Pt-confinement catalyst with dendritic hierarchical pores on excellent sulfur-resistance for hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene
2022-05-22XilongWngChengkunXioMohnndAlsiPengZhengZhengkiCoJinlinMeiYuShiAijunDunDoweiGoKuoWeiHungChunmingXu
Xilong Wng ,Chengkun Xio ,Mohnnd H.Alsi ,Peng Zheng ,Zhengki Co ,Jinlin Mei ,Yu Shi ,Aijun Dun ,*,Dowei Go ,Kuo-Wei Hung ,*,Chunming Xu ,*
a State Key Laboratory of Heavy Oil Processing,China University of Petroleum (Beijing),Beijing,102249,China
b KAUST Catalysis Center and Division of Physical Sciences and Engineering,King Abdullah University of Science and Technology,Thuwal,23955-6900,Saudi Arabia
c School of Chemistry and Chemical Engineering,University of Jinan,Jinan,250022,China
Abstract Metal confinement catalyst MoS2/Pt@TD-6%Ti (TD,TS-1/Dendritic mesoporous silica nanoparticles composite) in dendritic hierarchical pore structures was synthesized and showed excellent sulfur-resistance performance and stabilities in catalytic hydrodesulfurization reactions of probe sulfide molecules.The MoS2/Pt@TD-6%Ti catalyst combines the concepts of Pt-confinement effect and hydrogen spillover of Pt noble metal.The modified micropores of Mo/Pt@TD-6%Ti only allow the migration and dissociation of small H2 molecules (0.289 nm),and effectively keep the sulfur-containing compounds(e.g.H2S,0.362 nm)outside.Thus,the MoS2/Pt@TD-6%Ti catalyst exhibits higher DBT and 4,6-DMDBT HDS activities because of the synergistic effect of the strong H2 dissociation ability of Pt and desulfurization ability of MoS2 with a lower catalyst cost.This new concept combining H2 dissociation performance of noble metal catalyst with the desulfurization ability of transition metal sulfide MoS2 can protect the noble metal catalyst avoiding deactivation and poison,and finally guarantee the higher activities for DBT and 4,6-DMDBT HDS.
Keywords: Pt-confinement effect;Hydrogen spillover;Sulfur-resistance;Dibenzothiophenes;Hydrodesulfurization
1.Introduction
Hydrodesulfurization (HDS) has become an increasingly important research topic over the past decades due to the growing demand for transportation fuels[1-7].Environmental regulations in many countries worldwide demand the production of clean fuels with an ultra-low sulfur content of 10 ppm or less [8-10].As the traditional HDS catalysts,alumina-based Ni(Co)-W(Mo) catalysts are widely used in industrial processes due to their satisfying activity and low cost [11-16].However,in order to meet the requirements of deep HDS conversion,new efficient catalysts with high activity and selectivity should be developed to remove the refractory sulfur-containing compounds,e.g.,DBT and 4,6-DMDBT,from transportation fuels [17-21].
Compared to other transition metals,noble metals and the corresponding alloys (Pt or PtPd alloy) show better hydrogenation properties in the DBT and 4,6-DMDBT HDS reactions[22-26].Although noble metal catalysts display high catalytic performance in the hydrotreating process,they are relatively expensive and susceptible to sulfur poisoning even at very low sulfur contents feedstocks,which limit their practical applications in HDS reactions [27,28].Therefore,the research of noble catalysts with sulfur resistance properties arouses more interests of scientists in different research fields.Many preparation strategies,such as constructing a core-shell structure or alloying with a second metal (Pd,Ru or Ir,etc.),have been performed to enhance the sulfur tolerance of noble metal catalysts [29-31].For example,Pt noble metal can be encapsulated in the pores (around 0.5 nm) of zeolite only to allow the migration and dissociation of small hydrogen molecules (0.289 nm) and exclude the large sulfur-containing compounds [32-35].This method protected the noble metal catalyst from sulfur poisoning and provided activated hydrogen for the HDS reaction [36].Unfortunately,a fatal disadvantage of poisoning caused by the smaller hydrogen sulfide molecule (H2S,0.362 nm) poisoning cannot be prevented [37].Therefore,a critical problem to be solved is to isolate the H2S from the noble metal center,to achieve excellent sulfur-resistance performance in the presence of H2S.Besides,the noble metal catalyst with a high loading of noble metals will increase the production cost of the catalyst compared with those of the transition metal sulfide catalysts.As for noble metal catalysts,the approach to realize high HDS activity but cheap price would be the focus to explore.
Herein,a new dendritic MoS2/Pt@TD catalyst consisting of sulfur-resistance Pt@TS-1 and MoS2active phases was prepared.The pore-modified Pt@TS-1 seed with appropriate micropore sizes (between 0.289 nm and 0.362 nm) not only created activated H protons over the Pt noble metal sites anchored inside the internal channels of micropores,but also incentivized H+spilling over to the surface of MoS2active phases located in the external channels,which prohibited the direct contact between Pt noble metal and the sulfur-containing compounds (including H2S),consequently avoiding the poisoning of Pt active sites.Moreover,titanium element with various chemical states in the Pt@TS-1 can generate more delectron,which is be beneficial to the creation of more S vacancies of MoS2species.The dendritic MoS2/Pt@TD catalyst possessed the combining characteristics of noble metals of excellent hydrogenation performance at low temperatures and transition metals of low-cost.Thus,the as-prepared novel dendritic MoS2/Pt@TD catalyst showed superior activities and high sulfur-resistance stabilities for DBT and 4,6-DMDBT HDS.
2.Experimental
2.1.Synthesis of Pt@TD catalyst
2.1.1.Synthesis of Pt@TS-1 seed
The synthesis procedure of Pt@TS-1 seed (molar ratios of SiO2/TiO2=25) is as follows.40 g of tetraethyl orthosilicate(TEOS),2.64 g tetrabutyl titanate,and 46.72 g TPAOH(25 wt%)were added to 8.68 g of deionized water.The mixture was stirred to dissolve for 1 h in an ice water bath,then switched to a water bath at 70°C for 3 h.48 g of isopropanol was added to the mix and stirred for 1 h.The resulting solution was labeled as solution A.0.10 g of NaOH and 0.12 g 3-mercaptopropyltrimethoxysilane were added to 2.0 g of deionized water.3.08 mL of chloroplatinic acid (H2PtCl6,100 mmol L-1) was added to the mixture and stirred for 20 min.The resulting solution was labeled as solution B.Then the solution B was added to the solution A slowly,and continued to stir at 70°C for 30 min.The resulting solution C was transferred to a 200 mL autoclave at 170°C for four days.Finally,Pt@TS-1 seed was synthesized by centrifugation,washing,and drying.
2.1.2.Synthesis of Pt@TD catalyst
0.60 g triethanolamine (TEA) was dissolved in 220 mL deionized water and stirred at 80°C for 30 min.Then 3.34 g of cetyltrimethylammonium bromide(CTAB)and 1.48 g sodium salicylate(NaSal)were added and kept stirring for 1 h.35.2 g TEOS (28.4 wt% SiO2) was added dropwise and agitated for 30 min,then 2.0 g of the as-synthesized Pt@TS-1 seed was blended into the mixture solution D and kept continual stirring for 1.5 h.The resulting mixture E was transferred to a 500 mL autoclave at 100°C for 4 h.Finally,Pt@TD catalyst was prepared after washing with water and ethanol,filtrating,drying at 80°C for 12 h and calcining at 300°C for 2 h.Pure TS-1/DMSNs (TD) composite was prepared using the same method without H2PtCl6.
2.2.Preparation of the series pore-modified Pt@TD catalyst
The micropore of Pt@TD catalyst was modified by using the deposition-precipitation method.3.0 g of Pt@TD catalyst and a certain amount (0/0.126/0.396/0.816 g) of tetrabutyl titanate was added to 90 mL of ethanol.The mixture F was completely dissolved for 40 min by ultrasonator.Then the mixture was stirred slowly at 45°C until all the ethanol was completely evaporated.Finally,a series of pore-modified Pt@TD catalysts were synthesized by adjusting the TiO2amounts (0 wt%/1 wt%/3 wt%/6 wt%) incorporated into the system,and the products were noted as Pt@TD-xTi,which x represented the mass fractions of TiO2.
2.3.Preparation of corresponding MoS2/Pt@TD-xTi catalysts
Mo/Pt@TD-xTi catalysts with 12 wt% MoO3and 0.1 wt%Pt were synthesized via incipient wetness impregnation method using ammonium molybdate ((NH4)6Mo7O24·4H2O).The as-prepared catalysts were denoted as Mo/Pt@TD-0%Ti,Mo/Pt@TD-1%Ti,Mo/Pt@TD-3%Ti and Mo/Pt@TD-6%Ti,respectively.The corresponding MoS2/Pt@TD-xTi catalysts were obtained after presulfidation of Mo/Pt@TD-xTi catalysts using 3 wt% CS2in hexane.The reference catalysts of MoPt/TD and Mo/TD with 12 wt% MoO3and 0.1 wt% Pt were prepared through two-step incipient wetness impregnation of TD support using (NH4)6Mo7O24·4H2O and H2PtCl6(100 mmol L-1).
2.4.Catalysts characterization
X-ray diffraction (XRD) patterns were measured in wideangle between 5°-50°and small-angle between 1.3°-6°at 40 kV and 40 mA in Cu Kα radiation.Nitrogen adsorption/desorption isotherms were acquired at -196°C on a Micromeritics TriStar II 2020 instrument.Scanning electron microscopy (SEM) was taken on a Hitachi SU8010 apparatus.Transmission electron microscope (TEM) images were recorded on an FEI F20 apparatus.CO temperature-programmed desorption (CO-TPD)and H2-TPD were carried out on a quartz reactor with a quadrupole mass spectrometer(OmniSTAR TM).X-ray photoelectron spectroscopy (XPS)was collected on a Thermo Fisher K-Alpha spectrometer.
2.5.Evaluation of catalyst activity
The activity of MoS2/Pt@TD-xTi series catalysts and the reference catalysts were investigated within a fix-bed reactor for DBT or 4,6-DMDBT HDS.1.0 g Mo/Pt@TD-xTi catalysts precursors were loaded in the center of the reactor.Before the HDS reaction,the precursor was presulfided at 340°C for 4 h under the conditions of 4.0 MPa,volumetric ratio of H2/Oil of 200 (v/v) and weight hourly space velocity (WHSV) of 8.0 h-1.After the presulfidation was completed,fresh H2and liquid feed (DBT or 4,6-DMDBT) were switched into the reactor.DBT or 4,6-DMDBT HDS experiments were conducted at 340°C,4.0 MPa,200 (v/v) and 10-100 h-1.
Hence,the HDS conversions were determined by the following equation [38]:
whereSfis the initial concentration of liquid feed before reaction (ppm) andSpis the residual concentration of DBT or 4,6-DMDBT after reaction (ppm).
The HDS of DBT or 4,6-DMDBT is assumed to follow the pseudo-first-order reaction kinetics,and the rate constantkHDS(mol g-1h-1) can be presented as [39]:

whereFdenotes the feeding rate of DBT or 4,6-DMDBT(mol h-1),mrefers to the weight of catalysts(g),and τ refers to the HDS conversion of (%).
3.Results
3.1.Catalysts characterization
3.1.1.XRD
Five characteristic peaks of Pt@TD-xTi series catalysts appear at the ranges of 7.8-8.7°and 22-25°in the XRD patterns as shown in Fig.1,which correspond to the (011),(020),(051),(303),(313),and (532) planes of TS-1 zeolite[40].No discernible peak relating to Pt metal is identified,which is ascribed to the low loading and high dispersion of Pt.It is evident that peak intensities of Pt@TD-xTi series catalysts decrease in comparison of Pt@TS-1 seed,suggesting that Pt@TS-1 seeds are embedded into the skeletal structure of DMSNs.
3.1.2.HAADF-STEM-EDS
To better understand the Pt distributions in Pt@TD-6%Ti catalyst,HAADF-STEM,and EDS mappings were carried out to investigate the morphology and metal dispersion of catalysts,and the relevant photos are presented in Fig.2.The Pt@TD-6%Ti catalyst(Ultralow 0.1 wt%Pt content)shows no aggregation of bulk Pt particles,indicating that noble metal Pt particles are evenly distributed in the Pt@TD-6%Ti catalyst.In addition,Ti elements are also evenly distributed in the Pt@TD-6%Ti catalyst,demonstrating that Pt@TS-1 seeds are embedded into the skeletal structure of DMSNs.
3.1.3.N2 physisorption
N2adsorption-desorption isotherms and pore size distributions of the series Mo/Pt@TD-x%Ti catalysts,MoPt/TD and pure TD composite are presented in Fig.3.All the isotherm curves of the series Mo/Pt@TD-x%Ti catalysts,MoPt/TD and the pure TD composite display type-IV curves with H2 hysteresis loops.The hysteresis loops of the series Mo/Pt@TD-x%Ti catalysts and MoPt/TD became narrow after the addition of Pt and Mo active metals into TD composite.Moreover,the pore size distributions of the series Mo/Pt@TDx%Ti catalysts and MoPt/TD are relatively discrete compared with pure TD composite.
The texture properties of the series Mo/Pt@TD-x%Ti catalysts,MoPt/TD and the pure TD composite are presented in Table 1.As shown in Table 1,the surface areas and pore volumes decrease from Mo/Pt@TD-0%Ti (407 m2g-1,1.09 cm3g-1) to Mo/Pt@TD-1%Ti (399 m2g-1,1.07 cm3g-1),then reduce to Mo/Pt@TD-6%Ti(367 m2g-1,0.95 cm3g-1) as the TiO2amounts increase.Notably,MoPt/TD catalyst shows the lowest surface area,pore volume and the smallest pore size among these catalysts.The sufficient surface area and pore size can provide a favorable environment for excellent dispersion of Pt and Mo active metal species’ and a fast diffusion for the reactants and products.

Fig.1.XRD profiles of the as-prepared dendritic Pt-encapsulated catalysts.
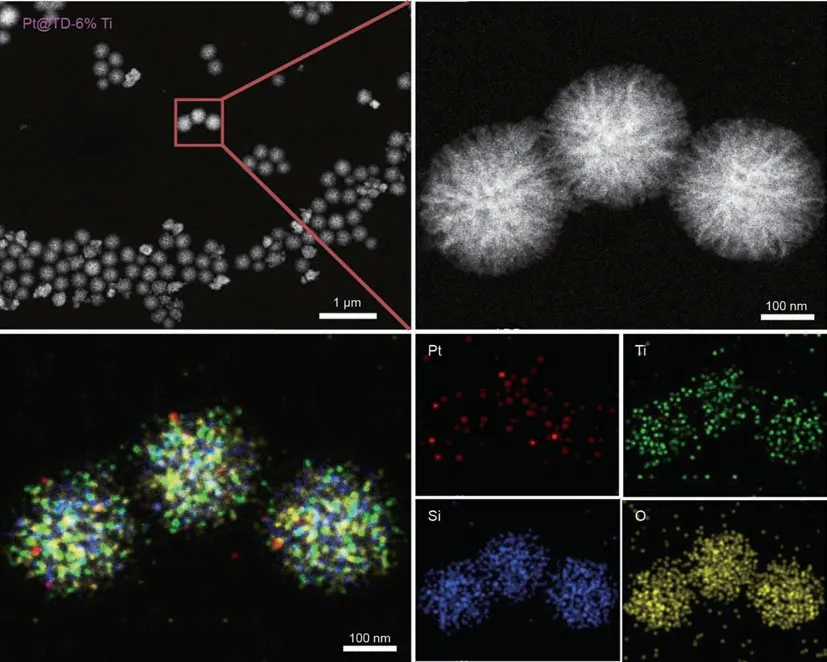
Fig.2.HAADF-STEM and EDS images (Pt,Ti,Si,O) of Pt@TD-6%Ti catalyst.
3.1.4.SEM
The SEM images of the series Mo/Pt@TD-x%Ti catalysts and Pt/TS-1 seed are shown in Fig.4.All the series Mo/Pt@TD-x%Ti catalysts display similar uniform wrinkled morphology,indicating that deposition of TiO2has little impact on the morphology of the series Mo/Pt@TD-x%Ti catalysts.The wrinkled surfaces of the series Mo/Pt@TD-x%Ti catalysts are tremendous advantages to the enhancement of the surface area.Besides,the series Mo/Pt@TD-x%Ti catalysts present no independent phases of Pt@TS-1 seeds,manifesting that Pt@TS-1 seeds are embedded into the skeletal structure of DMSNs successfully.
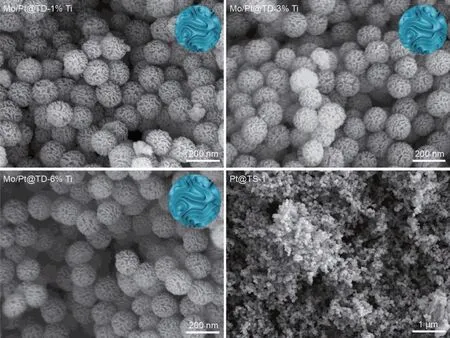
Fig.4.SEM images of the series catalysts.
3.1.5.TEM
The TEM images of the series Mo/Pt@TD-x%Ti catalysts and the pure TD composite are shown in Fig.5.All the series Mo/Pt@TD-x%Ti catalysts exhibit similar open dendritic center-radial pore channels as the pure TD composite,demonstrating that the dendritic pore structures are well maintained after the additions of Pt and Mo active metals into TD composite.The open dendritic pore structure facilitates the diffusion of reactants and products in the pore channels,which finally contribute to the improvement of catalytic performance of the HDS reaction.
3.1.6.H2-TPD
The H2-TPD data can be used to investigate the H2adsorption capacity of the catalyst.The H2adsorption capacity of the catalyst increases with the increase of the adsorption peak intensity.The adsorption peak area represents the adsorption amount of H2molecules of the catalyst.The H2-TPD curves of the series Mo/Pt@TD-x%Ti catalysts are displayed in Fig.6.In addition,MoPt/TD and Mo/TD catalyst were selected as reference catalysts for the H2-TPD characterization.From the H2-TPD curve of Mo/TD catalyst,it can be seen that the H2adsorption peak of Mo/TD catalyst is very weak,which indicates that adsorption of H2by the Mo metal and TD composite is very weak.The H2adsorption peak of MoPt/TD catalyst is also relatively weak,indicating that Pt particles on the MoPt/TD catalyst are too large and Pt metal dispersion is not even,resulting in the exposure of less H2adsorption site.From the H2-TPD curves of the series Mo/Pt@TD-x%Ti catalysts,it can be seen that with the increase of TiO2modification amount,the H2adsorption amount decreases slightly but not significantly,which indicates that the modified micropores of Mo/Pt@TD-6%Ti can still allow the migration and dissociation of small H2molecules.
3.1.7.CO-TPD
CO molecule(0.369 nm)and the H2S molecule(byproduct of the HDS reaction,0.362 nm) show the similar kinetic diameters [41],indicating that if the CO molecule cannot enter into the micropores of catalyst successfully,then H2S molecule will also be denied entry to the micropores of the catalyst,consequently no access to the active sites of Pt clusters.The CO-TPD data can be used to investigate the CO adsorption capacity of the catalyst.The CO-TPD curves of the series Mo/Pt@TD-x%Ti catalysts are displayed in Fig.7.In addition,MoPt/TD and Mo/TD catalysts were selected as reference catalysts for the CO-TPD characterization.From the CO-TPD curve of Mo/TD catalyst,it can be seen that the CO adsorption peak of Mo/TD catalyst is very weak,which indicates that the adsorption of CO by the Mo metal and TD composite is very weak.The CO adsorption peak of MoPt/TD catalyst is also relatively weak,which may be because Pt particles on the MoPt/TD catalyst are too large and Pt metal dispersion is not even,resulting in the exposure of less CO adsorption site.From the CO-TPD curves of the series Mo/Pt@TD-x%Ti catalysts,it can be seen that with the increase of TiO2modification amount,the CO adsorption amount decreases significantly,which indicates that most micropores of Mo/Pt@TD-6%Ti cannot allow the migration of H2S molecules from the products to the Pt site.
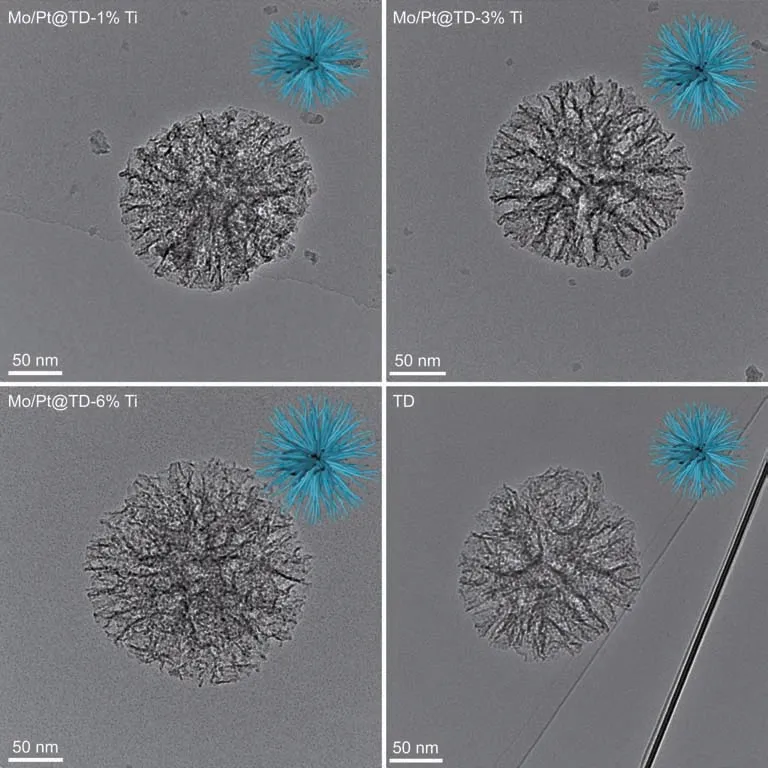
Fig.5.TEM images of the series catalysts.
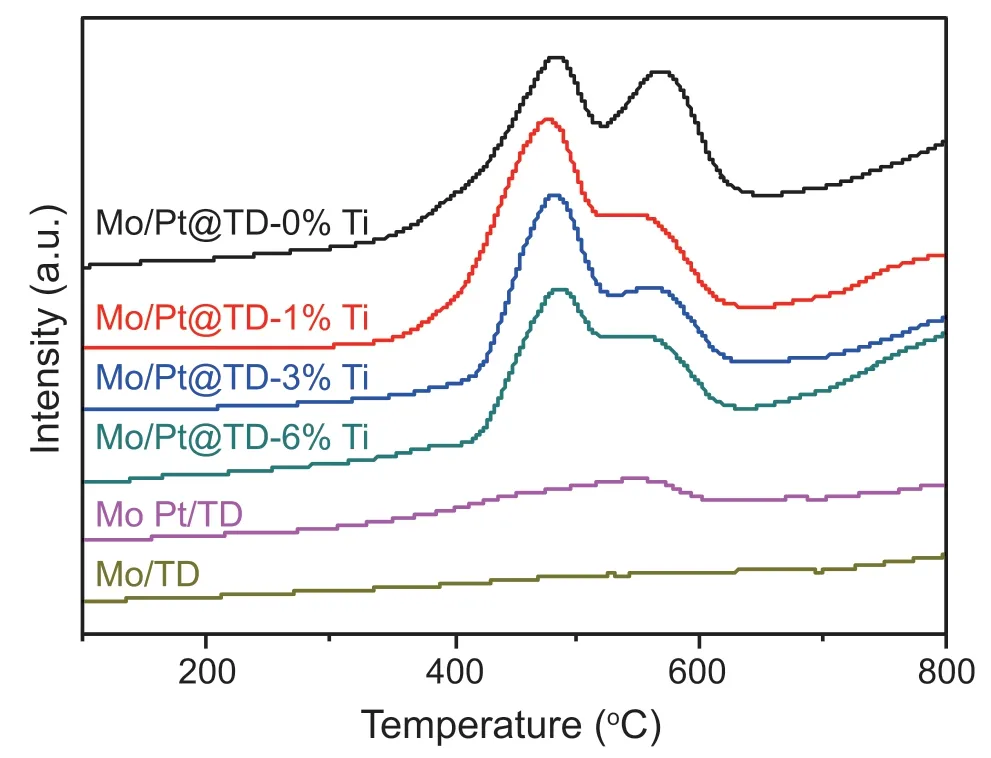
Fig.6.H2-TPD spectra of the series catalysts.
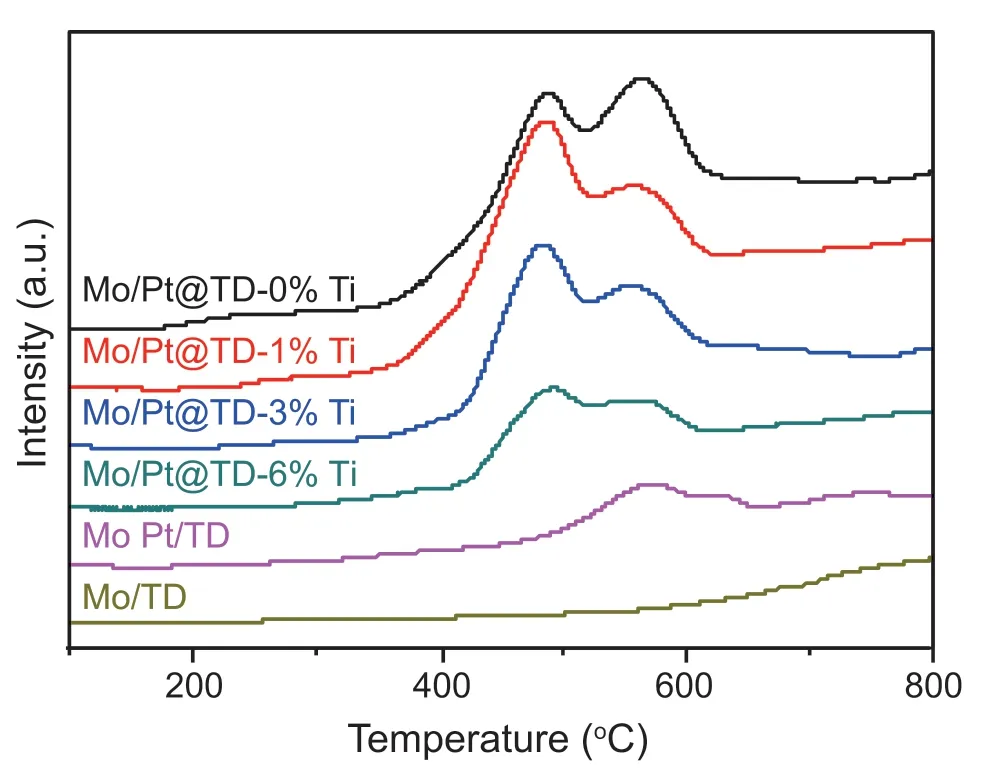
Fig.7.CO-TPD spectra of the series catalysts.
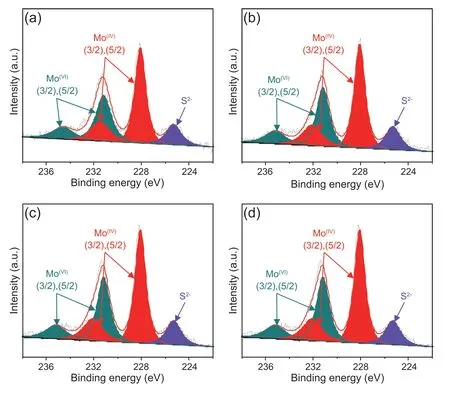
Fig.8.Mo 3d XPS of series catalysts:(a) MoS2/Pt@TD-1%Ti;(b) MoS2/Pt@TD-3%Ti;(c) MoS2/Pt@TD-6%Ti and (d) MoS2-Pt/TD.
It can be seen from the H2-TPD and CO-TPD characterization results that the Mo/Pt@TD-6%Ti catalyst can effectively prohibit the sulfur-containing compounds transferring into the inside of micropores due to the confined small pores after the Ti modification,thus avoiding the direct contact between the sulfur-containing compounds and Pt metals.At the same time,H2molecules can diffuse in and out of the micropore channels freely on the Mo/Pt@TD-6%Ti catalyst and hydrogen dissociation occurs on the surface of Pt sites,of which activated hydrogen spills over and transmit to MoS2active sites.
3.1.8.XPS
To investigate and further understand the chemical surface state of MoS2species on the series MoS2/Pt@TD-x%Ti and MoS2-Pt/TD catalysts,XPS measurements were performed and the relevant spectra are presented in Fig.8.The XPS fitting criterion is similar to the previous paper [42].Table 2 displays the Mo 3d statistical results of the series MoS2/Pt@TD-x%Ti and MoS2-Pt/TD catalysts.As shown in Table 2,the sulfidation degrees of the series MoS2/Pt@TD-x%Ti catalysts have a little change compared with MoS2-Pt/TD catalyst,indicated that the existing state of Pt noble metal has a minor effect on the reduction and sulfidation of Mo transition metal.
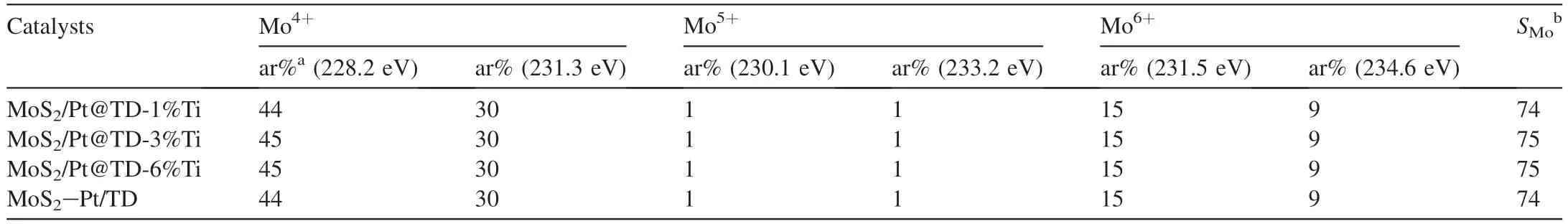
Table 2 Mo 3d XPS statistical results of the series catalysts.
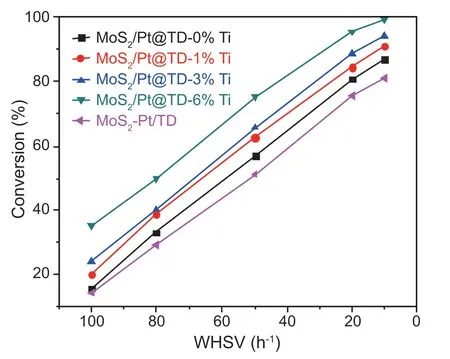
Fig.9.DBT HDS conversions over NiMo series catalysts.
3.2.HDS of DBT
The HDS activities of DBT over the series MoS2/Pt@TD-x%Ti and MoS2-Pt/TD catalysts with different WHSVs are presented in Fig.9.The DBT HDS conversions decrease with the increasing WHSVs.The DBT HDS conversions over the series catalysts follow the sequence of MoS2-Pt/TD <MoS2/Pt@TD-0%Ti < MoS2/Pt@TD-1%Ti < MoS2/Pt@TD-3%Ti < MoS2/Pt@TD-6%Ti in the WHSVs ranges of 10-100 h-1.MoS2/Pt@TD-6%Ti catalyst displays the best DBT HDS conversion than other MoS2/Pt@TD-x%Ti catalysts and MoS2-Pt/TD catalyst.
Further study on the product distributions shows that DBT reacts along direct desulfurization route (DDS) and hydrogenation route (HYD),and the corresponding HDS reaction mechanisms are shown in Fig.S1 (Supporting Information).Table 3 shows the DBT HDS product distributions of the series MoS2/Pt@TD-x%Ti and MoS2-Pt/TD catalysts.The HYD/DDS ratios of DBT over the series catalysts increase as the sequence of MoS2-Pt/TD (0.52) < MoS2/Pt@TD-0%Ti(0.69) <MoS2/Pt@TD-1%Ti (0.85) <MoS2/Pt@TD-3%Ti(1.04) <MoS2/Pt@TD-6%Ti (1.43),which is consistent with the trend of catalytic activity (kHDS).This result confirms that DBT mainly proceeds HDS via the HYD route,implying the HYD route made a considerable contribution to the total activity of DBT HDS over MoS2/Pt@TD-6%Ti catalyst.
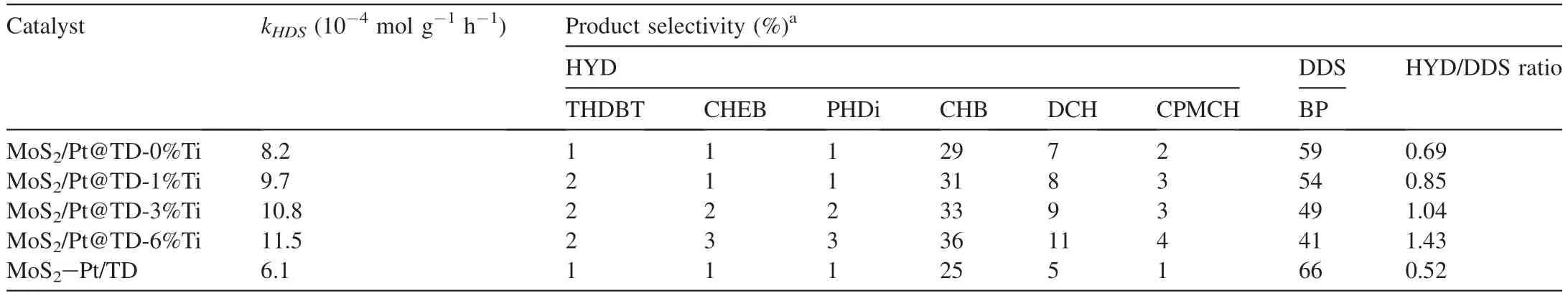
Table 3 Catalytic performances for DBT HDS over the series NiMo/TD catalysts.
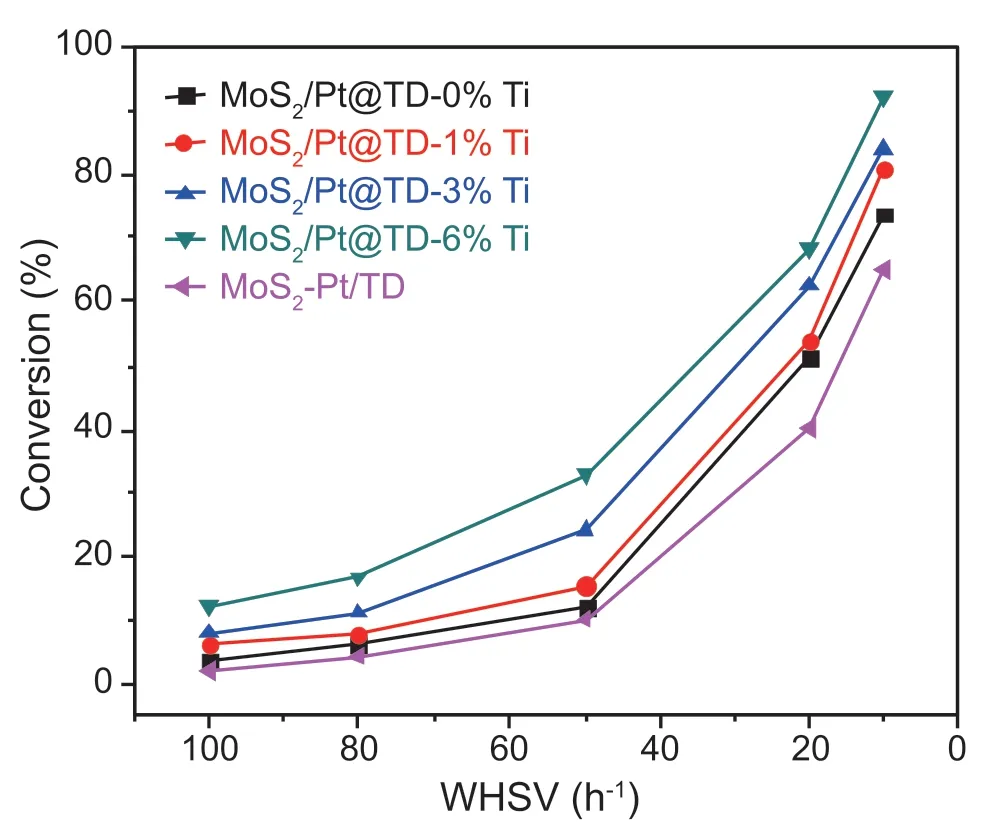
Fig.10.4,6-DMDBT HDS conversion over the series catalysts.
3.3.HDS of 4,6-DMDBT
The HDS activities of 4,6-DMDBT over the series MoS2/Pt@TD-x%Ti and MoS2-Pt/TD catalysts with different WHSVs are exhibited in Fig.10.The 4,6-DMDBT HDS conversions decrease with increasing WHSVs values.The 4,6-DMDBT HDS conversions over the series catalysts increase in the sequence of MoS2-Pt/TD <MoS2/Pt@TD-0%Ti <MoS2/Pt@TD-1%Ti <MoS2/Pt@TD-3%Ti <MoS2/Pt@TD-6%Ti during the WHSVs of 10-100 h-1.MoS2/Pt@TD-6%Ti catalyst shows the highest 4,6-DMDBT HDS conversion than other MoS2/Pt@TD-x%Ti catalysts and MoS2-Pt/TD catalyst.
In order to investigate the stability and sulfur-resistance performance of the series MoS2/Pt@TD-x%Ti and MoS2-Pt/TD catalysts,long-period (100 h) 4,6-DMDBT HDS reactive experiments over MoS2/Pt@TD-6%Ti and MoS2-Pt/TD catalysts were complemented.It can be seen from Fig.11,the 4,6-DMDBT HDS conversions over MoS2/Pt@TD-6%Ti catalyst were maintained at 92.1% at 100 h,In contrast,the 4,6-DMDBT HDS conversions over MoS2-Pt/TD catalyst decrease significantly,indicating that the Pt-confinementMoS2/Pt@TD-6%Ti catalyst possessed better sulfur-resistance performance and 4,6-DMDBT HDS catalytic stabilities.
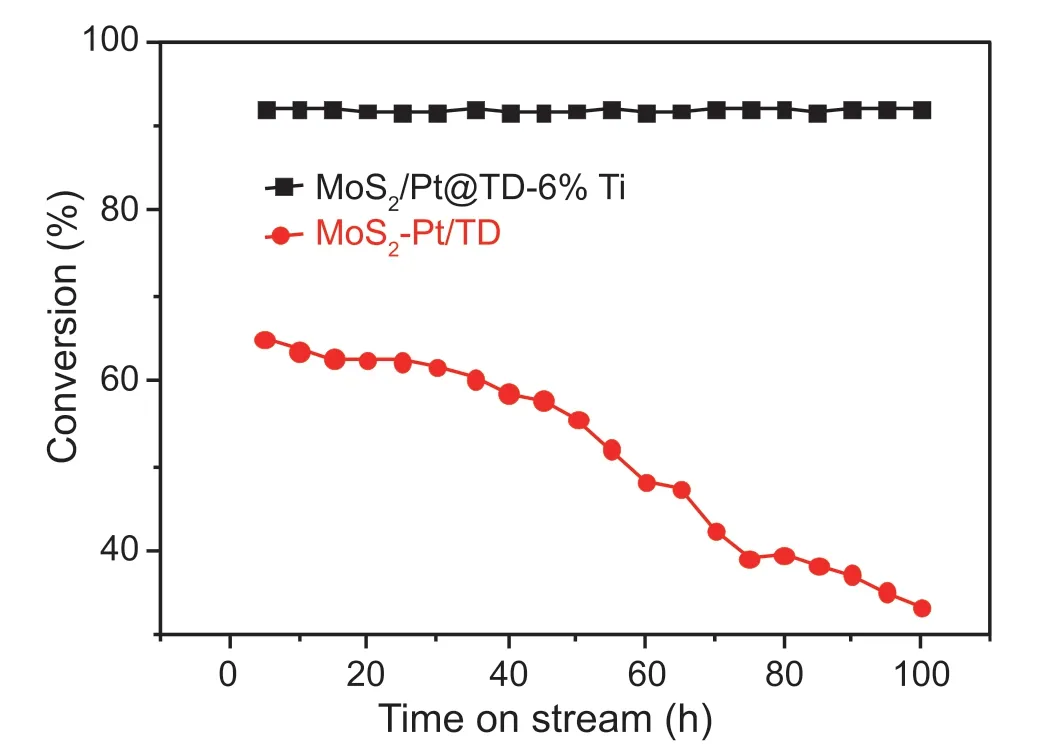
Fig.11.4,6-DMDBT HDS results with time on stream over the series catalysts.
Further study on the product distributions shows that 4,6-DMDBT reacts along DDS,HYD,and isomerization (ISO)routes,the corresponding HDS reaction mechanisms are shown in Fig.S2 (Supporting Information).Table 4 exhibits the 4,6-DMDBT HDS product distributions of the series MoS2/Pt@TD-x%Ti and MoS2-Pt/TD catalysts.The ISO selectivities of the series catalysts increase in the sequence of MoS2-Pt/TD (17%) <MoS2/Pt@TD-0%Ti (24%) <MoS2/Pt@TD-1%Ti (29%) <MoS2/Pt@TD-3%Ti (46%) <MoS2/Pt@TD-6%Ti (54%),which is in agreement with the order of catalytic activity(kHDS).This result confirms that 4,6-DMDBT mainly proceeds the ISO route in the HDS reaction process,proving that the ISO route contributes more to the total activity of 4,6-DMDBT HDS over MoS2/Pt@TD-6%Ti catalyst.
4.Discussion
Dendritic Pt-confinement MoS2/Pt@TD-6%Ti catalyst with excellent sulfur-resistance performance and HDS catalytic stabilities were successfully prepared.MoS2/Pt@TD-6%Ti catalyst displays much better DBT and 4,6-DMDBT HDS activities than those of other MoS2/Pt@TD-x%Ti and the MoS2-Pt/TD reference catalyst.Advanced characterization and activity evaluation were conducted to interrogate the structure-activity relationship and the results are discussed as follows:
Firstly,the modified micropore of Mo/Pt@TD-6%Ti can still allow the migration and dissociation of small H2molecules(Fig.6).H2undergoes dissociation on Pt sites to produce activated hydrogen protons.Then H+spills over to the MoS2active sites on the mesoporous surface,which can enhance the HYD route of DBT HDS and the ISO route of 4,6-DMDBT HDS (Tables 3 and 4).
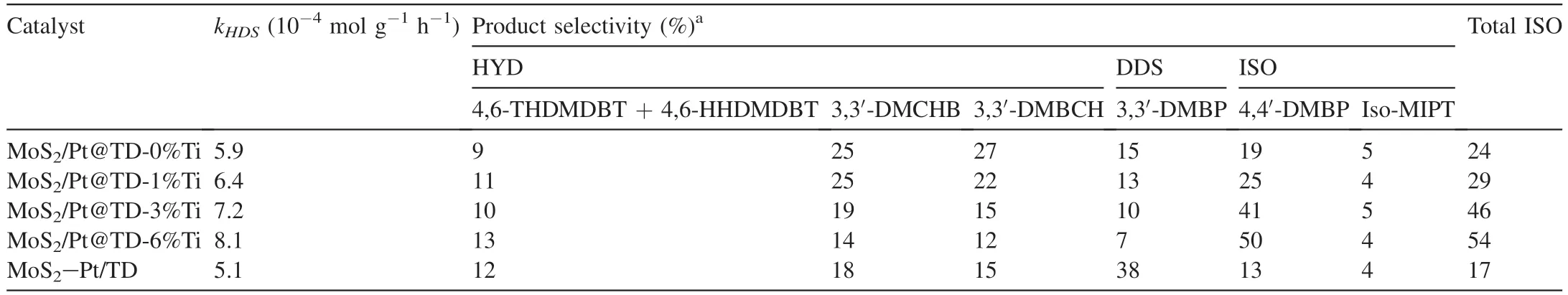
Table 4 Catalytic performances for HDS of 4,6-DMDBT over different catalysts.
Secondly,the modified micropores of Mo/Pt@TD-6%Ti catalyst possesses 0.289-0.362 nm in diameter,which can effectively confine Pt metal and prohibit the sulfur-containing compounds diffusing into the inside of the micropores(Fig.7),thus avoiding the direct contact between the sulfur-containing compounds and Pt metal.Thus,Mo/Pt@TD-6%Ti catalyst can effectively prevent the sulfur poisoning of noble metal Pt,thus improving the sulfur-resistance performance and HDS catalytic stabilities (Fig.11).
Thirdly,the higher DBT and 4,6-DMDBT HDS activities of the MoS2/Pt@TD-6%Ti catalyst are the results of the synergistic effect from the strong H2dissociation ability of noble metal Pt and the excellent desulfurization activity of transition metal sulfide MoS2.
Fourthly,Pt noble metal’s existing state shows little effect on the reduction and the sulfidation of Mo transition metal(Fig.8).The series MoS2/Pt@TD-x%Ti catalysts and MoS2-Pt/TD catalyst present almost the same sulfidation degrees,indicating that the higher DBT and 4,6-DMDBT HDS activities of MoS2/Pt@TD-x%Ti catalyst were mainly due to the stronger H2dissociation ability and the excellent sulfurresistance performance of pore-confinement Pt metal.
Fifthly,the uniform wrinkled surfaces(Fig.4)and the open dendritic pore structures(Fig.5)of Mo/Pt@TD-6%Ti catalyst can improve the accessibility of DBT and 4,6-DMDBT reactants to MoS2active sites and reduce the diffusion resistance of reactants and products in the DBT and 4,6-DMDBT HDS reaction process.
Sixthly,Ti species of Pt@TS-1 seeds can provide spillover of d-electrons for the transition metal oxide Mo species on the surface of the mesopore,which can create more sulfur vacancies and facilitate the reduction and sulfidation of the transition metal oxide Mo species.
5.Conclusions
The dendritic Pt-confinement MoS2/Pt@TD-6%Ti catalyst shows excellent sulfur-resistance performance and HDS catalytic stabilities in the DBT and 4,6-DMDBT HDS reactions.The MoS2/Pt@TD-6%Ti catalyst not only exhibits excellent DBT and 4,6-DMDBT HDS activity but also reduces the production cost.This new concept of combining H2dissociation performance of noble metal catalysts with the desulfurization ability of transition metal sulfide MoS2may open a unique perspective of the development of noble metal catalysts for the industrial HDS process of feedstocks with high sulfur contents.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was supported by the National Natural Science Foundation of China (No.21808079,21878330 and 21676298),Key Research and Development Program of Shandong Province (No.2019GSF109115),the National Science and Technology Major Project,the CNPC Key Research Project (2016E-0707),the King Abdullah University of Science and Technology(KAUST)Office of Sponsored Research(OSR) under Award (No.OSR-2019-CPF-4103.2) and the Project of National Key R&D Program of China(2019YFC1907700).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.10.012.
杂志排行
Green Energy & Environment的其它文章
- Investigating ionic liquids for optimizing lithium metal anode
- A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application
- Advanced silicon nanostructures derived from natural silicate minerals for energy storage and conversion
- B-doped activated carbon as a support for a high-performance Zn-based catalyst in acetylene acetoxylation
- High recycling Fe3O4-CdTe nanocomposites for the detection of organophosphorothioate pesticide chlorpyrifos
- Hierarchical Cu3P-based nanoarrays on nickel foam as efficient electrocatalysts for overall water splitting
