of a single Au NR.(c)The UV-Vis-NIR absorption spectra of Au NRs in a colloidal solution(0.1 mg/mL).(d)The typical cross-sectional and(e)top view SEM micrograph of Au NRs/V2C MXene membrane.(f)The UV-Vis-NIR absorption spectra of pristine V2C and Au NRs/V2C membranes with different mass ratios of Au NRs of 2.5%, 5%, 7.5%,10%.Inset depicts the digital photograph of Au NRs/V2C MXene.

Fig.4.(a)The schematic diagram of the experimental setup to measure the transient temperature.(b)The transient temperature(temperature rise and decay with on/off of laser pulse as a function of time) of V2C MXene based membranes under different laser intensity.For better comparison, the start time of each curve was set as the end time of the previous curve.(c) Comparison of peak temperatures in the transient temperature curves for different HTP generators.
The oxidation of the VC MXene could be promoted with addition of Au NRs.The Au NR/VC MXene shows nearly identical XPS spectrum to the pristine VC MXene,except for a slight increase of V-O bonds from 55.59% to 60.22% in V 2p peak, indicating retention of the most metastable V-C bonds in Au NR/VC MXene,which is essential for generating HTP with the physical/chemical coupled process.
After irradiance(47.8 W/cm),the peaks of C-V disappeared in C1s and V 2p spectrum and only Vbonds can be observed in V 2p spectrum.In addition,the percentage of O-V bond in O 1s peak of the post-irradiance products of Au NR/VC MXene increases to 63.27%, higher than the values of 56.42% for that of VC MXene.These results indicate that the oxidation of VC MXene could be greatly promoted by adding Au NRs, facilitating more complete chemical energy release, and thus increased peak temperature in HTP.

Fig.5.XPS spectra of V2C MXene and Au NRs/V2C MXene membranes before and after laser irradiation.(a) V 2p spectra, (b) C 1s spectra, and (c) O1s spectra.

Fig.6.(a)Schematic illustration of the laser ignition of CL-20 pellet with V2C MXene and Au NRs/V2C MXene membranes as HTP generators.The(b,d)regular and(c,e)schlieren imaging with Au NRs/V2C MXene (b, c) and V2C MXene (d, e) membranes.
ε phase CL-20 is currently the most powerful compound used in polymer-bonded explosives (PBX), propellants, and laser ignition applications [17,60].However, the application of CL-20 in laser ignition is still changing due to its high ignition threshold.Herein,VC based membranes were applied as light-to-heat converter for laser ignition of CL-20.Since the non-absorption of VIS-NIR light,CL-20 can only be ignited by a high-energy-density laser irradiance(254 W/cm, 500 ms, 1064 nm) without any photothermal additives.However,with covering of a layer of Au NRs/VC MXene membrane on top surface of the CL-20 powder,the complete laser ignition with shortened ignition delay and decreased laser threshold is achieved.
The schematic structure showing the combination mode of MXene membranes and CL-20 pellets for laser ignition is shown in Fig.6a.Fig.6b-e are the typical snapshots of the light ignition and combustion of CL-20.As shown in Fig.6b and c, the CL-20 were ignited,defined as first establishment of the primary flame,after a short delay time of 29 ms.The flame spreaded around the laser induced “hot pot” and established a steady luminous flame at 100 ms.From the schlieren images (Fig.6c), the typical sooty decomposition products evolution and large particulates expelling from the surface of CL-20 during laser ignition [61] was not observed.This is because the combustion of CL-20 is governed by condensed phase reactions at low ambient pressure [61]; and the pyrolysis reaction with gaseous products is needed to accumulating enough heat for the flame establishment.Since the heat release from the laser irradiated Au NRs/VC MXene membrane is large and rapid enough to compensate heat losses for the primary flame formation of CL-20,the pyrolysis reaction in condensed phase may be replaced.The CL-20/VC MXene were ignited in a manner similar to CL-20/Au NRs/VC MXene, but with a long delay time of 43 ms (Fig.6d and e), agreeing with their HTP performance showing in Fig.4b.
Fig.7 shows the laser ignition threshold of CL-20 with Au NR/VC MXene as HTP generator, and compared to other HTP generators.The laser ignition threshold here is defined as the statistical energy of the incident laser that triggers 50%of successful ignitions.It was measured by changing the duration of the laser pulse at fixed laser intensity of 31.8 and 79.6 W/cm.Fig.7a shows the required laser pulse duration for 50%of successful ignitions at the two fixed laser intensities.Fig.7b shows the corresponding laser ignition threshold.The laser threshold of Au NRs/VC MXene membrane outperforms not only the VC MXene, but also other reported HTP generators (such as the Au nanoparticle/TiCand GO).Note that the laser threshold at 31.8 W/cmis lower than that at 79.6 W/cm,which is a desired feature for laser ignition applications.

Fig.7.The laser pulse duration (a) and corresponding laser ignition threshold (b) for ignition of CL-20 with different HTP generators.The laser ignition threshold,defined as the statistical energy of the incident laser that triggers 50%of successful ignitions,were measured by changing the duration of the laser pulse at fixed laser intensity of 31.8 and 79.6 W/cm2.
4.Conclusions
In summary,the Au NRs/VC MXene membrane is developed as a novel laser induced HTP generator owing to its physical/chemical coupled light-to-heat conversions process and more complete chemical energy release.The light absorption of the membrane could be enhanced by Au NRs, leading to a drastic increase in temperature of the membrane by physical photothermal effect.The temperature rise can trigger the oxidation of the VC MXene and releases large amounts of heat,resulting in a further increase of the temperature.The HTP generation performance of VC MXene based membranes outperforms that of TiCTMXene due to the more complete chemical energy release of the VC MXene.Assembled as a laser igniter with Au NRs/VC MXene membrane as HTP generator and 1064 nm pulsed laser as ignition energy,the CL-20 pellet can be ignited with low laser threshold of 140 mJ, lower than the typical HTP generators based on TiCTMXene(220 mJ)and GO(240 mJ).
Xiaodong Li, Hongping Zhang and Guangcheng Yang designed the project.Bo Yang, Pengfei Tang, Chunjiao Liu and Rui Li performed the experiments.Jin Chen and Zhiqiang Qiao analyzed the data.Bo Yang and Xiaodong Li co-wrote the paper.All authors contributed to the general discussion.
Supporting data are available in the online version of the paper,including the SEM micrograph of VC MXene membrane,FTIR curve of VC and TiCin Oatmosphere,the TEM micrograph of Au NRs/VC MXene membrane after laser irradiation and curve fitting results for the XPS spectra of VC MXene,VC MXene-Post-irradiation products, Au NRs/ VC MXene and Au NRs/ VC MXene-Postirradiation products.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors acknowledge the National Natural Science Foundation of China (21703217, 11702264, 11702268, 11802276,11772307) for financial support.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.04.005.
杂志排行
Defence Technology的其它文章
- Defence Technology
- Roll angular rate extraction based on modified spline-kernelled chirplet transform
- Buckling of composite cylindrical shells with ovality and thickness variation subjected to hydrostatic pressure
- Preparation of the core-shell HMX@CS microparticles by biological excitation:Excellent hydrophobic-oleophilic properties and decreased impact sensitivity effectively
- Quantitative prediction and ranking of the shock sensitivity of explosives via reactive molecular dynamics simulations
- Hygrothermal effect on high-velocity impact resistance of woven composites
