SARS-CoV-2 infection rates after different vaccination schemes: An online survey in Turkey
2022-05-23OyaBaydarToprakSennurOzenBerkerOzturkBurcuOzturkEbruOzturkMehmetKitapciNurdanKokturk
Oya Baydar Toprak, Sennur Ozen, Berker Ozturk, Burcu Ozturk, Ebru Ozturk, Mehmet Kitapci, Nurdan Kokturk✉
1Chest Diseases Department, Faculty of Medicine, Cukurova University, Adana, Turkey
2Department of Pulmonary Medicine, Delta Hospital, Istanbul, Turkey
3Chest Diseases Department, Aksaray Training and Research Hospital, Aksaray, Turkey
4Department of Pulmonary Medicine, Faculty of Medicine, Gazi University, Ankara, Turkey
5Department of Biostatistics, Hacettepe University, Ankara, Turkey
6Department of Nuclear Medicine, Integra Medical Imaging Center, Ankara, Turkey
ABSTRACT
Objective: To identify effects of various nationwide vaccination protocols on the evolution of new SARS-CoV-2 infections among adult population and to evaluate the safety of mRNA (BioNTech/Pfizer) vaccine.
Methods: Totally 10 735 adult volunteers that received at least one dose of BioNTech/Pfizer or triple doses of CoronaVac participated in this cross-sectional-online survey between 1 and 10 September 2021. The information was collected covering a 5-month period from April 2021 to September 2021. Information about people who were vaccinated with only single and double dose CoronaVac were not included in this study.
Results:At least one side effect after single and double dose of BioNTech/Pfizer and triple doses of CoronaVac were observed in 42.1%, 42.5% and 10.9%, respectively. The most common side effects were shoulder/arm pain, weakness/fatigue, muscle/joint pain and headache. The side effects were the most frequent in single BioNTech/Pfizer, while it was the least in triple CoronaVac. The rate of positive PCR tests before vaccination was 17.6%, and decreased to 3.0% after vaccination. The rates of positive SARS CoV-2-PCR were 18.8%, 3.5%, 3.1%, 0.5% and 4.6% in single BioNTech/Pfizer,double BioNTech/Pfizer, double CoronaVac+single BioNTech/Pfizer,double CoronaVac+double BioNTech/Pfizer and triple CoronaVac,respectively. While 1.8% of PCR positive COVID-19 cases needed intensive unit care in the pre-vaccination period, intensive care unit was required in 0%, 1.5%, 2.4%, 0% and 4.2% after single BioNTech/Pfizer, double BioNTech/Pfizer, double CoronaVac+single BioNTech/Pfizer, double CoronaVac+double BioNTech/Pfizer and triple CoronaVac, respectively. Reinfection rate after vaccination was 0.4%.
Conclusions: The rarity of COVID-19 infection after vaccination suggests that efficacy of vaccines is maintained. On the other hand,the data underscore the critical importance of continued public health mitigation.
KEYWORDS: mRNA vaccine; COVID-19; SARS-CoV-2;Vaccine schemes; Reinfection; Positivity rate after vaccination
Significance
Vaccines have been shown to minimize transmission, spread,hospitalizations, and mortality of COVID-19. There have been limited investigations on the rate of COVID-19 emergence following immunization, as well as the clinical characteristics of acute infection after various vaccine protocols. This study reveals that Biontech/Pfizer and various schemes are tolerably safe and complete immunization against SARS-CoV-2 reduces case rates even when the virus is prevalent in the population and novel variations arise. The rarity of COVID-19 following vaccination is reassuring, suggesting that the vaccines' effectiveness has been maintained.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)is considered as a public health problem all over the world due to its easy and rapid spread, straining health systems and clinical management[1]. As a result, although the continued public health mitigation measures (masking, physical distancing and daily symptom screening etc.) have been taken, SARS-CoV-2 has adversely affected not only the infected people but also the entire population, the global economy and resources[2]. Therefore, it was urgent to develop vaccines to prevent the spread of SARS-CoV-2 infection in order to achieve herd immunity.
Vaccination reduces the risk of transmission and infection by causing a coordinated response in innate or adaptive immunity, as well as an immunological memory[3]. Since the vaccine reduces both the transmission and spread, and the hospitalizations and deaths in coronavirus disease 2019 (COVID-19), it is necessary to overcome the vaccine hesitancy and to spread the vaccination rapidly.
In Turkey, the first SARS-CoV-2 vaccine was launched in January 2021 with double dose inactivated CoronaVac vaccine performed in a four-week period. The routine protocol recommended until April 2021 was double dose CoronaVac only. In April, double dose BNT162b2 messenger ribonucleic acid (mRNA) vaccine(BioNTech/Pfizer) became available in Turkey. As of July 2021, in Turkey, there are two choices as a booster immunization with the third dose provided with either CoronaVac, or a BioNTech/Pfizer vaccine prioritizing health care workers and higher risk groups,later the stratified population. Due to the fluctuations in the course of the epidemic, the detection of new variants and the requirement of at least double dose BioNTech/Pfizer vaccine for travelers to some countries, the second dose of BioNTech/Pfizer vaccine started to be offered optionally in late July 2021 on the top of double dose CoronaVac+single BioNTech/Pfizer vaccine. As the different vaccine combinations (single CoronaVac, double CoronaVac, triple CoronaVac, single Biontech/Pfizer, double BioNTech/Pfizer, double CoronaVac+single BioNTech/Pfizer, double CoronaVac+double BioNTech/Pfizer) have being recommended officially, the confusion in the society has increased and the need for scientific data has become urgent. As far as we know, there are very limited studies that provide information about the rate of COVID-19 emerging after vaccination, and the clinical characteristics of the acute infection after combination of different vaccine protocols[4-8].
The primary outcome of this study is to describe the effects of various nation-wide vaccination protocols on the evolution of new SARS-CoV-2 infections among the general adult population and the secondary outcome is to evaluate the safety of the BNT162b2 mRNA (BioNTech/Pfizer) vaccine with an online questionnaire.
2. Subjects and methods
2.1. Ethical approval and participants’ consent statement
This is a cross-sectional study that was approved by the local institutional ethics committee of Cukurova University, Adana(approval No. 2021/114). An informed consent statement was required to be checked in at the beginning of the questionnaire. All procedures performed in the study involving human participants were in accordance with the ethical standards of the hospital,national research committee and the 1964 Helsinki declaration.
2.2. Study design and participants
An online questionnaire (https://docs.google.com/forms/d/e/1FAIpQLSdKTmjlbqC_XnvpQqYV6U4nuu3gbAcbjESrMZGm Fb2gmgE0wA/closedform) was applied via the internet to general population. The questions in the questionnaire were prepared by considering the side effect profiles and clinical observations previously mentioned in the literature[9-11]. None of the questions were open-ended. We performed a logic check and corrected any non-logical data. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Inclusion criteria: (1) Over 18 years of age; (2) Consent to participate; (3) Vaccinated with at least one dose of Biontech/Pfizer or triple doses of CoronaVac.
Exclusion criteria: (1) Refuse to participate (2) Vaccinated with only one dose of CoronaVac; (3) Vaccinated with only double doses of CoronaVac.
Out of 10 735 volunteers assessed for eligibility, 381 were excluded as they does not fit the inclusion criteria. Ten thousand three hundred and fifty-four participants over the age of 18 who had received at least one dose of BioNTech/Pfizer or triple doses of CoronaVac(triple CoronaVac, single or double BioNTech/Pfizer, double CoronaVac+single BioNTech/Pfizer, double CoronaVac+double BioNTech/Pfizer) were included in a cross-sectional survey study between 1 and 10 September 2021 (Figure 1). In our study, we evaluated definite combinations of vaccines, especially since we aimed to observe the side effects of Biontech/Pfizer and booster effects of vaccines. Single and two doses of CoronaVac were excluded from the study because they filled the effect protective period and the booster effect could not be evaluated. The information was collected covering a 5-months period from April 2021 to September 2021. Information about people who were vaccinated with only single and double dose CoronaVac were not included in this study. This group was evaluated in a separate survey study which has been submitted elsewhere. Vaccination with the third dose of Biontech/Pfizer in Turkey started on November 4,2021, after the study ended. Therefore, we could not find data on this group neither in this study.

Figure 1. Flow chart for selecting the participants and frequency of different vaccination protocols
Participation in the study was from all seven geographical regions and each 81 provinces of Turkey and categorized due to the first level of Nomenclature of Territorial Units for Statistics system. The participants received no financial benefits.
2.3. Variables, measurement and outcomes
The online questionnaire consists of detailed sociodemographic data, pre-vaccine COVID-19 history, vaccine side effects and, the rate and timing of positive SARS-CoV-2 polymerase chain reaction(PCR) before and after vaccination if obtained. The survey starts with a brief study information and approval of informed consent.After the approval, the participants were asked whether they had at least one dose of Biontech/Pfizer or triple doses CoronaVac vaccine;Those who answered “yes” were able to continue working. To make the survey easier to complete, options for most questions are “yes”or “no”.
The questionnaire is consisted of the following three sections:
(1) In the first section, sociodemographic data (includes age, sex,residence, smoking history and occupational history) and detailed comorbidity anamnesis were recorded.
(2) In the second section, all side effects that developed in the period starting from immediately after the first vaccination to the end of the 30th day after second vaccination were questioned.
(3) In the third section, questions regarding the PCR positivity before and after each dose of vaccines, the clinical characteristics of COVID-19 and the requirements of hospital/intensive care unit in pre- and post-vaccine periods were recorded in detail for each vaccination protocol.
The participants were asked to respond to appropriate questions according to their real situation. The PCR positivity status before and after vaccination were given based on the participants’declaration only.
2.4. Data analysis
All analysis was performed by using IBM SPSS version 23.Descriptive statistics for continuous variables are given as mean±standard deviation while for categorical variables were given as frequency and percentages. While the association among two dependent categorical variables was examined with McNemar’s test, for independent categorical variables, Pearson’s Chi-squared test or Fisher-Freeman-Halton test was examined. Moreover, if a significant difference was found among independent groups,the column proportions were compared for pairwise comparisons with the Bonferroni adjustment. Safety analyses were expressed as counts and percentages for side effects from inoculation of the 1st vaccination through the 30th day of second dose. The statistical significance was considered as P-value<0.05.
3. Results
3.1. Socio-demographic characteristics
Ten thousand seven hundred thirty-five (10 735) people participated in our survey study. Three hundred eighty-one (381, 3.5%) of 10 735 participants were excluded as they did not have at least one dose of BioNTech/Pfizer or triple CoronaVac. Six thousand four hundred eighty-eight (6 488, 62.7%) women and 3 866 (37.3%) men were included in the study. The mean age of the participants was(45.7±13.4) years. Five thousand two hundred forty-seven (5 247,50.7%) of the participants were health-care workers. All provinces of Turkey participated in the study and these data were categorized according to the first level of Nomenclature of Territorial Units for Statistics system.
Five thousand two hundred twenty-two (5 222, 50.4%) of the participants declared they had never smoked, 2 820 (27.2%) were active smokers, while 2 312 (22.3%) were ex-smokers. A total of 2 879 (27.8%)of the participants had a history of at least one doctor-diagnosed disease (Table 1).

Table 1. Sociodemographic and clinical characteristics of participants [n(%)].
3.2. Pre-vaccine SARS-CoV-2 infection
One thousand eight hundred twenty-six (1 826, 17.6%) participants in the whole population had declared positive PCR test for SARS-CoV-2 before vaccination. Within this group, 1 536 (84.1%) participants were treated at home, 257 (14.1%) people were treated at general ward and 33 (1.8%) were treated in the intensive care unit.
3.3. Safety of BioNTech/Pfizer
Side effects from inoculation of the 1st vaccination through the 30th day of second dose were recorded. Four thousand one hundred thirty-six (4 136, 42.1%) and 2 945 (42.5%) of the participants stated that they had at least one side effect after single or double dose of BioNTech/Pfizer, respectively. The most common side effects were shoulder/arm pain, weakness/fatigue, muscle/joint pain and headache. The periods during which side effects developed after vaccination has differed, and detailed data are shown in supplementary material.
Considering the side effects after the first dose of BioNTech/Pfizer (n=2 896), the most common side effects were shoulder-arm pain (1 123, 38.8%), weakness/fatigue (946, 32.7%), muscle/joint pain (724, 25.0%), headache (486, 16.8%), fever (370, 12.8%),dizziness (162, 5.6%), palpitations/heart rhythm problems (107,3.7%), nausea/vomiting (89, 3.1%), local lymphadenopathy (96,3.3%), diarrhea (92, 3.2%), chest pain (75, 2.6%), hypertension (66,2.3%), shortness of breath (63, 2.2%), local allergic reaction (46,1.6%), low blood pressure (43, 1.5%), clot (any part of the body) (11,0.4%), myocarditis (11, 0.4%), sudden fainting (11, 0.4%), thyroid disorders (11, 0.4%), zona zoster infection (11, 0.4%), pericarditis(9, 0.3%), myocardial infarction (6, 0.2%), stroke (6, 0.2%), facial palsy (6, 0.2%), respectively.
Considering the period after the second dose of BioNTech/Pfizer(n=6 933), the most common side effects were shoulder-arm pain(2 218, 32.0%), weakness/fatigue (2 176, 31.4%), muscle/joint pain (1 643, 23.7%), headache (1 137, 16.4%), fever (984, 14.2%),dizziness (339, 4.9%), palpitations/heart rhythm problems (222,3.2%), nausea-vomiting (194, 2.8%), diarrhea (180, 2.6%), chest pain (159, 2.3%), local lymphadenopathy (138, 2.0%), shortness of breath (111, 1.6%), hypertension (104, 1.5%), local allergic reaction(76, 1.1%), low blood pressure (70, 1.0%), thyroid disorders (21,0.3%), zona zoster infection (21, 0.3%), clot (any part of the body)(14, 0.2%), sudden fainting (14, 0.2%), myocarditis (14, 0.2%),pericarditis (7, 0.1%), myocardial infarction (7, 0.1%), facial paralysis (7, 0.1%) and stroke (6, 0.1%), respectively.
3.4. Safety of triple CoronaVac
At least one side effect after triple doses of CoronaVac were observed in fifty seven (10.9% ,) of the participants. Considering the side effects after the third dose of CoronaVac (n=525), the most common side effects were weakness/fatigue (36, 6.9%), muscle/joint pain (31, 5.9%), shoulder/arm pain (30, 5.7%), headache (22,4.2%), chest pain (10, 1.9%), palpitations/heart rhythm problem(10, 1.9%), diarrhea (10, 1.9%), dizziness (9, 1.7%), hypertension(9, 1.7%), fever (7, 1.3%), nausea/vomiting (5, 1.0%), allergic reaction (5, 1.0%), low blood pressure (4, 0.8%), shortness of breath(4, 0.8%), sudden fainting (2, 0.4%), thyroid disorders (2, 0.4%),local lymphadenopathy (2, 0.4%), myocardial infarction (2, 0.4%),pericarditis (2, 0.4%), myocarditis (2, 0.4%), facial palsy (1, 0.2%)while clot, stroke or zona zoster infection has never been seen.
Safety of various vaccine protocols can be seen in Table 2 in detail. The rate of low blood pressure, sudden fainting, myocardial infarction, facial palsy and zona zoster infection were similarin different protocols. When side effects were examined based on different protocols, all side effects (except fever, zona zoster infection, thyroid disorders and low blood pressure) were most commonly seen after the first dose of BioNTech/Pfizer. If different vaccination protocols were compared, prevalence of the side effects were higher in the single BioNTech/Pfizer group, while lower in the triple CoronaVac group for the majority of the side-effect categories although not all the differences were significant (Table 2).

Table 2. Side effects of different vaccine protocols.
3.5. Positive SARS-CoV-2 PCR and reinfection rates
The positive SARS-CoV-2 PCR test before any vaccination protocols was declared in 1 826 (17.6%) participants. This was significantly higher than the declared positive rate in 306 (3.0%)after vaccination (P<0.001). The number of participants who declared PCR positive both before and after vaccination (reinfection rate) was 40 (0.4%).
3.6. SARS-CoV-2 positive PCR rates after different vaccine protocols
In single BioNTech/Pfizer group, 49 (20.9%) had declared positive PCR tests after vaccination. Forty-two of them (85.7%) were treated at home, while 7 (14.3%) were followed in the general ward but none of those needed intensive unit care.
In double BioNTech/Pfizer group, 136 (3.5%) had declared positive PCR tests. One hundred thirty-three (97.8%) of them were treated at home, 1 (0.7%) were treated in the ward and 2 (1.5%)needed intensive care unit. One of the patients requiring intensive care had a severe cardiac failure and the other was found to be PCR positive incidentally while being followed in the intensive care unit due to a femoral fracture at an advanced age.
In double CoronaVac+single BioNTech/Pfizer group, 83 (3.1%)had declared positive PCR tests after vaccination. Among them, 80(96.4%) were treated at home, while 1 (1.2%) received treatment at the general ward and 2 (2.4%) in intensive care unit.
In double CoronaVac+double BioNTech/Pfizer, 14 (0.5%) had declared positive PCR tests after vaccination. Twelve (85.7%)people were treated at home, and 2 (14.3%) people were treated at the general ward and none of those needed intensive unit care.
In triple CoronaVac group, 24 (4.6%) had declared SARS-CoV-2 positive PCR test after vaccination. Nineteen (79.2%) of the group were treated at home, while 4 (16.7%) of the group admitted to general ward and 1 (4.2%) admitted intensive care unit.
When we focus on the association between vaccination protocol and the positive PCR rates after vaccination, the highest positive PCR rate comes from single BioNTech/Pfizer, followed by triple CoronaVac, double BioNTech/Pfizer and double CoronaVac+single BioNTech/Pfizer, respectively. Significant difference in the PCR positivity after vaccination was found between the vaccine protocols (Table 3). When the pairwise comparisons of column proportions were observed, the proportion of PCR positivity in the single Biotech/Pfizer group is statistically higher than in other four groups while the proportion of PCR positivity in the doble CoronaVac+double Biontech/Pfizer group is statistically lower than in other groups. The detection time of positive PCR test developing after different protocols of vaccines can be seen in detail in Table 3.
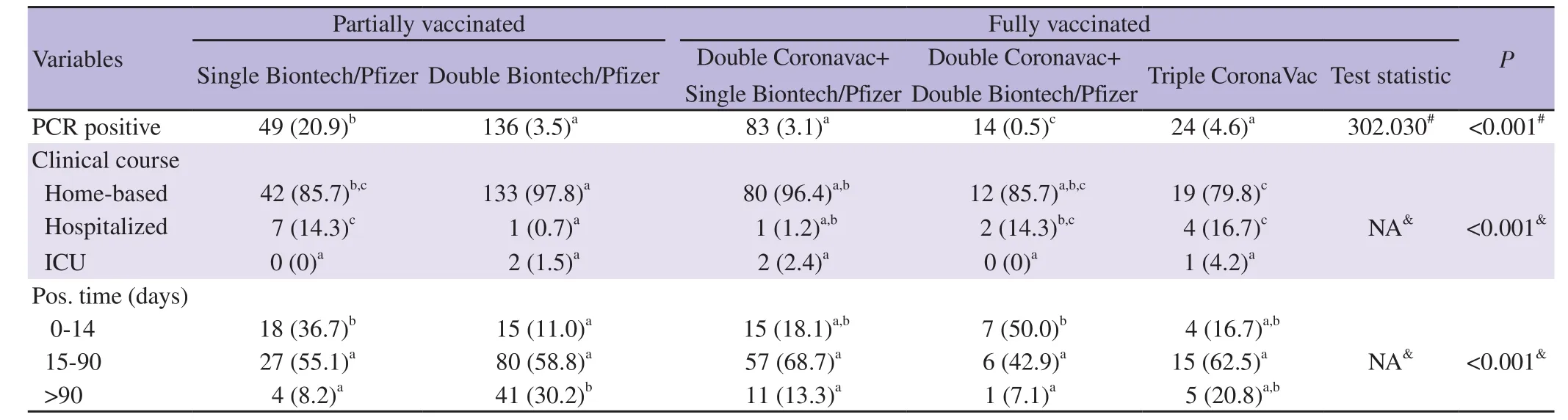
Table 3. The association among vaccination procedure and the results of SARS-CoV-2 PCR and clinical course after vaccination.
4. Discussion
In this study, the side effects reported after single or double doses of BioNTech/Pfizer or triple doses of CoronaVac inoculation and,SARS-CoV-2 PCR positivity rates after five different vaccine combinations (single dose BioNTech/Pfizer, double doses of BioNTech/Pfizer, double doses of CoronaVac+single dose of BioNTech/Pfizer, double doses of CoronaVac+double doses of BioNTech/Pfizer and triple doses of CoronaVac) and the clinical characteristics of the participants who declared positive SARSCoV-2 PCR pre and after vaccines were determined. At least one side effect after single and double dose of BioNTech/Pfizer and triple doses of CoronaVac were seen in 42.1%, 42.5% and 10.9% of the participants, respectively. The most common side effects were shoulder/arm pain, weakness/fatigue, muscle/joint pain and headache. Among the different vaccine protocols, the side effects were most frequent in single BioNTech/Pfizer arm, while it was the least in triple CoronaVac arm. The side effects after single and double dose of CoronaVac were not collected in this survey.The rate of side effects was found to be low in the group with triple CoronaVac. To the best of our knowledge, there has been no literature data that can be seen regarding this topic. It is thought that this low level may be due to the nature of inactivated vaccine that cannot produce an immune response as much as mRNA vaccines.At least one side effect after single and double dose of BioNTech/Pfizer were seen in 42.1% and 42.5% of the participants,respectively. Serious side effects (myocarditis, pericarditis,myocardial infarction and sudden fainting) were rare. Serious side effects were the most common in the single BioNTech/Pfizer group.In a study investigating the safety of the BNT162b2 mRNA vaccine in a large group of patients who were followed up with an electronic diary for an average of two months after vaccination, any reported adverse event frequency was 27%. In this study, only four serious side effects (shoulder injury, right axillary lymphadenopathy,paroxysmal ventricular arrhythmia, and right leg paresthesia) and no vaccine-related death were reported[9]. In another study, the most common side effects in older patients were headache, fatigue,myalgia, chills and injection-site pain, and no serious side effects were observed[10]. In similar studies, the incidence of side effects was over 50% on average and more frequent after the second dose; and serious side effects are very rare, in accordance with our findings[11-13]. Side effects was seen in 10.9% of the participants after triple dose of CoronaVac. The most common side effects were shoulder/arm pain, weakness/fatigue, muscle/joint pain and headache. No data were found investigating side effects after triple CoronaVac and considering the limited number of patient data in our study, it was seen that the rate of side effects was lower in this vaccine scheme. Likewise, the incidences of overall adverse reactions after the first and second injections of CoronaVac were 15.6% (238/1 526) and 14.6% (204/1 397), respectively in a previous study[14]. It was thought that this might be the result of the inactive vaccine does not stimulate the immune system as strongly as mRNA vaccines. Among the different vaccine protocols, side effects were most frequent in single BioNTech/Pfizer, while it was the least in triple CoronaVac. To the best of our knowledge, this is the first data about the side effects after different vaccine protocols.
The rate of declared positive PCR tests before vaccination was 17.6%, while it was 3.0% after any vaccination protocol. The rates of declared positive SARS-CoV-2 PCR were 20.9%, 3.5%, 3.1%,0.5% and 4.6% in single BioNTech/Pfizer, double BioNTech/Pfizer, double CoronaVac+single BioNTech/Pfizer, double CoronaVac+double BioNTech/Pfizer and triple CoronaVac groups,respectively. While 1.8% of PCR positive COVID-19 cases needed intensive unit care in the pre-vaccination period; intensive care unit was required in 0%, 1.5%, 2.4%, 0%, 4.2% after single BioNTech/Pfizer, double BioNTech/Pfizer, double CoronaVac+single BioNTech/Pfizer, double CoronaVac+double BioNTech/Pfizer and triple CoronaVac, respectively. Reinfection rate after vaccination was 0.4%.
Clinical trial data of BNT162b2 vaccine estimated an early vaccine efficacy in preventing COVID-19 of 52.4% before dose two, and 90.5% on days 2-7 after dose two[9]. In a population-based study of over eight million people, the PCR positivity rate was 0.68% and 3.68% in full vaccination and unvaccinated groups, respectively[15].In a study conducted on healthcare workers in New Jersey, the PCR positivity rate after vaccination was 0.58% in the group that received one or two doses of BioNTech/Pfizer or Moderna, while the positivity rate after two doses of vaccination was 0.15%[16]. In a study conducted on healthcare professionals, in which the frequency of COVID-19 was evaluated after mRNA vaccines (BioNTech/Pfizer+Moderna), PCR positivity was observed in 1.3% after the first dose of vaccination, and 0.3% after two doses of vaccination,while this rate was 9.5% in the unvaccinated participants[17]. In an Israel study that assess vaccine-associated rate reductions on healthcare workers, 1.87% of the participants had SARS-CoV-2 and symptomatic COVID-19 rate has dropped from 5 to 1.2 per 10 000 person-days after 14th day of the first dose of mRNA vaccine[18].Israel study conducted with active and passive surveillance by using questionnaires, hotlines and post-vaccination web-based questionnaires among healthcare workers, 0.54% had laboratoryconfirmed COVID-19 and the greater portion was after 1-14 days after vaccination with mRNA vaccine[19]. In another study published from Chicago, post-vaccine PCR positivity was seen in 3.5% of the participants; nearly two thirds were asymptomatic, 9% were hospitalized and only one with multiple comorbidities has died;the same study detected PCR positivity in 71% of unvaccinated persons, 23% of partially vaccinated persons, 2% of vaccinated but not immune persons, 4% of fully vaccinated people[20]. A California study dealing with healthcare workers with weekly PCR screening showed that after receiving both vaccinations, 0.13% health care workers tested positive, vast of majority was between 1 to 7 days after the second dose[21]. In a retrospective study from Israel health care workers who underwent periodic testing for SARS-CoV-2 infection, vaccination with the BNT162b2 vaccine was associated with an adjusted incidence rate ratio of 0.03 for symptomatic infection and 0.14 for asymptomatic infection more than 7 days after the second dose[8]. An Israel national surveillance study from the first four months of vaccination, 3.3% of SARS-CoV-2 infected people needed hospitalization, 1.9% had severe illness and critical hospitalization whereas the mortality rate was 0.48%[22]. An Israel study recently showed a 90% reduction in deaths with a third booster dose and during the study period, confirmed SARS-CoV-2 infection was observed in 0.38% of participants in the booster group with adjusted hazard ratio for SARS-CoV-2 infection in the booster group, as compared with the nonbooster group was 0.17[14].In the literature review, where most of the data were collected from healthcare professionals, it was shown that the PCR positivity rate decreased after vaccination, as in our study. The rate of prevaccine PCR positivity in our study was found to be relatively higher compared to literature, which was thought to be related to the fact that Turkey is one of the top countries in the COVID case list.Considering the population participating in the study, a significant decrease can be seen in post-vaccine PCR positivity, and also the decrease can be seen in all full vaccination protocols. Since there are full vaccination recommendations with different vaccination protocols in Turkey and these recommendations are unique to Turkey, this is the first data showing PCR positivity after different vaccination protocols, according to our current knowledge.
Our study has limitations due to the characteristics of a survey study. All data are dependent on the person's own notification, and the data of the deceased patients and the patients still in the intensive care unit cannot be included. Although there is participation from all provinces of Turkey, it still does not represent the whole country. Lack of active laboratory surveillance in the study may have resulted in an underestimation of asymptomatic or mild cases. Since the PCR test could not be performed on all patients participating in the study, post-vaccination positivity was of course made according to the person's notification, but it must be kept in mind that the guideline of Ministry of Health of the Republic of Turkey recommends SARS-CoV-2 testing, which is triggered by the presence of at least one symptom during daily screening or by an identified exposure, regardless of vaccination status through the entire period of pandemic.
We do not know yet whether protective immune responses are durable; we do not know whether primary series and booster doses can or should be different. After the first half of 2021, in many countries such as Turkey, England, Israel, France and many others,the third dose was approved, especially for risk groups, six months after the second dose. The different vaccine protocols and dosing regimens and variations in the robustness of natural and vaccinal immunity may result in various outcomes in near future. It is therefore urgent to determine the strength and duration of clinical protection through careful clinical evaluations in order to declare public policies. Our study indicates that full vaccination against SARS-CoV-2 decreases case rates even in a time of high prevalence in community and emergence of new variants. The rarity of positive test results 14 days after administration of vaccines is encouraging and suggests that the efficacy of these vaccines is maintained.On the other hand, the data underscore the critical importance of continued public health mitigation measures (masking, physical distancing, daily symptom screening, and regular testing), even in environments with a high incidence of vaccination, until herd immunity is reached at large.
Conflict of interest statement
The authors declare that they have no potential conflict of interest including any financial, personal or other relationships with the other people or organizations that could inappropriately influence,or be perceived to influence the presented work.
Funding
The authors received no extramural funding for the study.
Authors’ contributions
OB, NK, SO, MTK made substantial contributions to the conception and design of the work; OB, NK, EO, BO, BOS contributed to the acquisition, analysis, interpretation of data;OB, EO, BO contributed to the creation of new software used in the work; OB, NK, BO, SO, MTK, BO have drafted the work or substantively revised it and all authors have approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. All authors read and approved the final version of the manuscript.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Tuberculous meningitis and miliary tuberculosis in Iran: A review
- Surveillance system-based physician reporting of pneumonia of unknown etiology in China: A cross-sectional study
- Outcome of patients with severe COVID-19 pneumonia treated with high-dose corticosteroid pulse therapy: A retrospective study
- Diffuse alveolar hemorrhage complicating dengue haemorrhagic fever in a 15-yearold boy: A case report
- Membranous nephropathy associated with tuberculosis-a case report
- A hypothetical mechanism whereby malaria infection protects against COVID-19
