High recycling Fe3O4-CdTe nanocomposites for the detection of organophosphorothioate pesticide chlorpyrifos
2022-05-22YanxueGuoHuiLiuDongChenJianglanQuJunYang
Yanxue Guo ,Hui Liu ,Dong Chen ,Jianglan Qu ,Jun Yang ,c,d,
a Department of Applied Chemistry,Key Laboratory of Urban Agriculture (North China),Ministry of Agriculture and Rural Affairs,Beijing University of Agriculture,Beijing,102206,China
b State Key Laboratory of Multiphase Complex Systems,Institute of Process Engineering,Chinese Academy of Sciences,Beijing,100190,China
c University of Chinese Academy of Sciences,No.19A Yuquan Road,Beijing,100049,China
d Zhongke Langfang Institute of Process Engineering,Fenghua Road No 1,Langfang Economic&Technical Development Zone,Hebei Province,065001,China
Abstract To alleviate the secondary contamination of our environment when using quantum dots (QDs) to detect the organophosphorothioate pesticides (OPPs),we herein report a strategy to assemble magnetic Fe3O4 nanoparticles and luminescent CdTe quantum dots (QDs) into a composite nanosystem,which possesses both the magnetic property of Fe3O4 nanoparticles and the luminescent character of CdTe QDs,for the detection of chlorpyrifos,one of the typical OPPs.This strategy involves the isolated synthesis of magnetic Fe3O4 nanoparticles with positive charges and luminescent CdTe QDs with negative charges,and their subsequent assembly by electrostatic interaction.The as-prepared Fe3O4-CdTe nanocomposites have a detection limit as low as 10 ppb for chlorpyrifos,and are also selective for the OPPs with a phosphorothioate moiety(P=S bond).In specific,the Fe3O4-CdTe nanocomposites can be conveniently harvested by a normal magnet,and the recycling rate for both Cd and Fe determined by inductively coupled plasma atomic emission spectroscopy(ICP-AES)is higher than 96%,showing great potential in alleviating the Cd pollution on the environment.
Keywords: Magnetic nanoparticles;Quantum dots;Organophosphorothioate pesticides;Nanocomposites;Chlorpyrifos
1.Introduction
The efficient detection of organophosphorothioate pesticides(OPPs),i.e.simple,fast,sensitive,reliable,on-site and costeffective,is of great necessity due to the serious harm to human being of their residues in air,water,soil,vegetables and fruits[1-6].In comparison with the detection methods based on chromatography (e.g.gas chromatography (GC),high performance liquid chromatography (HPLC),or gas chromatography-mass spectrometry) [7-12],electrochemical analysis [13,14],hyperspectral imaging [15-17],colorimetry [18-21],single atom photocatalysis[22],and surface enhanced Roman spectroscopy(SERS) [23-25],which usually suffer from expensive instruments,time-consuming and operational complexity in sample pretreatment,the detection strategies based on luminescent materials have attracted a lot of attention due to their rapidness,convenience,superior sensitivity and cost-efficiency [26-33].The unique luminescent features offer a favorable analytical platform with low background signals and high sensitivity through the photoexcitation-emission combination[34-37].As a typical example,early in 2010,Zhang et al.reported a ligand replacement-induced luminescence switch of quantum dots(QDs) for ultrasensitive detection of OPPs [38].They firstly quench the green emission of CdTe QDs in a basic medium by coordinating the dithizone at their surfaces through a fluorescence resonance energy transfer(FRET)mechanism.Then upon addition of the OPPs,the original dithizone ligands at the surfaces of CdTe QDs are replaced by the hydrolysate of OPPs,leading to immediate recovery of the QD luminescence.This strategy has very low detection limit (ca.0.1 nmol L-1),and therefore is promising in developing luminescence-based chemosensors for OPPs.Unfortunately,QD luminescence-based OPPs detection is associated with an apparent disadvantage:the recycling of QDs is not considered,and the toxic QDs discarded may induce secondary contamination of our environment and agricultural products.In this regard,developing high recycling luminescent QDs for OPPs detection is definitely an increasing need.
Inspired by immobilizing biocatalysts on magnetic nanoparticles or by depositing magnetic nanoparticles on reduced graphene oxides for environmental remediation [39,40],we herein report the integration of magnetic Fe3O4and luminescent CdTe for the detection of OPPs,in which positively charged magnetic Fe3O4nanoparticles and negatively charged luminescent CdTe QDs are firstly prepared separately,and then Fe3O4-CdTe nanocomposites are assembled through electrostatic interaction.In this strategy,the high luminescence of CdTe quantum dots guarantees the detection sensitivity for OPPs,while the magnetization of Fe3O4is used to harvest the nanocomposites from the detection media by a magnet.We choose chlorpyrifos,one of the typical OPPs,as the detection object,and will show that a detection limit of 10 ppb and a recycling rate of more than 96% for Cd and Fe can be achieved.Combining with their high sensitivity,the high recycling property of Fe3O4-CdTe nanocomposites may promote the wide application of luminescence-based methods in real agricultural samples.
2.Experimental
2.1.Chemical reagents
Pesticides including chlorpyrifos (CP,C9H11Cl3NO3PS),dichlorvos (DL,C4H7Cl2O4P),ethoprophos (EP,C8H19O2PS2),and acetamiprid (AM,C10H11ClN4) were from Agro-Environmental Protection Institute,Ministry of Agriculture and Rural Affairs of the People's Republic of China.Analytical grade tellurium (Te) powder,cadmium chloride hemi(pentahydrate) (CdCl2·2.5H2O),sodium borohydride(NaBH4),sodium hydroxide (NaOH),ferrous chloride tetrahydrate (FeCl2·4H2O),ferric chloride (FeCl3),1,6-hexanediamine (HDA,C6H16N2),and dithizone (C13H12N4S) were from Sinopharm Chemical Reagent Co.,Ltd.Absolute ethanol(C2H5OH),methanol (CH3OH) and sulfuric acid (H2SO4)were from Beijing Chemical Works.Mercapto acetic acid(MAA,C2H4O2S)was from J&K Scientific Ltd.All glassware were washed by aqua regia(3/1 volume ratio for HCl/HNO3),followed by copious rinsing with ultrapure water,which was produced by a Millipore water purification system.
2.2.Synthesis of Fe3O4-CdTe nanocomposites
The CdTe QDs and Fe3O4magnetic particles were firstly prepared separately,and were assembled into Fe3O4-CdTe nanocomposites with both luminescence and magnetization through electrostatic attraction.The MAA-protected CdTe QDs were prepared in aqueous phase using a method reported previously with slight modification[41].In brief,to a 100-mL three-necked flask,0.114 g of CdCl2·2.5H2O (0.5 mmol) and 50 mL of deoxygenated ultrapure water were added,followed by dropwise addition of 66 μL of MAA under vigorous stirring and N2atmosphere.Then the pH of the mixture was adjusted to 11 using 1 mol L-1NaOH solution for further use.For the preparation of Te precursors,0.024 g of Te powder and 0.038 g of NaBH4were added to 2 mL of deoxygenated ultrapure water,and the mixture was heated at 60°C for 30 min under N2atmosphere to form purple NaHTe solution.Subsequently,the freshly obtained NaHTe solution was transferred into the MAA-CdCl2solution,and the mixture was heated to 100°C under N2atmosphere and maintained there for 20 min for forming desired CdTe QDs.The as-prepared CdTe QDs were cooled down to room temperature,precipitated and washed thrice by absolute ethanol,and redispersed into 50 mL of water.
The magnetic Fe3O4nanoparticles were prepared using the co-precipitation method developed before [42,43].1.49 g of FeCl2·4H2O (7.5 mmol)and 2.43 g of FeCl3(15 mmol)were firstly dissolved into ultrapure water to form 0.15 mol L-1and 0.3 mol L-1aqueous FeCl2and FeCl3solutions,respectively.Then 0.5 mL of FeCl2solution and 0.5 mL of FeCl3solution were sufficiently mixed in a 50-mL three-necked flask,followed by dropwise addition of 1 mol L-1NaOH solution to form black precipitates.The precipitates were completely disappeared with further addition of NaOH solution until the pH value was 11.Subsequently,the FeCl2-FeCl3-NaOH solution was heated at 50°C for 1 h to obtain magnetic Fe3O4nanoparticles,which were collected by centrifugation,washed with absolute methanol thrice,dried in vacuum,and redispersed into 100 mL of ultrapure water.
For the synthesis of Fe3O4-CdTe nanocomposites,the surface of the magnetic Fe3O4nanoparticles were firstly modified by 1,6-hexanediamine (HAD).To achieve this,50 mL of aqueous HDA solution(0.05 mol L-1)was added to the 100-mL Fe3O4colloidal solution prepared above,and after stirring,the pH value of the mixture was adjusted to 5 using dilute H2SO4solution.Subsequently,in a 25-mL three-necked flask,5 mL of CdTe QD solution and 5 mL of HDA-modified Fe3O4solution were mixed together,followed by addition of dilute H2SO4to adjust the pH of the mixture to 6.Finally,the mixture was heated at 30°C for 1 h to obtain Fe3O4-CdTe nanocomposites with both luminescence and magnetization,which could be conveniently recovered by a normal magnet and redispersed in water.
2.3.Detection of organophosphorothioate pesticides(OPPs)
To prepare magnetic QD-based probe for OPPs,an appropriate amount of Fe3O4-CdTe nanocomposites recovered by a magnet was redispersed into ultrapure water to obtain a nominal CdTe concentration of 10 mmol L-1,while a 1 mmol L-1methanolic solution of dithizone was also prepared.Then 100 μL of aqueous Fe3O4-CdTe solution was diluted with 3 mL of 10 mmol L-1NaOH solution,followed by addition of 30 μL of methanolic dithizone solution to quench the CdTe luminescence.The magnetic QD-dithizone(MQD-DZ) system thus obtained is the luminescent probe for detecting OPPs.
For the detection of OPPs,5 μL of a pesticide solution in ethanol with known concentrations was mixed with the MQDDZ probe,and then the luminescent spectra were recorded at room temperature under the excitation wavelength of 400 nm.
2.4.Instrumentation
The luminescent spectra of the Fe3O4-CdTe nanocomposites were recorded on a Horiba Fluoromax-4 spectrofluorometer,while the magnetization measurements were performed on a Quantum Design MPMS-3 at room temperature.A JEOL JEM-2100F electron microscope running at 200 kV was employed for transmission electron microscopy(TEM) images.X-ray diffraction (XRD) patterns of the Fe3O4-CdTe nanocomposites were obtained on a Bruker D8 X-ray diffractometer using Cu-K radiation (λ=1.5406 Å).Inductively coupled plasma atomic emission spectroscopy(ICP-AES,PerkinElmer Optima 6300DV) was used to measure the content of Cd and Fe in the MQD-DZ probe solution before and after magnetic recycling.
3.Results and discussion
Fig.1 schematically shows the synthesis of Fe3O4-CdTe nanocomposites by electrostatic attraction.After surface modification by 1,6-hexanediamine (HAD) in aqueous solution with a pH value of 5,the protonated amino groupsin HDA render the surface of Fe3O4nanoparticles positively charged,while the mercapto acetic acid (MAA)makes the surface of CdTe QDs negatively charged.Upon mixing these two kinds of particles,the electrostatic attraction between two opposite charges assembles them into Fe3O4-CdTe nanocomposites with both the luminescent feature of CdTe QDs and the magnetic property of Fe3O4particles.

Fig.1.Schematic illustration showing the synthesis of Fe3O4-CdTe nanocomposites with both luminescence and magnetization via electrostatic attraction between positively charged Fe3O4 magnetic particles and negatively charged CdTe QDs.
3.1.Synthesis and characterization of Fe3O4-CdTe nanocomposites
Fig.S1 in Supplementary Material (SM) shows the XRD pattern of the Fe3O4-CdTe nanocomposites prepared in aqueous phase via electrostatic attraction,in which the diffraction peaks corresponding to (220),(311) and (400)planes of face-centered cubic Fe3O4and (111) and (220)planes of face-centered cubic CdTe could be easily identified,suggesting the presence of Fe3O4and CdTe phases in the nanocomposites.In addition,the diffraction peaks indexed to CdTe QDs are relatively weak and wide,suggesting that the synthesized CdTe QDs have fine particle sizes.The TEM image of the as-prepared Fe3O4-CdTe nanocomposites was shown in SM Fig.S2,in which a certain level of particle aggregation is observed,probably due to the presence of magnetic Fe3O4particles in the nanocomposites.
As exhibited in Fig.2a,the bare CdTe QDs show an emission peak at ca.559 nm.After integrating with magnetic Fe3O4nanoparticles through electrostatic attraction,the asformed Fe3O4-CdTe nanocomposites display an emission peak with different features from those of the bare CdTe QDs:(i)a slight red-shift for the peak(ca.577 nm)is observed;(ii)the excitonic peak intensity becomes less pronounced.This is associated with the aggregation of the nanocomposites observed in the TEM image,which usually induces the redshift and weakening of the QD luminescence.It is noteworthy that although a certain level of aggregation of the Fe3O4-CdTe nanocomposites cannot be avoided due to the magnetic property of their Fe3O4domains,the overall luminescence of the Fe3O4-CdTe nanocomposites is highly reproducible due to their synthetic simplicity.As evinced by SM Fig.S3,the relative changes in luminescence intensity of Fe3O4-CdTe nanocomposites synthesized in five batches are lower than 15%,suggesting the good control of the synthesis,which is essential for latter detection of chlorpyrifos,one of the typical OPPs.
The magnetic property of the as-prepared Fe3O4-CdTe nanocomposites was shown in Fig.2b,in which the fielddependent magnetization plots illustrate that both Fe3O4particles and Fe3O4-CdTe nanocomposites are superparamagnetic at room temperature.In particular,the saturation magnetization value of the Fe3O4-CdTe nanocomposites is 18 emu g-1at room temperature,lower than that of Fe3O4nanoparticles(46 emu g-1).The reduction in magnetization of Fe3O4-CdTe nanocomposites from their Fe3O4counterparts could be reasonably attributed to the decrease of Fe3O4ratio in the nanocomposites and the aggregation of the same.Anyway,both the magnetic property and luminescent feature equip the Fe3O4-CdTe nanocomposites with recycling capability and high sensitivity for the detection of OPPs.

Fig.2.(a) Luminescence spectra at excitation wavelength of 400 nm of CdTe QDs (red) and Fe3O4-CdTe nanocomposites (black);(b) Magnetic properties of Fe3O4 nanoparticles (circle dots) and Fe3O4-CdTe nanocomposites (pentagrams) measured at room temperature.
3.2.Recycling of Fe3O4-CdTe nanocomposites by a normal magnet
Owing to their magnetization,the Fe3O4-CdTe nanocomposites could be easily recycled by a normal magnet for reuse.Fig.3 shows the emission spectra of the supernatant of Fe3O4-CdTe colloidal solution before (black curve) and after magnetic harvesting of the composite particles (red curve),in which the significant reduction in luminescence intensity of the supernatant after magnetic harvesting illustrates the efficient separation of the Fe3O4-CdTe composite particles with the magnet.In addition,as shown by the photograph of the Fe3O4-CdTe colloidal sample before and after magnetic separation (Inset in Fig.3),the supernatant turns into clear while the composite particles attracted to the container wall are observed under the magnetic field,also suggesting effective harvesting of the nanocomposites from the solution.
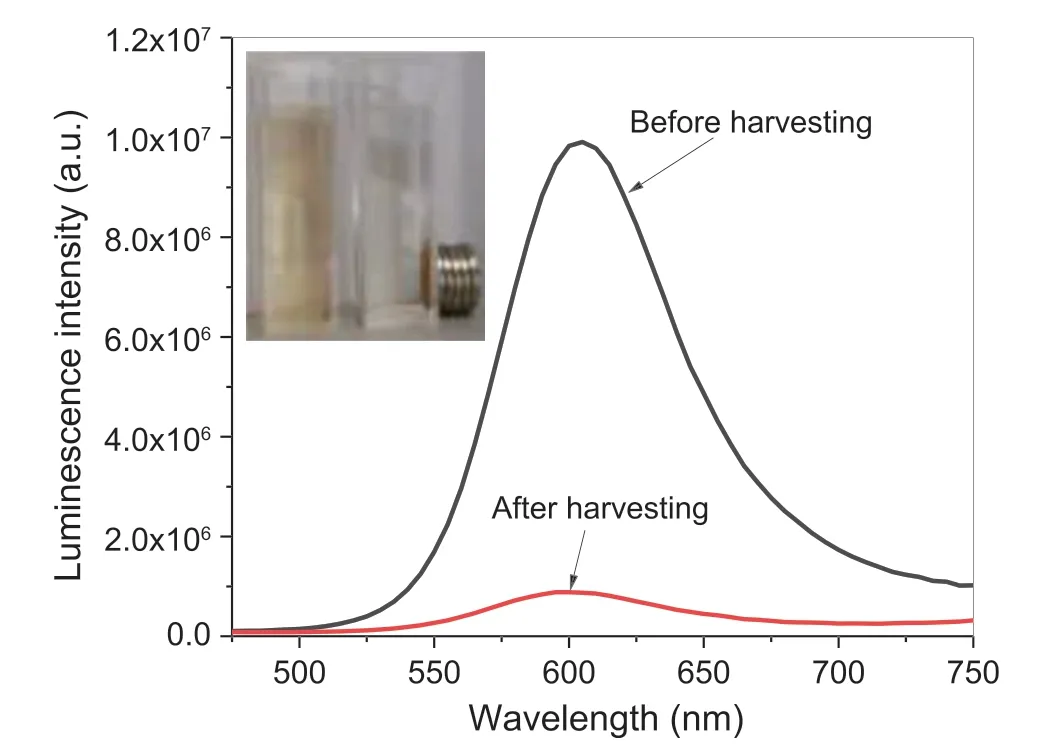
Fig.3.Luminescence spectra of the supernatant of Fe3O4-CdTe colloidal solution before and after magnetic harvesting of the composite particles.Inset is the photograph of the Fe3O4-CdTe colloidal sample before and after magnetic separation.
For the quantitative analysis of the magnetic recycling of the Fe3O4-CdTe composite particles from solution,we determined the Cd and Fe concentration in the solution before and after magnetic separation using ICP-AES.The Cd and Fe contents in the solid recovered by the magnet were also analyzed.The ICP-AES results for the Cd and Fe contents in the supernatant and solid were summarized in SM Table S1,which demonstrate that the recycling rates for Cd and Fe by the magnet can reach 96.84% and 96.86%,respectively,proving that the luminescent Fe3O4-CdTe nanocomposites could be effectively recycled by a magnetic field,favorable for reducing the Cd contamination on the environment.The recycled Fe3O4-CdTe nanocomposites can be readily redispersed in water,and after recycled 10 times,the luminescence intensity of Fe3O4-CdTe nanocomposites still retains 84.2%of the original intensity,as shown in SM Fig.S4,suggesting its high recycling stability.
3.3.Detection of OPPs by Fe3O4-CdTe nanocomposites
The detection of chlorpyrifos,one of the typical OPPs,by luminescent QDs has been well documented in literature[38].In brief,under alkaline conditions,the introduced dithizone,a bidentate chelator,coordinates with the excessive Cd2+ions on the surface of CdTe QDs [44,45],leading to the luminescence quenching of CdTe QDs due to the spectral overlap between the emission of QDs and the absorption of dithizone-Cd2+coordinating compounds,also defined as a mechanism of fluorescence resonance energy transfer (FRET) [46-49].Then,after adding chlorpyrifos into the strongly basic QDdithizone system,the chlorpyrifos could be rapidly decomposed into diethyphosphorothioate (DEP) and trichloro-2-pyridinol(TCP)[38,50,51].The former(i.e.DEP moiety)with a P=S bond can replace the dithizone from the QD surfaces due to its stronger coordinating capability with the Cd2+ions[52,53],resulting in the recovery of QD luminescence by closing the FRET pathway.The luminescence recovery of CdTe QDs is immediate,offering a quick and sensitive tool for the detection of chlorpyrifos.

Fig.4.(a)Luminescence quenching of Fe3O4-CdTe nanocomposites by dithizone(DZ);(b)luminescence recovery of magnetic QD-dithizone(MQD-DZ)probe by chlorpyrifos (inset shows the relationship between the luminescence enhancement and the concentrations of chlorpyrifos,labeled as CP).

Fig.5.Histogram showing the luminescence enhancement of MQD-DZ probe by various OPPs at concentration of 10 ppb:chlorpyrifos (CP),Dichlorvos (DL),Ethoprophos(EP),Acetamiprid(AM),and their mixture.I:luminescence intensity of MQD-DZ probe with various OPPs,I0:luminescence intensity of MQD-DZ probe without OPPs.
Fig.4a shows the quenching of the luminescence for the Fe3O4-CdTe nanocomposites by dithizone through the FRET mechanism under the conditions described in the experimental section.The luminescence intensity decreases dramatically upon the addition of 30 μmmol of dithizone.Further,with the introduction of chlorpyrifos (abbreviated as CP) with known concentrations to the MQD-DZ probe solution,the luminescence of the Fe3O4-CdTe nanocomposites recovers continuously with the gradual increase of chlorpyrifos amount,as exhibited by Fig.4b,in whichIandI0mean the luminescence intensity of the Fe3O4-CdTe nanocomposites with and without chlorpyrifos,respectively.It is noteworthy that at a chlorpyrifos concentration as low as 10 ppb,the limit for chlorpyrifos allowed by Chinese Food and Drug Administration (GB 2763-209),the recovery in luminescence can also be clearly identified by the spectrofluorometer,suggesting that the response of the MQD-DZ probe to the chlorpyrifos pesticide is very sensitive.In particular,as shown by the inset in Fig.4b,within the test scope,the relationship between the luminescence enhancement and the concentration of chlorpyrifos has very good linear character with an adjust R square of 0.9992,which could be conveniently used to calculate the chlorpyrifos content in given samples.
3.4.Selectivity of Fe3O4-CdTe nanocomposites for the detection of OPPs
The MQD-DZ probe has good selectivity for the detection of chlorpyrifos.As shown by Fig.5,in comparison with other three typical pesticides,i.e.dichlorvos(DL),ethoprophos(EP)and acetamiprid (AM),only chlorpyrifos or pesticide mixture containing chlorpyrifos has the capability to significantly enhance the luminescence of MQD-DZ probe solution.This is because the pesticides such as dichlorvos,ethoprophos and acetamiprid without the phosphorothioate moiety(P=S bond)cannot be hydrolyzed into stronger coordinating ligands to replace the dithizone (DZ) from the surface of the Fe3O4-CdTe nanocomposites,and thus cannot lead to any luminescence enhancement of the MQD-DZ probe solution.In addition,their presence does not have perceptible effect on the detection of chlorpyrifos by the MQD-DZ probe.The luminescence enhancement of a mixture containing chlorpyrifos,dichlorvos,ethoprophos and acetamiprid with concentration of 10 ppb is quite analogous to that of sole chlorpyrifos for the MQD-DZ probe solution,also illustrated by Fig.5.
4.Conclusions
In summary,we developed an electrostatic attraction-based strategy to assemble magnetic Fe3O4and luminescent CdTe into a composite nanosystem for the detection of organophosphorothioate pesticides (OPPs).We first prepared magnetic Fe3O4nanoparticles with positive charges and luminescent CdTe QDs with negative charges independently in aqueous phase,and then assembled them through electrostatic interaction.The Fe3O4-CdTe nanocomposites have both magnetic property of Fe3O4nanoparticles and luminescent character of CdTe quantum dots(QDs),which guarantees their high recycling function by a normal magnet and sensitive detection capability for OPPs.The detection of chlorpyrifos and other pesticides without P=S bonds proves that the Fe3O4-CdTe nanocomposites are sensitive and selective for OPPs.In specific,the recycling rate for both Cd and Fe determined by ICP-AES is higher than 96%,which is definitely beneficial for alleviating the Cd pollution on the environment.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (CIT&TCD201704049),the Development of Beijing Excellent Talents Project(2016000026833ZK01),Beijing Municipal Natural Science Foundation (2202015),the Technology Innovation Project of Beijing Municipal Institutions(KM201610020001),the National Natural Science Foundation of China(21706265),and State Key Laboratory of Multiphase Complex Systems,Institute of Process Engineering,Chinese Academy of Sciences (MPCS-2019-A-09).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.09.001.
杂志排行
Green Energy & Environment的其它文章
- Investigating ionic liquids for optimizing lithium metal anode
- A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application
- Advanced silicon nanostructures derived from natural silicate minerals for energy storage and conversion
- B-doped activated carbon as a support for a high-performance Zn-based catalyst in acetylene acetoxylation
- Hierarchical Cu3P-based nanoarrays on nickel foam as efficient electrocatalysts for overall water splitting
- Constructing Ti3C2 MXene/ZnIn2S4 heterostructure as a Schottky catalyst for photocatalytic environmental remediation
