Thermoresponsive Al3+-crosslinked poly(N-isopropylacrylamide)/alginate composite for green recovery of lithium from Li-spiked seawater
2022-05-22SungHoParkSangJoonLee
Sung Ho Park,Sang Joon Lee
Department of Mechanical Engineering,Pohang University of Science and Technology (POSTECH),Pohang,790-784,South Korea
Abstract With the rapid increase in the demand for lithium as an energy-critical element,the recovery of Li+ ions from seawater is a worldwide challenging issue.Herein,we propose a new facile and fast selective recovery approach of Li+ using an Al3+-crosslinked poly(N-isopropylacrylamide) (PNIPAAm)/alginate (Alg) (PNP/Alg(Al)) adsorbent.The in situ TEM images indicate that Alg-Al3+ coordination is reorganized via the rearrangement of PNIPAAm and Alg networks,as the temperature increases.The reorganization eventually leads to the formation of polycrystalline structure.The in situ FTIR results exhibit that PNP/Alg(Al) composite has peculiar phase transitions,which includes a retrogressive phase change from hydrophobic to hydrophilic.The synergetic effect of the strong repulsion force of Al3+ ions and the attractive force of negatively charged polymeric chains enables the efficient adsorption of Li+ions with a low affinity from Li-spiked seawater.7.3%of Li+ions are recovered from Li-spiked seawater although the concentration of Li-spiked seawater is very high.In addition,Li+ions can be extracted from PNP/Alg(Al) composite with the use of a small thermal energy.The proposed thermoresponsive IPN gel provides a strong potential in practical applications for Li+ recovery as an innovative energy-material strategy.
Keywords: Alginate;Poly(N-isopropylacrylamide);Lithium;Adsorption;Seawater
1.Introduction
The demand for the essential strategic element Li+has gradually increased in various applications,mostly related to lithium-ion batteries.Thus,the recovery of Li+from seawater and brine is a major concern in many industrialized countries where the reserves of ground Li+resources are scarce.Various Li+separation techniques,including electrodialysis [1],solvent extraction [2],coprecipitation [3],and adsorption [4],have been introduced to extract Li+ions from seawater and brine.However,the low concentration of Li+in seawater and the coexistence of other cations make it difficult to improve the Li+recovery efficiency[5].In addition,the requirement of a large amount of input energy and the generation of acidic or organic pollutants during the recovery process are less environmentally favorable.Although conventional lithium extraction techniques suffer from these inevitable shortcomings,adsorption has been considered as the most efficient approach due to the employment of a simple sorption process and high recovery efficiency.
Alginate(Alg)biopolymer is an anionic polysaccharide,as a main component of brown algae,which contains β-D-mannuronic (M) and α-L-glucuronic acid (G) residues.Alg can ionically crosslink polyvalent cations in a form of“egg-box”structure,which enables its wide applicability to various applications,such as drug delivery[6]and tissue engineering[7].Furthermore,the“egg-box”structure can be utilized to fabricate highly porous materials by generating mesopores around 2-4 nm with increasing specific surface area to develop high-rate capacitors [8],and to fabricate various types of porous materials for energy storage with a high performance depending on the crosslinking cations [9].Especially,due to the high contents of carboxylic acids and hydroxyl groups in anionic alginate,Alg has been used for the effective adsorption of toxic heavy metal ions [10] and anionic dyes [11].Benefiting from facile fabrication,easy functionalization,biocompatibility and highly porous materials,Alg works as a promising adsorptive material.
Alg composites composed of interpenetrating networks(IPNs) structure have been developed to provide proper functional and structural properties to Alg gels with a single network.The incorporation of functional polymers,including poly(vinyl alcohol) and polyacrylamide,into Alg networks can make IPN composites strong,stretchable,and tough[12,13].In addition,the incorporation of poly(N-isopropylacrylamide) (PNIPAAm) whose structure is variable on the basis of a lower critical solution temperature (LCST) introduces a thermal responsiveness to Alg composite,which leads to an eco-friendly recovery without the use of acidic treatment for desorption[14,15].Manufacturing IPN structure plays an important role for determining functionality of Alg composites.Thus,it is essential for understanding a basic knowledge of IPNs structure in Alg composite to give the insight on the development of an efficient adsorbent.
Recently,we demonstrated a new adsorptive Li+separation approach by using an Al3+-crosslinked phosphonate metal organic framework (pMOF)/Alg composite [16].In general,cations with a high adsorption affinity are easily adsorbed to negatively charged groups.However,a reversed ion selectivity where Li+ions with a low affinity are effectively adsorbed was anomalously observed in the composite possessing a low degree of pMOF.This effect is mainly attributed to the unique environment of Alg-Al3+coordination,which causes a strong repulsion force of cations by Al3+ions (Fig.1a).The strong repulsion force hinders the chemisorption,such as covalent and ionic bonding,of dehydrated cations that have a high affinity to negatively charged polymeric chains.Thus,cations with a high affinity are adversely rejected,even though they are attracted by the negatively charged polymeric chains.In contrast,Li+ions can be effectively adsorbed via physisorption in the form of hydrated ions due to their low adsorption affinity.
In this study,we propose a strategy for efficient Li+recovery by combining (1) the use of Alg-Al3+coordination that exerts a strong repulsive force,especially for cations with a high affinity,to provide a high ion selectivity for Li+ions and(2)the incorporation of PNIPAAm to introduce a thermalswitchable property for practical Li+extraction in an ecofriendly energy-saving manner.In particular,thermal responsive properties of IPN structure of the fabricated Al3+-crosslinked PNIPAAm/Alg (PNP/Alg(Al)) composite are characterized in detail,and the thermoresponsive Li+adsorption characteristics by evaluating its ionic selectivity from Li-spiked seawater are evaluated.
2.Materials and methods
2.1.Materials
Aluminum chloride hexahydrate (99%),lithium chloride(>99.0%),sodium alginate (no.A2033,M/G~1.56,≥2000 cp of medium viscosity at 2%,3.5×105g mol-1of molecular weight[17];FTIR and1H NMR results in Fig.S1),deuterium oxide (99%),ammonium peroxodisulfate (APS),N,N′-methylenebisacrylamide (MBAA) andN,N′-tetramethylethylenediamine (TEMED) were purchased from Merck,Germany.Potassium chloride (99.0%),sodium chloride (99.0%),calcium chloride(96.0%),and magnesium chloride(98.0%)were purchased from SAMCHUN,Korea.All chemicals were used without further purification.
2.2.Preparation of PNP/Alg(Al) composite
2800 mg ofN-isopropylacrylamide(NIPAAm)and sodium alginate,and 1400 mg of MBAA were dissolved in 150 mL of deionized (DI) water.NIPAAm in NIPAAm/Alg mixture was copolymerized by adding 10 mL of 0.1mol L-1aqueous APS solution and 5 mL of 0.1 mol L-1aqueous TEMED solution as redox initiators.Polymerization was performed during 24 h in a refrigerator at 4°C.Alg chains in PNIPAAm/Alg mixture were subsequently ionically crosslinked to make PNP/Alg(Al)hydrogels by immersing a drop of mixture in 2.5% aqueous aluminum chloride hexahydrate solution in a dropwise manner using a syringe pump(Harvard Apparatus,Harvard PHD2000)at a flow rate of 150 μL min-1.The fabricated hydrogels were meticulously washed with ethanol and deionized water until the conductivity of washed solution is comparable to the conductivity of DI water,and then incubated in an oven at 60°C several times to remove residual metal ions.Finally,PNP/Alg(Al) composite was prepared by drying it at room temperature.Pristine PNIPAAm was prepared by using the same amount of NIPAAm,TEMED,and APS in the absence of sodium alginate and polymerized for 24 h in a refrigerator at 4°C.The fabricated pristine PNIPAAm was subsequently dried at RT for FTIR analysis.
To compare structural variation of PNP/Alg composites depending on the PNIPAAm networks,a half and a quarter of amounts of NIPAAm and MBAA were mixed with 2800 mg of sodium alginate.The Alg composites containing a half and a quarter of amounts of NIPAAm and MBAA were named as PNP(1/2)/Alg(Al) and PNP(1/4)/Alg(Al),respectively.In addition,the variation of Alg-Al3+coordination was also evaluated by using 5% and 10% aluminum chloride solution instead of the 2.5% aluminum chloride solution.The Alg composites prepared with 5% and 10% aluminum solution were named as PNP/Alg(Al)@Al5 and PNP/Alg(Al)@Al10,respectively.Other procedures except the contents of NIPAAm,MBAA,and aluminum chloride were same with the procedures in fabrication of PNP/Alg(Al) composite.

Fig.1.Conceptual illustration of the present study.(a) Selective lithium adsorption strategy of the proposed adsorbent.(b)Adsorption and desorption process of hydrated cations by using thermal-switchable structural change in the PNP/Alg(Al) composite according to temperature based on the lower critical solution temperature.
2.3.Characterization and analyses
PNP/Alg(Al)composite was prepared in a form of bead and powder by using a mortar and pestle to analyze their characteristics.SEM images were obtained using FEI Helios 600 at an acceleration voltage of 5 keV to obtain the structural information of the composite.In situheating TEM study was performed for the PNP/Alg(Al) specimen,which was initially prepared by focused ion beam (FEI,Helios 600) for allowing the structure of the specimen to be movable in response to the thermal variation,with the employment of ultra-high voltage transmission electron microscope (HVEM,JEM ARMI1300S).The specimen was heated at a rate of 10°C min-1with an interval of 5°C from 30 to 60°C by a smart set hot stage controller (MODEL 901).TEM images were acquired when a given temperature was achieved and after a few minute for the stabilization.Powder XRD patterns were obtained by using a monochromatic X-ray beam of Cu Kα line (E=8.0470 keV) from the 1D X-ray scattering beamline of the Pohang Accelerator Laboratory (PAL) in Korea.Data were acquired at room temperature from 2θ ≈10 to 50°with an interval of 0.03°at a scanning rate of 0.6°min-1.In situFTIR measurement was conducted by using a Bruker Vertex 80/v FTIR spectrometer at the 12D IRS beamline of PAL.The powder composite was placed on the heater equipped with liquid nitrogen,and the temperature was controlled in the range of 25-60°C with a scanning rate of 10°C min-1.The FTIR spectra were recorded at a spatial resolution of 8 cm-1using a 64 × 64 mercury cadmium telluride focal plane array detector with a 15× objective lens operating at transmission mode.Each spectrum was subtracted by the background intensity to get accurate information at a given temperature.The obtained spectra were normalized by the intensity in the range of 1140-1000 cm-1.The FTIR spectrum of pristine sodium alginate was obtained from RIST of Korea.1H NMR result of sodium alginate dissolved in D2O was obtained by using 600 MHz FT-NMR spectrometer(Bruker,Avance III 600)at 300 K.The amounts of metal ions in the solutions were measured with an inductively coupled plasma-atomic emission spectrometer of RIST of Korea.DSC(TA Instruments,model Q20) experiment was conducted at a heating and cooling rate of 10°C min-1.TG(TA Instruments,model Q50) experiment was performed at a heating rate of 10°C min-1.
2.4.Evaluation of hydration and adsorption characteristics of PNP/Alg(Al)
The fabricated PNP/Alg(Al) composite was immersed in deionized water.The hydration process was monitored by using a camera (Nikon Inc.,D700).The PNP/Alg(Al) composites were immersed in a Li-spiked seawater solution containing 60 mg L-1of Li+ions for 1 h and 24 h.Multiple adsorption/desorption cycles test was conducted by initially immersing the fabricated PNP/Alg(Al) composite into the Lispiked seawater during 12 h.The PNP/Alg(Al)composite was subsequently immersed into DI water at 60°C during 6 h without washing process.Otherwise,cations might be physically adsorbed,thereby the washing process may unexpectedly remove the residual cations before desorption of cations.The initial concentrations of Li+,Na+,Mg2+,K+,and Ca2+ions were 60,10,900,1,300,390,390 mg L-1,respectively.pH was regulated by the addition of a small amount of 1 mol L-1HCl or NaOH solution to compare the effect of pH on Li+adsorption.The amount of the adsorbed ions was quantitatively calculated using the equation(1).The recovery ratio was also calculated by using the equation (2).The selectivity separation factor and extraction efficiency were estimated by using the equations (3) and (4).

whereqis the amount of adsorbed ions;Ciis the initial concentration of Li-spiked seawater;Cfis the final concentration of Li-spiked seawater after adsorption;Vis the volume of the metal solution;Wis the weight of PNP/Alg(Al);QLiandQM(mmol g-1) are the adsorption capacities of PNP/Alg(Al)composite for Li+ion and other cation,respectively;CLiandCM(mg L-1) are the initial concentration of the Li-spiked seawater for Li+ion and other cation,respectively;andCd,Liis the concentration of desorbed Li+ions from PNP/Alg composite dissolved in DI water.
3.Results and discussion
3.1.Synthesis and structural characterization of PNP/Alg(Al) composite
The IPN structure comprises PNIPAAm chains as a thermoresponsive component and sodium alginate (Alg) chains(Fig.1b).Pristine PNIPAAm,which is a thermoresponsive polymer,has a LCST of 32°C.The hydrophilic/hydrophobic structure is drastically changed by varying the temperature around the LCST.The hydrophilic compartment attracts hydrated cations when the temperature is below the LCST,while the hydrophobic compartment can reject the adsorbed hydrated cations above the LCST.First,a mixture ofN-isopropylacrylamide (NIPAAm) and sodium Alg is chemically polymerized byN,N′-methylenebisacrylamide (MBAA).The PNP/Alg(Al)composite with an IPN structure is subsequently prepared by physical crosslinking of Alg with Al3+ions.The IPN structure allows selective adsorption for hydrated cations with a low adsorption affinity and exhibits thermally switchable behavior to recover the adsorbed cations.
Optical and scanning electron microscopy(SEM)images of the fabricated PNP/Alg(Al) composite show a biconcave-like shape with rough surface structures of approximately 500 nm(Fig.2).Powder X-ray diffraction (XRD) results exhibit that the incorporation of PNIPAAm into normal Alg(Al) changes amorphous phases in IPNs structure(Fig.3a).Normal Alg(Al)exhibits two broad amorphous peaks in the regions of 2θ ≈ 10-34°and 34-50°.On the other hand,a newly developed amorphous phase of the PNP/Alg(Al) composite occupies approximately at 10°,while the front amorphous peak observed in normal Alg(Al)is shifted to the region of 14-34°.This implies that the incorporation of PNIPAAm leads to a specifically intertwined IPNs structure at a given structural order.However,the Bragg peaks of the crystalline structure in the XRD pattern of normal Alg(Al) disappear in this time,which is different from our previous result [16].This change results from the decreased X-ray beam current leading to a low signal-to-noise ratio.
The variation of amorphous IPNs structure is clearly observed depending on the amount of PNIPAAm (Fig.3b).The intensity of front peak in the region of 14-34°of PNP(1/2)/Alg(Al) and PNP(1/4)/Alg(Al) gradually decreases compared with PNP/Alg(Al),as the intensity of rear peak corresponding to Alg-Al3+coordination remains for all Alg composites.In addition,the short-range structural disorder decreases,as the intensity of peak approximately at 10°significantly decreases.This suggests that the decrease in the degree of PNIPAAm networks in Alg-Al3+coordination leads to a less intertwined IPNs structure.However,the IPNs structures of Alg composites are not significantly altered depending on the concentration of aluminum chloride crosslinking solution (Fig.3c).This suggests that the mechanical properties of PNP/Alg(Al) composites may be modulated depending on the degree of crosslinked Alg-Al3+coordination[18,19],while the structural orientation of Alg-Al3+coordination still retains.Magnified XRD peaks in the region of 10-30°clearly exhibit the structural changes (Fig.S2).
3.2.In situ TEM characterization of the thermoresponsiveness of IPNs structure

Fig.2.Structural characterization of the fabricated PNP/Alg(Al)composite.(a)Optical image of the composite.SEM images of the composite with magnification of (a) 200× and (b) 12,000×.Scale bars indicate 500 μm and 5 μm.
Thermoresponsive structural change of the PNP/Alg(Al)composite was evaluated by usingin situTEM analysis.The IPNs structure of PNP/Alg(Al) is entirely amorphous at a room temperature (RT) (Fig.4a).Normal Alg(Al) has a polycrystalline structure with lattices of 0.17 nm [16],although the Bragg peaks were not observed in XRD results(Fig.3a).The result implies that the incorporation of PNIPAAm chains into Alg hinders the formation of repetitive Alg-Al3+coordination having observable lattices.However,the IPNs structure is significantly changed,as the temperature increases above a LCST (Fig.4b).Darker region corresponds to a thicken IPN structure where a part of components in the thicken structure are derived from brighter region.This indicates that IPN structure of brighter region is thinner than that of darker region.Moreover,the crystallization of Alg-Al3+coordination having distinct lattices is observed at a specific dividing line (Fig.4c),as the temperature increases.The corresponding fast Fourier transform image exhibits that PNP/Alg(Al) composite gives rise to have polycrystalline structure with lattices of 0.2 nm.
The expected mechanism on structural change of PNP/Alg(Al) composite in response to a thermal energy is illustrated in Fig.4e.At a RT,randomly distributed PNIPAAm chains hinder the formation of organized Alg-Al3+coordination.The crosslinking of NIPAAm with MBAA initially decreases the conformational entropy of polymeric network[20,21].This implies that alginate chains in the polymeric network are structurally restricted by the formation of PNIPAAm.The restricted network environment makes the crosslinking process of Alg chains with Al3+ions in the presence of PNIPAAm network unfavorable compared to the crosslinking process of alginate chains themselves without interference of PNIPAAm network.Thus,the decrease in conformational entropy by the incorporation of PNIPAAm hinders the intact formation of Al3+-Alg coordination.Meanwhile,the least amount of conformational entropy of alginate chains decreases during the crosslinking,because Alg chains are structurally pre-confined by surrounding PNIPAAm [22].This eventually makes IPNs structure of PNP/Alg(Al) composite fully amorphous.
As the temperature increases,PNIPAAm chains begin to shrink according to a favorable orientation in IPNs structure.In addition,Alg-Al3+coordination has a significant motion due to its irregularity with exclusively adopting six-fold octahedral coordination,compared with Alg coordination crosslinked with other cations [23].This can induce partial hydrolysis of Alg chains with the aid of thermal energy due to its intrinsic characteristics of Alg-Al3+coordination [16],making Alg-Al3+coordination movable.With the synergetic effect of movement of PNIPAAm and Alg-Al3+coordination,the IPNs structure moves to a specific orientation where the structure is thickening or thinning on the basis of a dividing line.Differential scanning calorimetry (DSC) result also corroborates that the Alg-Al3+coordination of both normal Alg(Al)and PNP/Alg(Al)is structurally rearranged via partial hydrolysis at approximately 35.3 and 35.8°C,respectively(Fig.S3).The disappearance of endothermic peaks in the 2nd cycle might be attributed to the structural stabilization after the rearrangement.The phase transition of the PNIPAAm structure at the LCST in the fabricated PNP/Alg(Al) composite is not observed compared with that of other PNIPAAm composites where the endothermic peak appears around the LCST[24,25].This result might be attributed to the peculiar characteristics of Alg-Al3+coordination and the high degree of intertwinement of the IPNs structure.Accordingly,the apparent structural rearrangement of the PNIPAAm network via endothermic reaction is hindered.
As the temperature increases,the degree of reacted PNIPAAm increases,which can induce the aggregation of Alg-Al3+coordination at the dividing line.The aggregated Alg-Al3+coordination will have specific orientation to minimize structural energy by rearranging its coordination with a help of thermal energy.Eventually,reacted PNP/Alg(Al) composite has a polycrystalline structure with slightly longer lattices of 0.2 nm due to the structural hindrance of PNIPAAm chains,compared with of 0.17 nm of normal Alg(Al) [16].The reconfiguration of Alg-Al3+coordination itself in response to the increase of temperature is more favorable to amorphous structure rather than crystallization [16].In addition,PNIPAAm network can not form crystalline structure due to the intrinsic amorphous structural characteristics and no ability to crosslink Al3+ions.Thus,for the crystallization of Alg-Al3+coordination,the networks of PNIPAAm and Alg-Al3+coordination should move simultaneously.The rearrangement of PNIPAAm network with increasing temperature is helpful for the formation of crystalline structure of Alg-Al3+coordination by structural interaction as Alg-Al3+coordination moves.The reorganized Alg-Al3+coordination forming crystalline structure inside PNP/Alg(Al) with a high M/G ratio may enhance the mechanical strength of the Alg composite,because rigid G residues are aggregated.In addition,aggregated Al3+ions would have strong repulsive force compared with randomly distributed Al3+ions,leading to an improved ion selectivity.
Thermogravimetric analysis (TGA) indicates that the fabricated PNP/Alg(Al) composite is more thermally stable than normal Alg(Al) (Fig.S4).Powder XRD result also corroborates the thermal stability in the range of temperatures from 50 to 80°C for 24 h reaction in deionized (DI) water(Fig.S5).The results demonstrate that the IPNs structure of PNP/Alg(Al)composite is thermally stable in the region from RT to 60°C.

Fig.3.Comparisons of powder XRD patterns of the fabricated Alg composites.(a) Powder XRD patterns of normal Alg(Al) and PNP/Alg(Al) composites.(b)Powder XRD patterns of PNP/Alg(Al)composites with varying the degree of PNIPAAm and MBAA.(c)Powder XRD patterns of PNP/Alg(Al)composites with varying the concentration of aluminum chloride crosslinking solution.
3.3.In situ FTIR characterization of the thermoresponsiveness of IPNs structure
The structural changes of the PNP/Alg(Al) composite are further evaluated throughin situFourier transform infrared(FTIR) characterization.The spectrum of the PNP/Alg(Al)composite exhibits two distinct peaks of amide I and II bands at approximately 1641 and 1529 cm-1,respectively(Fig.S6).The amide I band corresponds to C=O stretching vibrations,while the amide II band represents the contribution of N-H bending and C-N stretching vibrations [26].Surprisingly,thein situFTIR results exhibit peculiar phase changes in the amide I and II bands during the heating and cooling process,as illustrated in Fig.5.Here,the structural changes are partially retrogressive,although apparent phase change via endothermic reaction is not observed in the DSC results.The first transition(transition I) occurs at a temperature of approximately 36°C where the intensity of FTIR peaks is significantly changed(Fig.5b,left and S7).This transition might be attributed to the incorporation of hydrophilic units of Alg chains,which increases the temperature of the volume phase transition [27].Thus,the significant interaction caused by Alg-Al3+coordination seems contribute to the shift of the LCST from 32°C to a higher temperature.The amide I band is broadened and shifted to a lower frequency range from 1641 to 1631 cm-1,while the amide II band is shifted to a higher frequency range from 1529 to 1531 cm-1.In addition,the peak intensities of both the amide I and II bands are significantly increased.

Fig.4.In situ TEM characterization of the fabricated PNP/Alg(Al) composite.TEM images of the composite at (a) a room temperature,(b) a 35 °C,and (c) a 50 °C.(d) Corresponding FFT image of (c).(e) Conceptual illustration of the thermoresponsive structural change of IPNs structure in the composite.Scale bars indicate 10 nm and 5 nm.
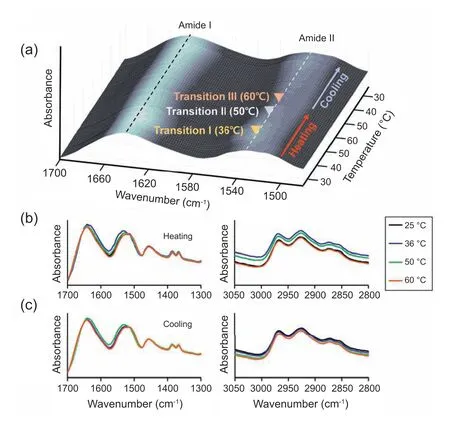
Fig.5.In situ FTIR characterization of the fabricated PNP/Alg(Al) composite.(a) The structural variation of the amide I and II bands in the temperature range between 25 and 60 °C at intervals of 1 °C during a heating/cooling cycle.(b) The structural variation of the amide I and II bands (left) and the hydrophobic moieties of C-H bonds(right)extracted at specific temperatures of 25,36,50,and 60 °C during the heating process.(c)The structural variation of the amide I and II bands (left) and the hydrophobic moieties of C-H bonds (right) extracted at specific temperatures of 25,36,50,and 60 °C during the cooling process.
The direction of peak shifts of the amide I and II bands is opposite compared with that of the band shifts of the conventional copolymerized PNIPAAm composites in response to thermal variation[28,29].It was reported that the C=O group of an amide group of PNIPAAm can form hydrogen bonds with the N-H group of a neighboring amide group or water with a lower frequency of amide I [26,28].The lower frequency shift of amide I indicates that the number of hydrogen bondings increases in the initial heating process.The different direction of peak shift is mainly attributed to the presence of intertwined alginate networks.Amide I of pristine PNIPAAm is shifted to a higher frequency,as temperature increases from RT to 60°C(Fig.S8a).The reconfiguration of amide I induces to destroy intra-and intermolecular hydrogen bondings.However,the coexistence of alginate initially makes amide I shifted to a lower frequency upon reaching 36°C,as the C=O groups of amide I form hydrogen bonds with carboxylic acid groups of alginate and the N-H group of a neighboring amide group.The reconfiguration of the IPN structure can induce the formation of the hydrophobic moieties of C-H bonds.The formation of hydrophobic moieties is observed with the increase in peak intensities in the region of 3000-2850 cm-1as temperature increases (Fig.5b,right)[30].It is well consistent with the formation of hydrophobic moieties of pristine PNIPAAm (Fig.S8b).This hydrophobic reconfiguration of the IPN structure can be utilized to block the penetration of water.
Transition II occurs at approximately 50°C,where the hydrogen bonds are destroyed,and the structure returns to that observed at 25°C (Fig.5b,left).Although the spectra somehow remain in the range above 50°C,both the amide I and II bands are drastically shifted at approximately 60°C(transition III) with a decrease in the peak intensities.Interestingly,the peak locations are oppositely shifted,compared with the shifting directions in the amide I and II bands observed at transition I.The amide I band shifts to a higher frequency with a decrease in peak intensity,while the peak position of the amide II band is shifted from 1529 to 1513 cm-1.These shifts indicate that the hydrogen bonds of carboxylic acid and neighboring amide groups are no longer retained.The hydrophobic moieties of C-H bonds are also destroyed as the temperature continuously increases(Fig.5b,right).This result suggests that the PNP/Alg(Al) composite may have hydrophilicity at 60°C.In general,hydrogen bondings are destroyed with increasing the number of hydrophobic moieties.However,the complicated interaction with intertwined alginate networks reduces the number of hydrophobic moieties.The different composition of IPN structure also gives rise to structural changes.PNP/Alg(Al)@Al5 composite whose alginate structure is more tightened via crosslinks with a large amount of Al3+ions exhibits unidirectional structural change where hydrogen bondings are destroyed and the number of hydrophobic moieties decreases as temperature increases(Fig.S9).The structure of PNP(1/2)/Alg(Al) becomes highly unstable,because it is difficult to reach an equilibrium as thermal energy is applied (Data are not included).Thein situFTIR results demonstrate that the fabricated composites exhibit phase changes from the original state to a hydrophobic state at transition I,from a hydrophobic state to the original state at transition II,and from the original state to a hydrophilic state at transition III.
These phase changes occur differently during the cooling process.As the temperature decreases to 50°C,the structure is quickly changed to the hydrophobic phase,whose structure is similar to that in transition I observed during the heating process (Fig.5c,left).However,the hydrophobic moieties of C-H bonds are still destroyed (Fig.5c,right).In addition,the amide I and II bands obtained at 25°C and 36°C cannot be fully returned to their original states,while the hydrophobic moieties of C-H bonds are reversely formed as the temperature decreases.The hysteresis might be attributed to the residual stress inside the IPN structure as the moieties return to the original structure.However,the residual stress can be easily alleviated by thermal annealing,with restoration of structural irregularity during the 2nd and 3rd heating/cooling cycles(Fig.S10).This result suggests that the IPN structure of the PNP/Alg(Al) composite is highly reversible during repetitive heating and cooling cycles.
The peculiar behavior of Alg chains on Al3+ions plays an important role for determining the thermoresponsive characteristics of IPNs structure.Due to an irregular interaction of Alg with Al3+ions and surrounding copolymer,the movement of IPNs structure is somehow restricted.This eventually makes the IPN structure initially become hydrophobic;However,the IPN structure is retrogressively returned to hydrophilic state via irregular interaction with the surrounding Alg chains.The detailed structural analyses should be required for understanding the behavior of IPNs structure.It might be helpful for developing a composite with IPNs structure having multi-functional properties in the practical applications,such as a drug delivery [31,32].
3.4.Adsorption of Li+ from Li-spiked seawater
Water molecules and ions diffuse into the inner networks of the dried composite to be adsorbed.Here,diffusion is an important process in the determination of the adsorption efficiency.As the fabricated dried composite is rehydrated during the hydration process,the volume of the composite is expanded,and the color is changed to bright white (Fig.6a).Rapid hydration initially occurs because the concentration gradient and surface area are initially maximized(Fig.6b).In addition,hydrophilic property of Alg polysaccharide assists the rapid hydration of the PNP/Alg(Al) composite.The diffusion of water molecules into the inner networks of the composite reaches to the saturation within 60 min.This rapid hydration behavior of the composite enables hydrated cations to be adsorbed very fast.
The adsorption kinetics were subsequently evaluated after 1 h and 24 h with Li-spiked seawater containing 60,10,900,1,300,390,and 390 mg L-1of Li+,Na+,Mg2+,K+,and Ca2+ions,respectively(Fig.6c).The Li+adsorption capacity of the composite incubated for 1 h at RT in Li-spiked seawater is 0.031 mmol g-1,while that of the composite incubated for 24 h is 0.029 mmol g-1.The difference might be attributed to the complicated interactions between the IPN structure of PNP/Alg(Al) composite and other cations with high adsorption affinity.However,their adsorption capacities are not significantly different.This result implies that Li+ions are very rapidly adsorbed within 1 h of the hydration process.
The thermoresponsiveness in the adsorption of cations was also evaluated at four different temperatures of 25,40,50,and 60°C (Fig.6d).The Li+adsorption capacity increases from 0.031 to 0.033 mmol g-1as the incubation temperature increases from 25 to 40°C.On the other hand,the Li+adsorption capacity significantly decreases from 0.033 to 0.024 mmol g-1,as the temperature increases from 40 to 50°C.Although the DSC result indicates the absence of an apparent phase transition of the LCST via endothermic reaction,thein situFTIR results show the presence of phase transitions.This thermoresponsive adsorption also corroborates that the IPN structure has a phase transition between 40 and 50°C.Thus,the hydrated PNP/Alg(Al) composite may have an increased LCST,compared with the transition I of the dried PNP/Alg(Al) composite (Fig.5).This difference results from the reconfiguration of hydratable networks in the IPN structure,composed of PNIPAAm and Alg chains.The hydratable network is more facilely movable than the buried polymeric chains in the dried composite,thereby inducing different molecular interactions inside the IPNs structure[33].The result indicates that phase transition III identified from the FTIR results is not observed during the adsorption experiment due to the presence of the hydratable network,with only one exceptional phase transition in Li+adsorption.Moreover,the facilely movable network can facilitate the diffusion of ions and molecules (Fig.6a-c) [34].
The Li+adsorption capacity at 60°C slightly increases from 0.024 to 0.025 mmol g-1.However,the value is still smaller than the capacities obtained below the LCST.This result suggests that the fabricated PNP/Alg(Al)has a thermalswitchable property in terms of switching between hydrophobicity and hydrophilicity on the basis of the LCST,which ranges between 40 and 50°C.The polymeric chains are collapsed and shrunk with sequestration of water molecules with the aid of the phase transition from hydrophilic state to hydrophobic state [35,36].In the fabricated PNP/Alg(Al)composites,hydrated cations are adsorbed via physisorption instead of chemisorption,because chemisorption is limited by the strong repulsion force of Al3+ions.Thus,the thermosensitive switching of wetting property gives rise to the rejection of hydrated cations by the virtue of sequestration of water molecules from the hydrophobic IPN structure.This property enables the efficient desorption of the adsorbed hydrated cations,while dehydrated cations are rejected by the strong repulsion force of Al3+ions in advance.In addition,a small amount of Al3+ions is leached from the fabricated PNP/Alg(Al) composite in the adsorption/desorption processes and even after the long-term acid treatment (Fig.S11).This indicates that the fabricated PNP/Alg(Al) composites have a highly stable structure during the repetitive adsorption/desorption processes without a significant leaching effect of Al3+ions.Furthermore,the extraction efficiencies for Li+,Na+,and Mg2+cations are stably maintained during 3 adsorption/desorption cycles (Fig.S12),although they are slightly smaller than the recovery ratio.This might be attributed to the remaining Li+ions in the composite,which are not fully extracted from the fabricated composite.This implies that the fabricated composite is structurally stable with maintaining adsorption/desorption performance without significant loss of crosslinked Al3+ions.This effect demonstrates the feasibility of efficient recovery of Li+ions by using a small amount of thermal energy without the use of acidic treatment in the desorption of Li+ions.

Fig.6.Adsorption characteristics of the fabricated PNP/Alg(Al).(a) Sequential optical images of the PNP/Alg(Al) composite immersed in deionized water.(b)Variation in the projected area of the bead during the diffusion of water molecules in the hydration process.(c)Comparison of the Li+adsorption capacities of the PNP/Alg(Al)composite for 1 and 24 h immersed in the Li-spiked seawater containing 60,10900,1300,390,and 390 ppm of Li+,Na+,Mg2+,K+,and Ca2+ions,respectively.(d)Effect of incubation temperature on the Li+adsorption capacity of the PNP/Alg(Al)composite immersed in the Li-spiked seawater.(e)Adsorption capacities and recovery ratios for Li+,Na+,K+,and Mg2+ ions from the Li-spiked seawater.
The adsorption capacities of other cations were also assessed to compare the recovery efficiencies (Fig.6e).In general,the adsorption affinity increases with decreasing hydrated radius of cations having the same ion valence[37].The hydrated radii of Li+,Na+,K+,and Mg2+are 0.38,0.36,0.33,and 0.43 nm,respectively [38].The adsorption capacities of Li+,Na+,K+,and Mg2+ions are 0.031,1.859,0.016,and 0.338,respectively.Due to the high concentration of Na+ions,the adsorption capacity for Na+ions is significantly higher than that for Li+ions.However,the adsorption capacity for K+ions is much lower than that for other cations due to the low concentration,even though K+ions have a high adsorption affinity.In addition,the adsorption capacity for divalent Mg2+ions is not significantly higher than that for monovalent other cations despite their strong affinity.The selectivity separation factors ofwere further evaluated for detailed comparison with other cations based on their initial concentrations in the Li-spiked seawater.The fabricated composite hasvalues of 3.06,13.05,and 2.00 for M ions of Na+,K+,and Mg2+,respectively.This result indicates that the proposed composite has a relatively high selectivity given that the initial concentration is very high,although the adsorption capacities for Na+and Mg2+ions are significantly higher than that for Li+ions.The Li+ion imprinted polymer whose cavity size is comparable to the ionic size of Li+ions capture Li+ions effectively withvalues of 50.88,42.38,22.5,and 22.2 for Na+,K+,Cu2+,and Zn2+ions,respectively,although competitive adsorption occurs in the mixture containing 100 mg L-1of Na+,K+,Cu2+,and Zn2+cations[39].On the contrary,the photo-controllable Li imprinted adsorbent hasvalues of 14.03,25.04,and 7.90 for Na+,K+,and Mg2+cations,respectively [40].Thus,the Li imprinted adsorbent enables efficient and eco-friendly recovery of Li+ions without the use of acidic treatment from the mixture containing 20 mg L-1of Mg2+,Na+,and K+cations.These selectivity separation factors of the fabricated PNP/Alg(Al) composite are smaller than those of other adsorbents.This is attributed to significant interactions of the large amount of other cations with Li+ions.Given that the concentration of impurities is significantly high,the present method would have a potential in the use of industrial application,such as Li+recovery from seawater which contain impurities of high concentration.
The complexity of the IPN structure with a strong repulsive force of cations by Al3+ions induces an effective rejection of divalent cations as well as monovalent cations with a high affinity.In our previous study,Mg2+ions were completely rejected by using normal Alg(Al)[16].This result is attributed to the absence of rehydratable networks in the structure of normal Alg(Al),thereby making the Alg-Al3+network more complicated and more efficiently rejecting divalent and monovalent cations with a high affinity.In addition,we observed that the fabricated Cu2+-crosslinked Alg beads do not reject Mg2+ions,while Li+ions are not adsorbed in the Cu2+-crosslinked Alg beads [41].This indicates that ion valence plays an important role in the determination of ion selectivity.However,the exact ion adsorption mechanism still remains to be investigated due to complicated interactions between the randomly distributed amorphous IPN structure and cations.Thus,it is required to elucidate the complicated interactions by adopting a new experimental approach,such as surface force apparatus measurement.
Although the Li+adsorption capacity is still low,7.3% of Li+ions are recovered from Li-spiked seawater containing 60 mg L-1of Li+ions.In addition,7.9,3.1,and 12.7% of Na+,K+,and Mg2+ions are recovered.These recovery rates for other cations are relatively comparable to the amount of recovered Li+ions.In particular,the quantity of recovered Na+ions is relatively small,despite the high concentration and strong adsorption affinity of Na+,compared with that of Li+ions.This result indicates that the adsorption of other cations is significantly hindered,even though a large amount of cations with a high affinity is dissolved in seawater.The adsorption capacities of PNP/Alg(Al)composites immersed in the Li-spiked seawater with pH values of 4,7,and 10 for 24 h were compared (Fig.S13).The adsorption capacities are 0.030,0.022,and 0.036 mmol g-1in the solutions with pH 4,7,and 10,respectively.The corresponding recovery ratios are evaluated to be 7.162,5.184,and 8.311.The adsorption capacity for pH 7 solution is lowest,while Li adsorption capacity at pH 4 is still high comparable with that at pH 10.This indicates that acid treatment,which is usually used for the extraction of ions from conventional adsorbents,insignificantly affects the extraction of Li+ions from the fabricated composite.This might be attributed to the complicated ion interactions of cations dissolved in the multicomponent solution with the IPN structure.The detailed ion adsorption mechanism is still unelucidated.It is required to analyze the ion interactions between PNP/Alg(Al)composite and different types of cation in detail.This future study might be helpful for developing a new Li+recovery technique with high efficiency.
Various types of selective Li+adsorbent with a high selectivity have been developed for the Li+recovery from seawater [42,43].For selective Li+separation,most adsorbents have pores with a size comparable to that of Li+ions.The small pore size enables the rejection of other cations that are larger than Li+ions,thereby achieving a high Li+selectivity.However,the intrinsic morphological structure of these conventional Li+adsorbents worsens the diffusion rate for Li+ions due to the extremely small pore sizes[44].In addition,the ion exchange rate is largely limited because the adsorption affinity of Li+ions is lower than that of other cations,and Li+ions competitively interact with cations with a high affinity.Recently,a photocontrollable Li+recovery strategy with an adsorption capacity of 0.091 mmol g-1from the mixture containing 20 mg L-1of Li+,Na+,K+,and Mg2+was newly highlighted as a method without the use of acidic treatment[40].However,there is currently no efficient adsorption strategy that can resolve the intrinsically limited challenges.
On the contrary,the present result provides a potential applicability despite the low Li+adsorption capacity in that Li+ions with a low adsorption affinity are quickly and effectively recovered from seawater where the amount of Li+ions is extremely small in the presence of a large amount of impurities,including Na+,K+,and Mg2+,by applying thermal energy.In addition,the performance of PNP/Alg(Al) composite can be optimized by varying structural properties to appropriately reject other cations.The Li+recovery without acidic treatment is also largely unexplored,expecting to resolve the intrinsically limited challenges and to give unique chemical and physical properties beyond the characteristics of conventional Li+adsorbents.
4.Conclusions
We have proposed a PNIPAAm-incorporated Al3+-crosslinked Alg IPNs composite for the effective recovery of Li+ions from seawater.Thein situTEM results exhibit that IPNs structure is shrunken to a favorable orientation via a rearrangement of PNIPAAm and partial hydrolysis of Alg chains,as the temperature increases.In addition,the rearrangement of polymers induces the aggregation of Alg-Al3+coordination,which leads to the formation of polycrystalline structure inside IPNs structure.Thein situFTIR results indicate that the PNP/Alg(Al) composite has peculiar three phase transitions:a phase change from hydrophilic to hydrophobic and retrogressive phase changes from hydrophobic to hydrophilic occur at once during the heating or cooling process.The practical application of the PNP/Alg(Al) composite to Li-spiked seawater was evaluated,which shows that the composite is able to quickly and selectively adsorb Li+ions.In addition,the composite can desorb the adsorbed Li+ions with the aid of a phase transition from hydrophilic to hydrophobic at a LCST.Using a small amount of thermal energy,Li+ions can be extracted from the proposed PNP/Alg(Al) composites.This enables the eco-friendly recovery of Li+ions from seawater or saline water without acidic treatment for desorption.A total of 7.3% of Li+ions are recovered from Li-spiked seawater containing a large amount of impurities and an extremely small concentration of Li+ions,although the adsorption capacity for Li+ions is still lower than that for other cations.The proposed thermal-switchable PNP/Alg(Al) composite can be effectively utilized for fast selective Li+recovery with a small amount of thermal energy.Conventional selective Li+adsorption techniques nearly reach the uppermost limit in the improvement of Li+recovery efficiency due to the intrinsic morphological structure of adsorbents.Due to the facile synthesis,eco-friendly process,and high recovery rate of Li+ions,the proposed thermoresponsive IPN gel has strong potential in practical Li+recovery as an innovative strategy.
Conflict of interest
There is no conflict of interest.
Acknowledgments
We appreciated the help of HVEM experiment by S.J.Y.at the KBSI.This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2017R1A2B3005415).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.10.006.
杂志排行
Green Energy & Environment的其它文章
- Investigating ionic liquids for optimizing lithium metal anode
- A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application
- Advanced silicon nanostructures derived from natural silicate minerals for energy storage and conversion
- B-doped activated carbon as a support for a high-performance Zn-based catalyst in acetylene acetoxylation
- High recycling Fe3O4-CdTe nanocomposites for the detection of organophosphorothioate pesticide chlorpyrifos
- Hierarchical Cu3P-based nanoarrays on nickel foam as efficient electrocatalysts for overall water splitting
