Fabrication of Cu+sites in confined spaces for adsorptive desulfurization by series connection double-solvent strategy
2022-05-22ShuShiYuXiaLiShuaiShuaiLiXiaoQinLiuLinBingSun
Shu Shi,Yu-Xia Li,Shuai-Shuai Li,Xiao-Qin Liu,Lin-Bing Sun
State Key Laboratory of Materials-Oriented Chemical Engineering,Jiangsu National Synergetic Innovation Center for Advanced Materials(SICAM),College of Chemical Engineering,Nanjing Tech University,30 South Puzhu Road,Nanjing 211816,China
Abstract Cu+-containing materials have shown various application prospects especially in adsorption and catalysis,because they are versatile,nontoxic and low cost.To date,developing a mild and controllable approach for the fabrication of Cu+sites has remained a pronounced challenge.Herein,we report a series connection double-solvent strategy (SCDS) for fabricating Cu+ sites within MIL-101(Cr),a typical metal-organic framework.By employing the SCDS in which vitamin C is chosen as the environmentally benign reducing agent,Cu2+ was incorporated in the pores and then transformed to Cu+in the confined spaces.Compared to the conventional high-temperature autoreduction method conducted under harsh environment(700 °C for 12 h)with a low Cu+yield(less than 50%),SCDS can selectively reduce Cu2+to Cu+at room temperature without generating any Cu0.The resulting Cu+ modified MIL-101(Cr) exhibits good desulfurization performance in view of both uptake and recyclability.
Keywords: Double-solvent method;π-complexation adsorption;Selective reduction;Deep desulfurization
1.Introduction
Sulfur oxides from the combustion of sulfur-containing liquid hydrocarbon fuels cause a series of problems,which includes triggering diseases such as the human respiratory tract to endanger human health and forming severe ecological hazards like acid rain [1,2].Moreover,sulfur in fuel cells can lead to the poisoning of fuel cell cathodes [3-7].With concerns related to these serious consequences,various countries are issuing stringent rules on limiting the sulfur levels in fuels[8,9].Aromatic sulfur compounds (ASCs) which account for more than 60%of total sulfides are a major obstacle to achieve deep desulfurization [10,11].The traditional hydrodesulfurization (HDS) technology is less effective in the removal of ASCs [12-17],which fails by degrees to comply with increasingly stringent sulfur emission regulations.Therefore,some alternative desulfurization techniques including adsorption,oxidation,and biological desulfurization are developed [18-22].Among these techniques,adsorptive desulfurization has become a promising alternative with regard to the advantages of high selectivity,low cost and easy regeneration of adsorbents [9,20,23].The focus of adsorptive desulfurization is to develop efficient adsorbents [23-25].It has been reported that Cu+-containing adsorbents exhibit good performance in the removal of ASCs by π-complexation[20,26-28].Because π-complexation interaction is stronger than the van der Waals force but weaker than the chemical bond,materials with π-complexation can form unique superiority with good adsorption capacity,high selectivity,and easy desorption regeneration [8,29].In addition,Cu+has the advantages of cheap and non-toxic [26,30,31],which also makes it have broad application prospects.
Generally,the synthesis of the Cu+-containing adsorbents includes two necessary steps,namely introduction of Cu2+precursors into the porous material and reduction of Cu2+to Cu+as reported previously by our and other groups[8,31-35].Wet impregnation is frequently adopted to introduce Cu2+precursors [36,37],whereas only a part of Cu2+precursors enter the porous supports,and the remaining Cu2+inevitably gathers outside the pores,which is unfavorable to subsequent reduction of Cu2+[38,39].For the second step,two approaches have been adopted mainly.The first approach is high-temperature autoreduction (HTA).This method demands tough conditions (e.g.,700°C for 12 h) [13,40],so that the structure of the porous carrier with relatively poor thermal stability may be destroyed,for instance,metal-organic frameworks (MOFs).Furthermore,the yield of Cu+generated by HTA is usually less than 50%,which is far from satisfaction [41].The second approach is liquid-phase reduction.This approach requires a favorable reducing agent and the reduction occurs in solution.However,during the reduction process,the diffusion of reducing agent in the solution will also give rise to the Cu2+species to diffuse outside of the pores,thereby aggregating on the outer surface of the porous materials.In spite of significant efforts that have been made,developing a controllable approach to efficiently construct Cu+-containing adsorbents is still full of challenges.
Herein,we report a series connection double-solvent strategy(SCDS,Fig.1)for the preparation of an efficient adsorbent for desulfurization.MIL-101(Cr),a typical MOF,featuring a hydrophobic outer surface and a hydrophilic inner surface of the pores,is used as the porous carrier suitable for the double-solvent (DS) method.Compared to most other MOFs,MIL-101(Cr) possesses higher porosity and better stability.By employing the SCDS,Cu2+and reducing agent are loaded into the pores.A vast amount ofn-hexane and a trace of aqueous solutions are adopted to achieve the DS system,which guarantees that the introduced Cu2+are confined to MIL-101(Cr)pores and the conversionto Cu+takes place in that confined spaces.As a polyhydroxy compound,vitamin C has the enediol group in its molecular structurewhich is easily oxidized and plays vital roles in the redox reaction of human body[42].Vitamin C is selected as a green reducing agent due to its harmless to the environment.The SCDS results in selective conversion to Cu+at 25°C without generation of any Cu0which is impossible for traditional HTA.Because Cu0cannot work as active sites as Cu+which can form π-complexes with ASCs,the absence of Cu0is important for our materials applied in adsorptive desulfurization.It is demonstrated that the obtained Cu+-containing adsorbent can capture 332 μmol g-1thiophene and 358 μmol g-1benzothiophene(BT)under ambient conditions,superior to MIL-101(Cr) (136 μmol g-1for thiophene and 207 μmol g-1for BT),and the adsorption performance is well maintained after three cycles.
2.Experimental
2.1.Materials
Copper chloride (CuCl2·2H2O,>99.5%),chromium nitrate (Cr(NO3)3·9H2O,>99%),vitamin C (>99%),hydrofluoric acid (HF,40%),ethanol (> 99%),N,Ndimethylformamide (DMF,>99%),andn-hexane (>99%)were bought from Sinopharm Chemical Reagent Co.,Ltd.Terephthalic acid (PTA,>98%) was obtained from Aldrich Industrial Co.,Ltd.Thiophene,BT,and isooctane were got from Sigma.
2.2.Sample preparation
2.2.1.Preparation of MIL-101(Cr)
The synthesis of MIL-101(Cr) takes place based on the reports with some modifications [43].Typically,Cr(NO3)3·9H2O(1660 mg),PTA(664 mg),HF(0.8 mL),and H2O (19.2 mL) were sealed in a beaker under stirring for 2 h.Afterward,it was diverted into a Teflon hydrothermal autoclave reactor followed by heating at 220°C for 8 h.The as-obtained sample was dispersed in boiled DMF and ethanol for 12 h respectively and dried under vacuum.
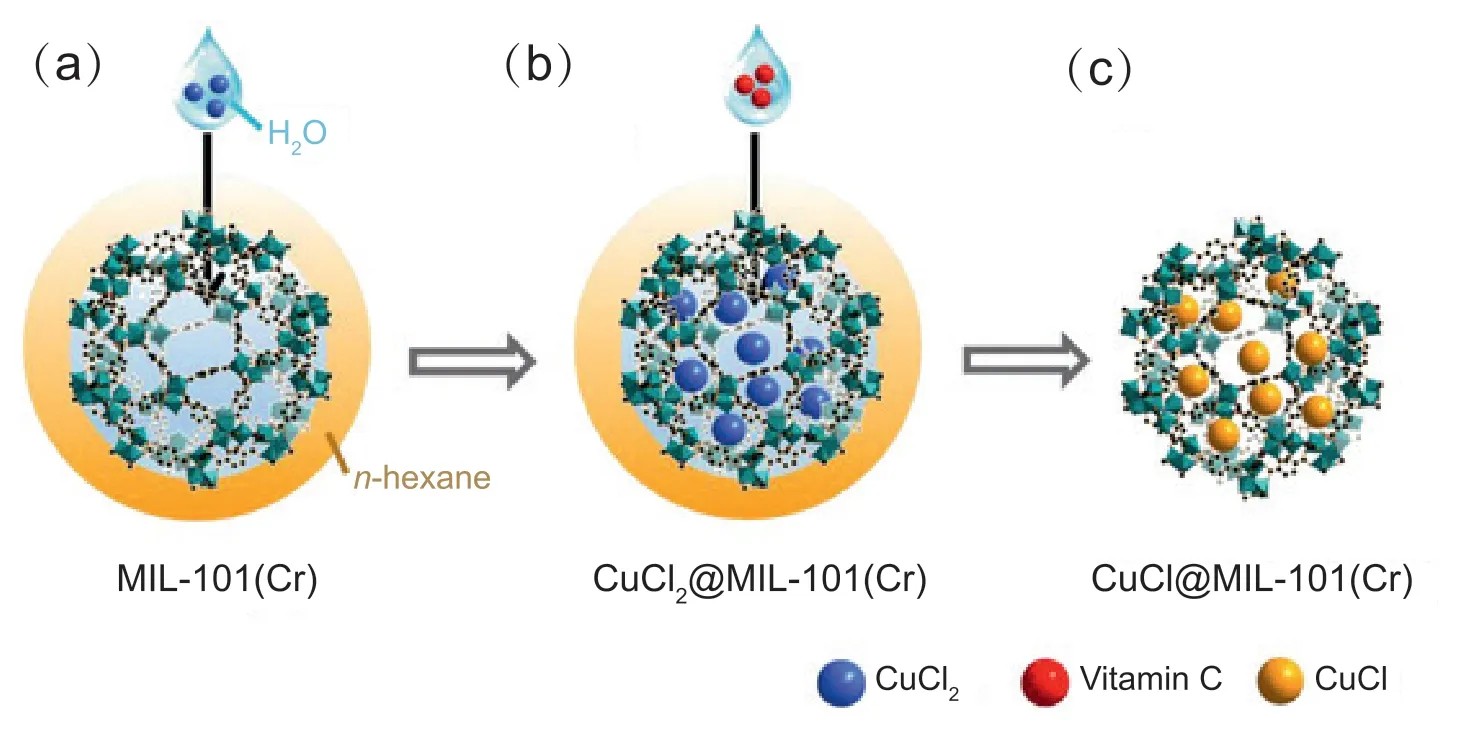
Fig.1.Schematic diagram of the fabrication of Cu+sites in MIL-101(Cr)using a series connection double-solvent strategy(SCDS).(a)Introduction of hydrophilic CuCl2 aqueous solution to the hydrophobic n-hexane dispersed with MIL-101(Cr);(b) introduction of hydrophilic aqueous reducing agent (vitamin C) into hydrophobic n-hexane dispersed with CuCl2@MIL-101(Cr);(c) formation of CuCl@MIL-101(Cr) containing Cu+ sites.
2.2.2.Preparation of CuCl2@MIL-101(Cr)
Typically,purified MIL-101(Cr) (200 mg) andn-hexane(40 mL) were added into a plastic centrifuge tube and then dispersed by ultrasonication for 15 min.After 2 h of lower speed stirring,320 μL of 0.625,1.25,2.5,or 3.75 mol L-1CuCl2aqueous solution was added dropwise under vigorous stirring in 16 min and continue to stir for 2 h.Then,the final sample was obtained after drying in a 100°C oven and namednCuCl2@MIL-101(Cr) (n,the different amount of CuCl2varied from 2 to 6 mmol g-1).
2.2.3.Preparation of CuCl@MIL-101(Cr)
CuCl2@MIL-101(Cr)(200 mg)andn-hexane(40 mL)were mixed under 15 min of ultrasonication.After 2 h of lower speed stirring,equimolar amounts of 0.63 mol L-1vitamin C aqueous solution (120,140,200,or 220 μL) were added dropwise into the mixture under vigorous stirring followed continuing to stir for 20 min.After filtering,the precipitate was washed with an appropriate amount of deionized water and ethanol,and dried at 80°C under vacuum.The resulted samples were namednCuCl@MIL-101(Cr) (n,the different loadings of CuCl varied from 2 to 6 mmol g-1).
3.Results and discussion
3.1.Structural and surface properties
The X-ray diffraction(XRD)profiles of all Cu2+-and Cu+-containing materials retain the prime diffraction character of pristine MIL-101(Cr) (Fig.2) [26,44],which illustrates the well maintained crystal structure of support by use of SCDS.When the Cu2+loading is less than 6 mmol g-1,the characteristic diffraction peaks attributed to CuCl2are absent in the XRD patterns.This is because CuCl2was well dispersed in MIL-101(Cr),as a result of the confining effect by using DS method.For 6CuCl2@MIL-101(Cr),a new diffraction peak at 16.3°which is attributed to CuCl2appears.After reduction,for 2CuCl@MIL-101(Cr) and 4CuCl@MIL-101(Cr),no visible CuCl peaks demonstrates good dispersion of CuCl on the support.For 6CuCl@MIL-101(Cr),characteristic diffraction peaks of CuCl at 28.5°and 47.5°become apparent,which is caused by excessive loading that exceeds the channel capacity threshold [45].In addition,for CuCl@MIL-101(Cr) samples,no diffraction associated with Cu0is detected.

Fig.2.XRD patterns of (a) CuCl2@MIL-101(Cr) and (b) CuCl@MIL-101(Cr).
The valence states of Cu species and quantitative analysis were investigated with X-ray photoelectron spectroscopy(XPS).As shown in Fig.3,Cu2+-containing materials show the Cu 2p3/2binding energy at 935.2 eV assigned to Cu2+.For CuCl@MIL-101(Cr),the Cu 2p3/2binding energy at 933.2 eV corresponds to Cu+[37],and no peak is observed at 935.2 eV.This indicates that Cu2+is nonexistent in the samples.In addition,titration was conducted to determine the content of Cu+.The data from titration in Table 1 show that there are 1.83,3.62,and 5.33 mmol g-1of Cu+presented in 2CuCl@MIL-101(Cr),4CuCl@MIL-101(Cr),and 6CuCl@-MIL-101(Cr) respectively,indicating the successful fabrication of Cu+on support by SCDS.The valence states of Cu species before and after reduction are also determined by UVvis spectra (Fig.4).The support displays two broad bands situated at 450 and 607 nm,which are attributed to the4A2g→4T1gand4A2g→4T2gtransition of Cr3+,respectively[46,47].After the introduction of CuCl2,the intensity of these two broad bands declines sharply and undergoes a certain degree of blue shift,which is because of the saturation of Cr3+caused by the ascent of Cu2+amount accompanied by the rise of electron transition energy.Besides,there is an obvious absorption band due to the d-d transition of Cu2+ranging from 700 to 850 nm,and its intensity gradually ascends with the rise of CuCl2content.For CuCl@MIL-101(Cr),the absorption peak attributed to Cu2+is significantly weakened;meanwhile,a peak that belongs to Cu+emerges at 386 nm.Hence,UV-vis results consistent with the results of XRD,XPS,and titration,confirm that Cu2+on MIL-101(Cr) is completely converted into Cu+without forming any Cu0through SCDS.
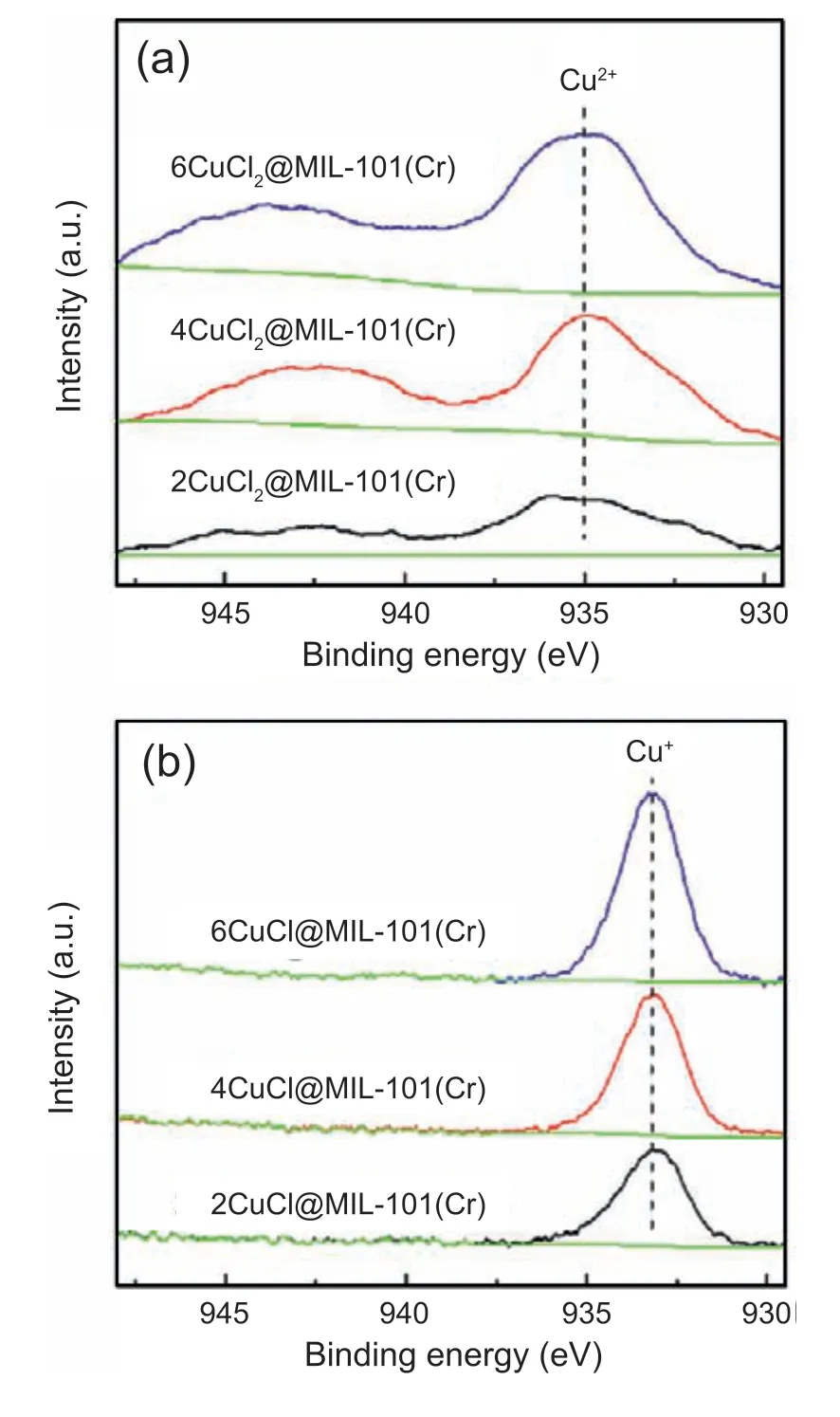
Fig.3.XPS spectra of(a)CuCl2@MIL-101(Cr)and(b)CuCl@MIL-101(Cr).
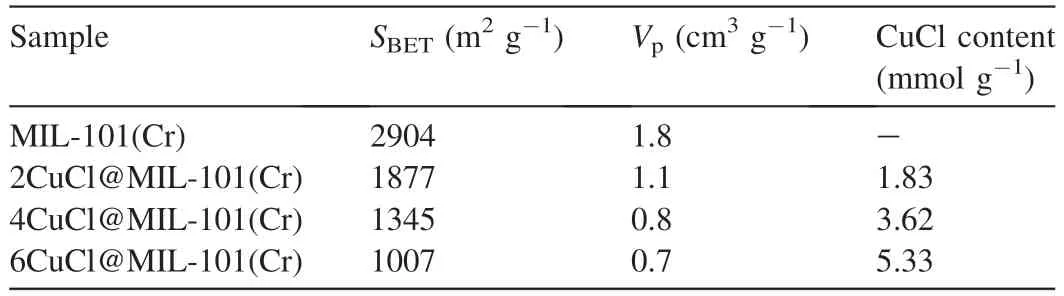
Table 1 Textural properties and Cu content of different samples.

Fig.4.UV-vis spectra of (a) CuCl2@MIL-101(Cr) and (b) CuCl@MIL-101(Cr).
N2adsorption measurements monitor the porosity of samples(Fig.5).For MIL-101(Cr),there are secondary uptakes at lower relative pressures,which are in line with the existence of two nanopore windows in structure[44,48,49].The Brunauer-Emmett-Teller (BET) surface area and total pore volume are 2904 m2g-1and 1.8 cm3g-1,respectively (Table 1),which are concordant with those have been well documented[44,50].Notwithstanding decreased N2adsorption amount,the isotherm shapes of Cu+-containing materials are equivalent to the pure MIL-101(Cr),which indicate the preserved pore structure by SCDS.As Cu content increases,pore volume gradually declines owing to the occupancy of pores by guest.This further proves the incorporation of Cu+within pores,especially for 2CuCl@MIL-101(Cr) and 4CuCl@MIL-101(Cr),without signal of Cu+species appearing in XRD patterns.IR spectrum (Fig.S1) of vitamin C shows a peak at 1754 cm-1assigned to the C-O stretching mode.There is no peak from vitamin C in 4CuCl@MIL-101(Cr),which signifies the complete removal of vitamin C.Moreover,the peak at 1700 cm-1ascribed to C=O decreases after the introduction of CuCl,which is due to the host-guest interaction between CuCl and the carboxyl group of PTA in MIL-101(Cr) [28].Scanning electron microscopy (SEM) images of Cu+-containing materials demonstrate regular octahedral structure,which are consistent with the topographic structure of support(Fig.S2).The dimension of crystals varies within 0.3-0.4 μm.To further determine the structural characteristics of the prepared adsorbents,we perform thermogravimetry(TG)analysis(Fig.S3).The obvious weight loss at about 300-450°C is caused by the destruction of MIL-101(Cr),while the premier loss is the removal of solvent and crystal water.Obviously,the shape of the weight loss curves of Cu+-containing materials is consistent with that of MIL-101(Cr),which coincides with previous results that MOF structure is well retained after the fabrication of Cu+by SCDS.Fig.6 depicts transmission electron microscopy (TEM) images of samples.The rhombohedra morphology of 4CuCl@MIL-101(Cr) can further verify the well preserved MIL-101(Cr) structure after modification of Cu+.In addition,C,Cr,Cu,and Cl elements are homogeneously scattered inside skeletons where C and Cr elements are derived from MIL-101(Cr).Therefore,these results prove that CuCl is successfully introduced into MIL-101(Cr) and is evenly distributed in the pores of support.

Fig.5.N2 adsorption-desorption isotherms of MIL-101(Cr) and CuCl@MIL-101(Cr).
Based on the above-mentioned results,Cu+sites are successfully fabricated in MIL-101(Cr) by SCDS.Since the SCDS process can be realized under mild conditions,the preservation of the support structure is guaranteed.The introduced Cu2+is selectively converted to Cu+without generation of any Cu0by using the green reducing agent vitamin C.Due to the introduction and reduction of Cu2+taking place in the confined spaces,Cu+species are well dispersed on MIL-101(Cr).These characteristics endow our materials with potentials for adsorptive desulfurization as explained in the following section.
3.2.Adsorptive desulfurization performance
As Cu+can form π-complexation with ASCs,the as-obtained Cu+-containing samples,namely CuCl@MIL-101(Cr),were exploited to remove thiophene and BT,which are representative ASCs whereas troublesome to remove by conventional HDS.The molecular sizes of thiophene and BT are 0.56 nm × 0.77 nm and 0.65 nm × 0.89 nm,respectively.
Figs.7a and S4 show the resultant breakthrough curves of thiophene.It can be intuitively found that the breakthrough of thiophene on 4CuCl@MIL-101(Cr) is 23 mL g-1,which is close to four times of the original MIL-101(Cr) (6 mL g-1).The calculation results are further listed in the Table S1.Obviously,compared with MIL-101(Cr),Cu+-containing materials exhibit larger thiophene uptakes due to the presence of Cu+.For instance,the adsorption capacities at breakthrough and saturation of 4CuCl@MIL-101(Cr) reaching 247 and 332 μmol g-1respectively,are nearly three times of pure MIL-101(Cr) (87 and 136 μmol g-1).Notably,the adsorption capacity is out of proportion to the corresponding Cu+loading.The adsorbent 4CuCl@MIL-101(Cr)with 3.62 mmol g-1Cu+displays the optimal adsorption effect in Cu+-containing samples.The Cu+content in 6CuCl@MIL-101(Cr) is 5.33 mmol g-1,which is the highest,yet its thiophene adsorption capacity is low compared to 4CuCl@MIL-101(Cr).This signifies the efficiency of desulfurization affected by both pores and active sites.The introduction of excessive Cu+can encumber partial pores of MIL-101(Cr),causing diffusion resistance and inaccessibility of some Cu+sites,which will have a negative effect on adsorption.
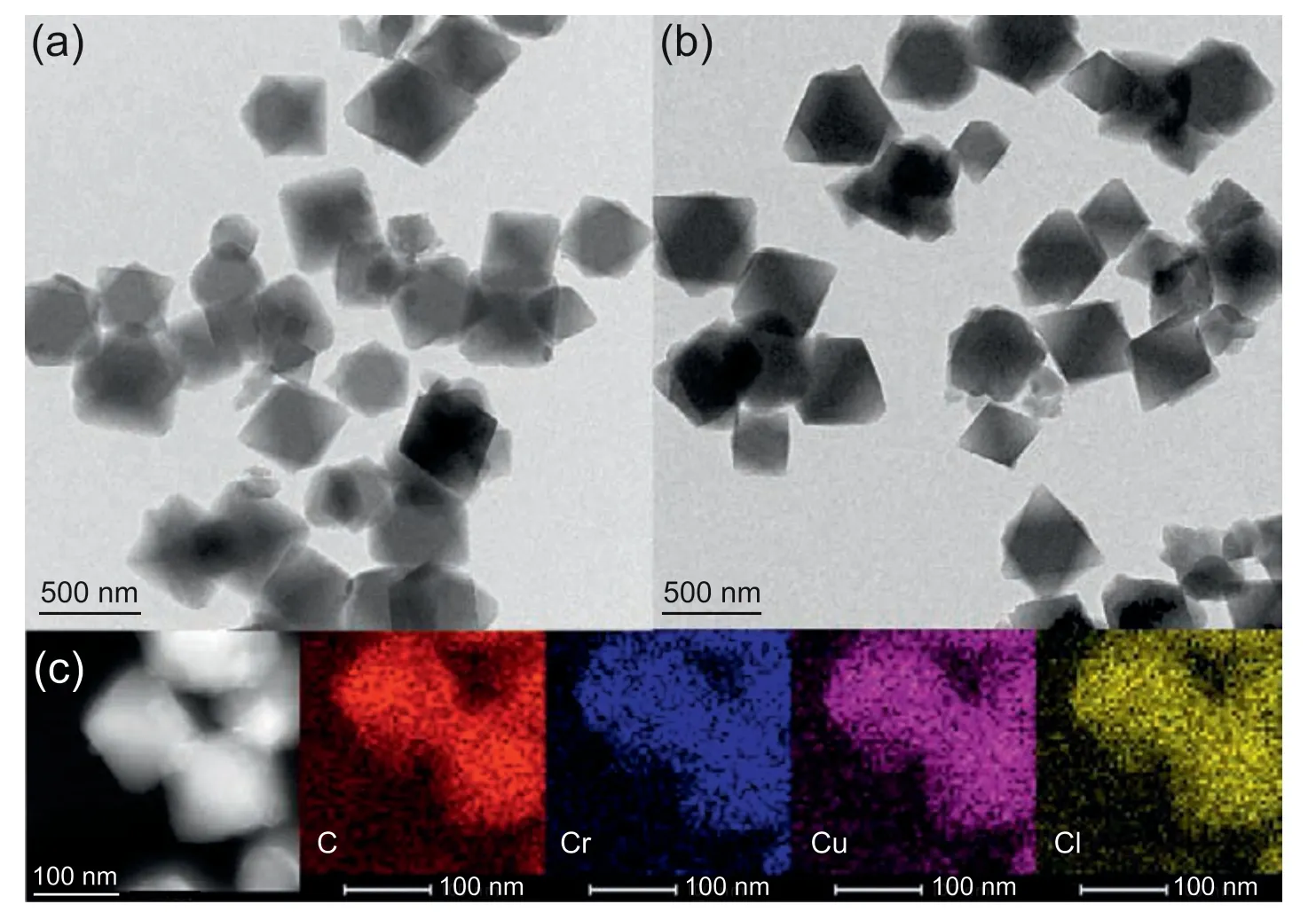
Fig.6.TEM images of(a) MIL-101(Cr) and(b)4CuCl@MIL-101(Cr) with different magnification;(c)dark-field TEM and EDX mapping images of C,Cr,Cu,and Cl elements of 4CuCl@MIL-101(Cr).
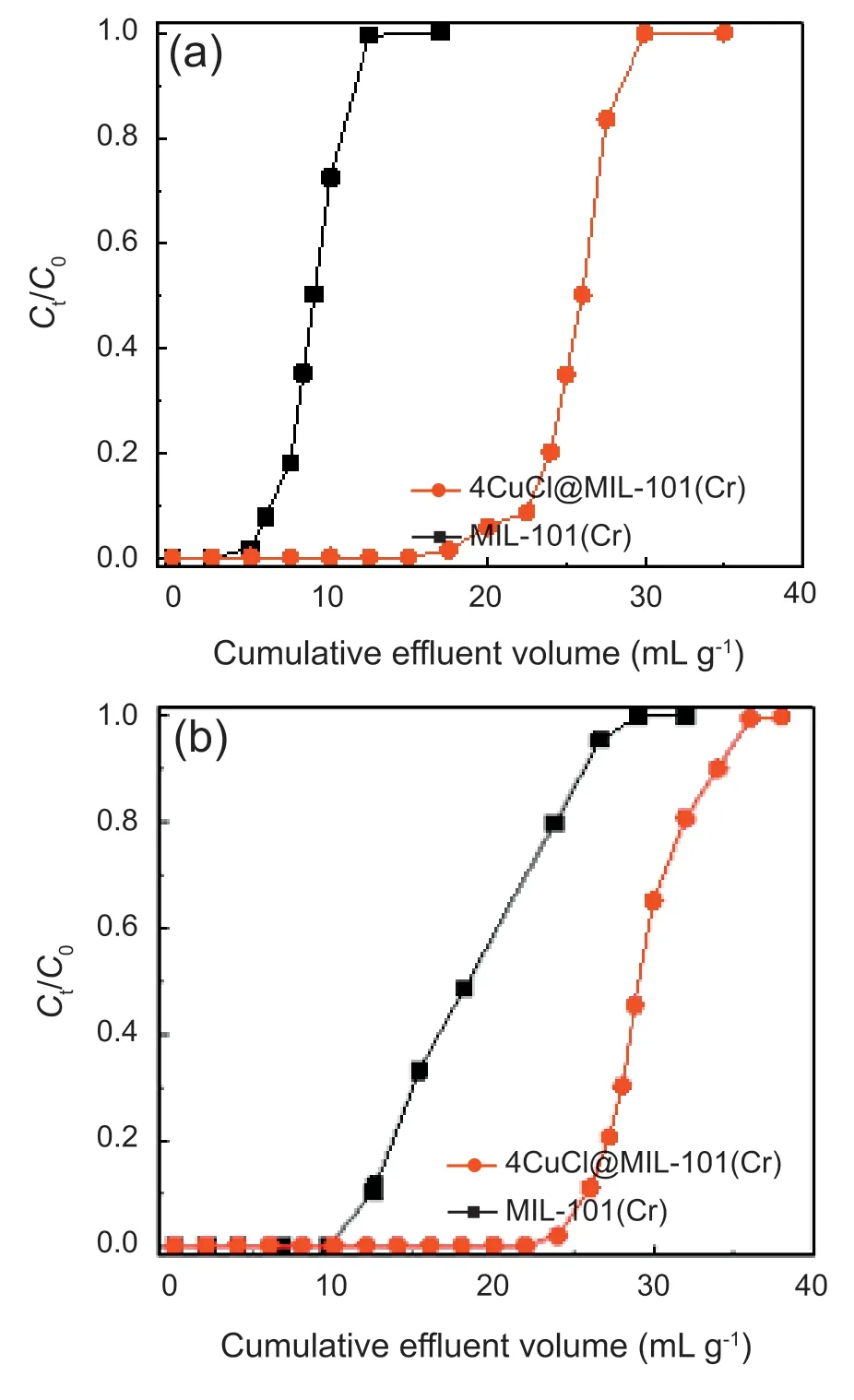
Fig.7.Breakthrough curves of (a) thiophene and (b) BT in a fixed-bed adsorber with MIL-101(Cr) and 4CuCl@MIL-101(Cr).

Fig.8.Reusability of 4CuCl@MIL-101(Cr) for thiophene capture.
The CuCl@MIL-101(Cr)have also been applied to capture BT,another larger molecule of typical ASCs(Figs.7b and S5).It is noticeable that the breakthrough curves and the calculations reveal that the adsorption capacities of BT over pristine and modified samples are larger than that of thiophene,which may be caused by the stronger interplay between BT and adsorbents.Pristine and Cu+-containing MIL-101(Cr) able to interact with benzene ring with higher electron density in BT,lead to stronger interplay between adsorbents and BT.Therefore,the breakthrough curve of BT is smoother relative to thiophene.The 4CuCl@MIL-101(Cr) sample manifests the optimal adsorption effect and the adsorption capacities at breakthrough and saturation reach 292 and 358 μmol g-1,which are much superior to pure MIL-101(Cr) (108 and 207 μmol g-1).Moreover,considering the significance of recyclability in realistic applications,4CuCl@MIL-101(Cr)was chosen to examine its regeneration performance.Fig.8 records the results of six cycles of regeneration.No significant decline in the desulfurization performance of the adsorbent is observed for each cycle.After sixth recycling,the content of Cu+was also monitored.The recycled adsorbent shows 3.57 mmol g-1Cu+,which is identical to the content of 3.62 mmol g-1before cycle experiments.Therefore,Cu+-containing adsorbents fabricated by SCDS possess good reusability.
4.Conclusions
Our present study provides SCDS for functionalization of MOFs with Cu+,in which both the introduction and following reduction of Cu2+are confined within the pores of support.The reducing condition is at 25°C under a green reducing agent vitamin C,overcoming the limitation of HTA that is unsuited to the construction of Cu+sites on MOFs.Cu2+confined in the pores of MIL-101(Cr) can be efficiently and selectively converted to Cu+without forming any Cu0.In view of the π-complexation between Cu+and ASCs,Cu+-containing adsorbent exhibits excellent performance in terms of removal of thiophene and BT with regardless of the adsorption capacity and recyclability.We anticipate that this strategy may pave the way to construct active sites possessing good dispersibility and requiring valence transformation on supports,especially those with relatively poor thermal stability.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21722606,21676138,21576137,and 21878149),the China Postdoctoral Science Foundation(51201370),and the Project of Priority Academic Program Development of Jiangsu Higher Education Institutions.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.10.009.
杂志排行
Green Energy & Environment的其它文章
- Investigating ionic liquids for optimizing lithium metal anode
- A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application
- Advanced silicon nanostructures derived from natural silicate minerals for energy storage and conversion
- B-doped activated carbon as a support for a high-performance Zn-based catalyst in acetylene acetoxylation
- High recycling Fe3O4-CdTe nanocomposites for the detection of organophosphorothioate pesticide chlorpyrifos
- Hierarchical Cu3P-based nanoarrays on nickel foam as efficient electrocatalysts for overall water splitting
