First principles investigation on Li or Sn codoped hexagonal tungsten bronzes as the near-infrared shielding material
2022-05-16BoShenZhou周博深HaoRanGao高浩然YuChenLiu刘雨辰ZiMuLi李子木YangYangHuang黄阳阳FuChunLiu刘福春andXiaoChunWang王晓春
Bo-Shen Zhou(周博深) Hao-Ran Gao(高浩然) Yu-Chen Liu(刘雨辰) Zi-Mu Li(李子木)Yang-Yang Huang(黄阳阳) Fu-Chun Liu(刘福春) and Xiao-Chun Wang(王晓春)
1Institute of Atomic and Molecular Physics,Jilin University,Changchun 130012,China
2College of Physics,Jilin University,Changchun 130012,China
Keywords: density functional theory,electronic structure,near-infrared radiation shielding material
1. Introduction
With higher demands for living quality,the expenditure of energy to maintain a comfortable indoor environment is everincreasing. To control the indoor temperature within an appropriate interval, over 10% of total electricity consumption is consumed on air conditions and fans, and a corresponding amount of global greenhouse gas has been released.[1]Much good research has been performed on clean energy materials to decrease the greenhouse-gas emissions.[2—9]Thermal losses and solar irradiation through glass window account for 60% of the total energy expenditure of air-conditioned and heating systems.[10]Furthermore, solar radiation consists of roughly 5%ultraviolet radiation(UV,10—400 nm),43%visible radiation (400—780 nm), and 52% near-infrared radiation(NIR, 780—2500 nm),[11]which implies that the NIR spectrum accounts for the bulk source of thermal energy from the Sun. Therefore,with the expectation to reduce the emission of greenhouse gas,the utilization of new NIR-shielding material in green building areas is indispensable.
Some corresponding materials have attracted a lot of attention such as vanadium dioxide (VO2),[12,13]rare-earth hexaboride nanoparticles (RB6),[14,15]tin-doped indium oxide(ITO),[16,17]and hexagonal tungsten bronze(HTB).[18—25]However,VO2shows poor transmittance(30%)[13]in the visible light range. RB6(transmittance over 50% in the 2000—2500 nm range)[15]and ITO (near 0 absorption coefficient in 780—1000 nm)[16,17]shields only part of NIR which indicates that the NIR-shielding ability is limited. Although the CsxWO3[26]exhibits a wide NIR shielding range and acceptable transmittance in the visible light range, it also exhibits chromatic instabilities when heated in a humid environment or being exposed to strong ultraviolet (UV) light,which renders detrimental effects on its long-life commercial application. Recently SnxWO3was reported as a nontoxic theranostic agent for imaging-guided cancer therapy,because of its absorption ability of NIR.[25]However,compared with the CsxWO3, the NIR-shielding ability of SnxWO3is not so satisfying(transmittance over 20%in the near-infrared range). Therefore,there is a necessity to find a less toxic,and chromatic stable material simultaneously with a wide NIRshielding range and acceptable visible transmittance.
Among these materials, HTB exhibits high visible light transmissivity and a wide NIR-shielding range (780—2500 nm). Recent studies have revealed the changing mechanism of the optical properties of HTB,which is highly correlated with the band structures and concentration of free carriers. In more detail,when photons in the NIR spectrum irradiate these materials,the photons could be absorbed to complete the transition of electrons in the valence bands (VBs) to the conductor bands(CBs).[19,27]On the other hand,the localized surface plasma resonance (LSPR) can be induced by the aggregated free electrons in the CBs to shield the near-infrared ray.[27—29]In recent research,it was found that the empty trigonal cavity could be occupied by light metallic elements,e.g.,Li.[19,30]Therefore,there is a possibility to improve the NIRshielding ability of materials by inserting Li and Sn atoms at trigonal and hexagonal cavities of HTB, respectively, which renders the rise of free carrier concentration in materials. As far as I know,rare studies have reported LixSnyWO3as one of the promising materials for the future energy-saving window.
In this work,the LixSnyWO3was investigated using density functional theory(DFT).First and foremost,the geometry structures of LixSnyWO3with six different doping concentrations had been constructed. Thereafter, binding energy was calculated for comparing the chemical stability of these structures. To further illustrate the thermal stability, the molecular dynamics study of Sn0.33WO3was performed. And then,the electronic properties of structures were investigated for a better understanding of the changing of optical properties.Finally, the optical properties of LixSnyWO3were calculated considering local field effects based on the random-phase approximation(RPA).[31]The results show that wheny=0 the NIR shielding ability increased with thexincrease,and wheny=0.33 the NIR shielding ability first increases and then decreases with thexincrease. Whenx=0 andy=0.33,the material exhibits the strongest NIR-shielding ability,is less toxic,has satisfying chemical stability, wide NIR-shielding range(780—2500 nm),and an acceptable visible transmittance which means it can be applied on the future energy-saving smart window.
2. Calculational method
In this paper, all calculation results were obtained via the Viennaab initiosimulation package (VASP).[32]We employed the generalized gradient approximation (GGA) with the Perdew—Burke—Ernzerh(PBE).[33]and used the projector augmented wave (PAW)[34]method. The influence of spin—orbit coupling effect (SOC) on electronic and optical properties was concerned. The valence electrons configurations for O, W, Li, Sn atoms were 2s22p4, 5d46s2, 2s1and 5s25p2, respectively. We adopted a Brillouin zone of 9×9×15 with aΓcentered Monkhorst—Packk-point mesh with cut-off energy of 500 eV during geometry optimization. And all of the structures were relaxed until the force on each atom was less than 0.02 eV/°A. And molecular dynamics simulations were performed at the time step of 2 fs,canonical ensemble(NVT),on the 2×2×2 supercell at the temperature of 300 K. Selfconsistent computations were performed with a convergence criterion of 10-8eV in energy.And the optical properties were concerned with approximation including local field effects in the RPA.[31]When calculating optical properties, nearly 100 bands were set to ensure that the empty bands were included in the calculations.
3. Results and discussion
3.1. Geometry structures and thermal stability
Table 1 shows the lattice parameters of optimized h-WO3,which were in accord with previous experimental results (xray diffraction data of h-WO3with a space group ofP6/mmm(No.191)).[35]
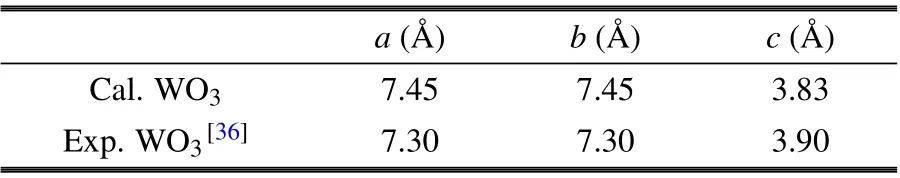
Table 1. Calculated and experimental crystal structure parameters ofh-WO3.
In this research,six structures of LixSnyWO3(withx=0,0.33 or 0.66;y=0 or 0.33)were concerned. For each structure, all spatial arrangements for Sn and Li atoms were considered,and only the most stable one was singled out as representative of every structure. Then,the binding energy of those six structures was calculated as below:
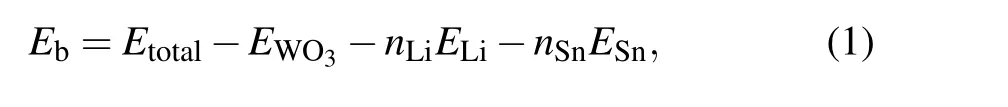
whereEtotalis the total energy of the system,EWO3is the energy of the pure h-WO3,nLiis the number of the Li atoms,ELiis the energy of the isolated Li atom,nSnis the number of the Sn atoms, andESnis the energy of the isolated Sn atom. In Fig.2,it was found that the binding energy declines while thexandyincrease. As is well known,this indicates the increase of stability. The energy evolution of the 10 ps MD simulation of Sn0.33WO3under the room temperature was exhibited in Fig.3. And the average energy of each atom was nearly a constant. This means Sn0.33WO3is stable at room temperature.
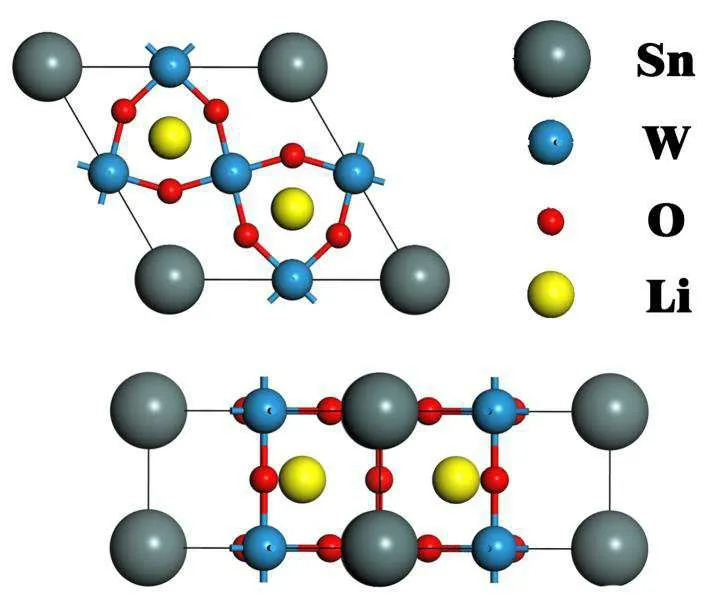
Fig.1. The top view and side view of Li0.66Sn0.33WO3.
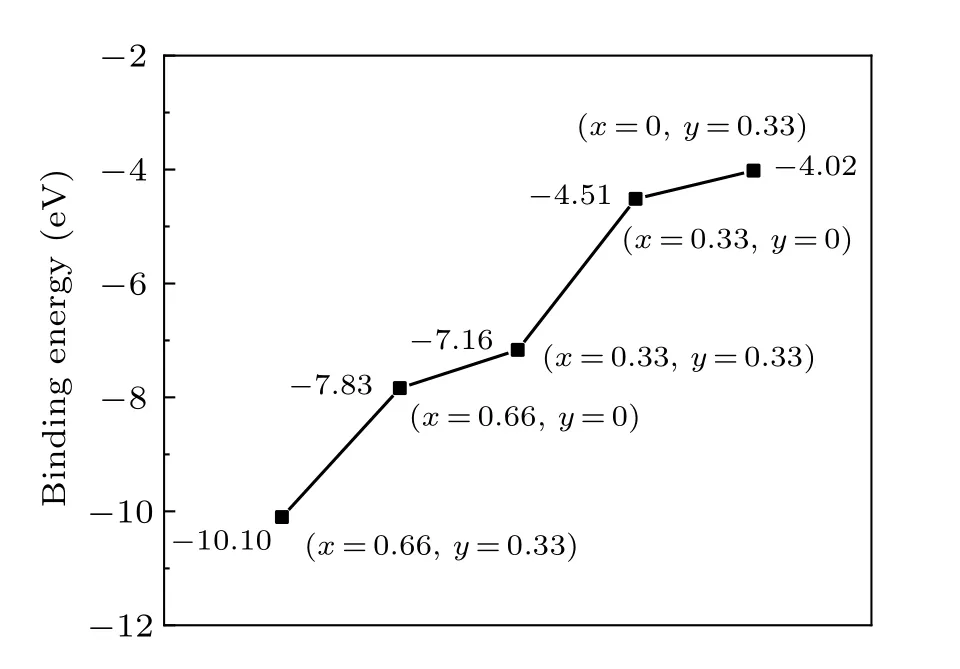
Fig.2. The binding energy of LixSnyWO3 (x=0,0.33,0.66;y=0,0.33).
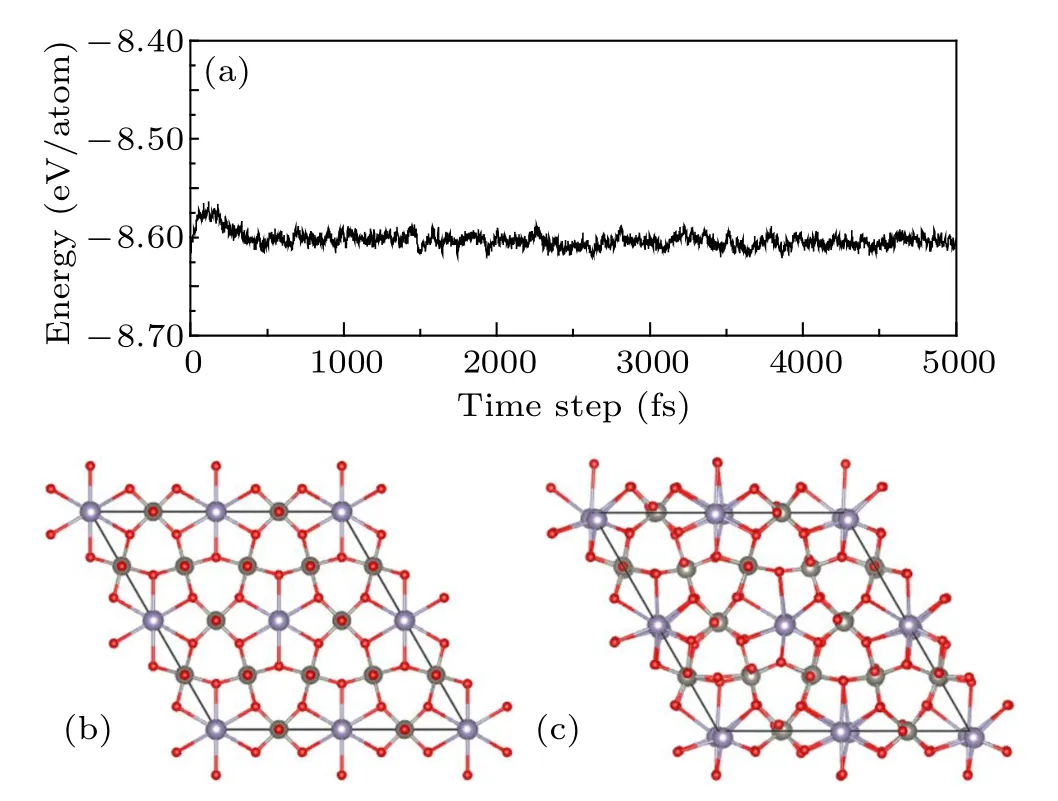
Fig. 3. (a) The energy evolution of Sn0.33WO3 during 10 ps MD simulations at room temperature,(b)and(c)are the structures before and after MD simulations.
3.2. Electronic structure
In Fig.4,it was found that h-WO3is an indirect bandgap semiconductor since whose valence band maximum (VBM)and conduction band minimum (CBM) do not at the same point,which occurs atApoint andΓpoint,respectively. The calculated bandgap of h-WO3is 0.44 eV, which approaches the previous result,[37]as results obtained through DFT theory are always underestimated. The band structure after doping is very similar to the pure h-WO3. Besides, it is found that the Fermi energy has been upshifted into the conduction bands(CBs) and the electrons occupied the bands that were empty before.
The density of states of LixSnyWO3is shown in Fig. 5.The DOS of pure h-WO3is in line with Liu’s result.[18]For pure h-WO3, the valence bands (VBs) mainly consist of the O-2p states,and the conduction bands(CBs)are mainly composed of the W-5d states,which are consistent with the experimental characterizations.[38]Since one W atom is surrounded by six O atoms forming an octahedral structure, W-5d states are split into t2g(low energy)and eg(high energy)states,[39,40]which could be observed through several peaks shown on PDOS curves of h-WO3, contributed from W-5d states. And the strong hybridization between O-2p states and W-5d states also indicates a strong covalency of the bonding. On the one hand, when Sn is doped in the h-WO3, the Fermi energy is upshifted in the conduction band,and the material exhibits the metal-like behavior,while electrons in Sn-5p mainly act as the free electrons. As the result, the materials display the n-type electronic conductivity. On the other hand,albeit Li-2s states are nearly zero, once Li is doped into h-WO3, the Fermi energy was upshifted, which improves the free carrier concentration of the material. In more detail, as shown in Table 2,it was found that the more ions are doped,the higher the free carrier concentration of the material will be. Compared with other structures,the free carrier concentration of Li0.33WO3is rather low, which is the reason why its NIR-shielding ability is almost zero.phase approximation(RPA).[31]And the real part of the dielectric function was calculated through the Kramer—Kronig relation. The reflectivityR, absorption coefficientα, and transmittanceTcould be derived from the dielectric function by the following formula:[18,42,43]
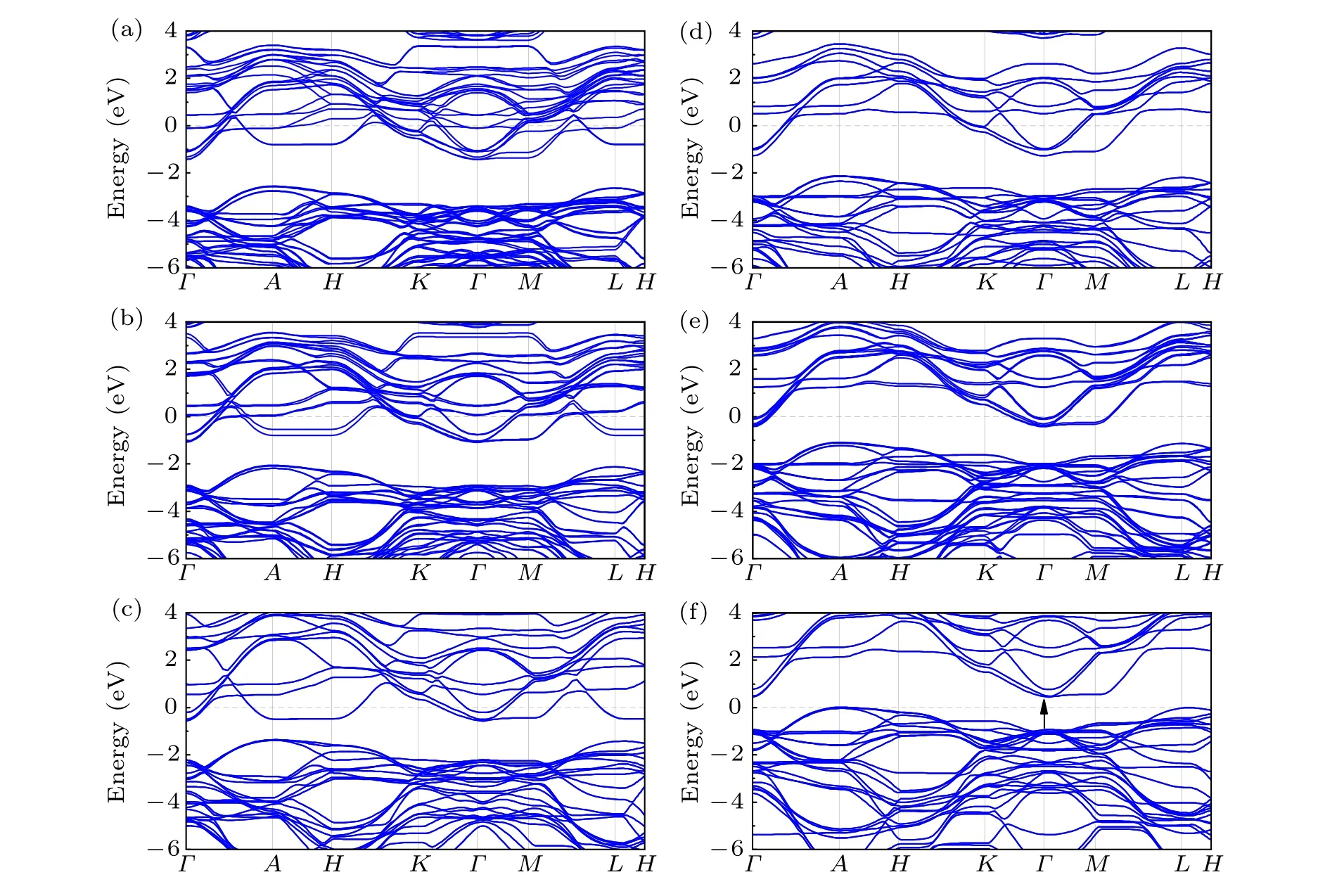
Fig.4. The band structures of(a)Li0.66Sn0.33WO3,(b)Li0.33Sn0.33WO3,(c)Sn0.33WO3,(d)Li0.66WO3,(e)Li0.33WO3 and(f)WO3.
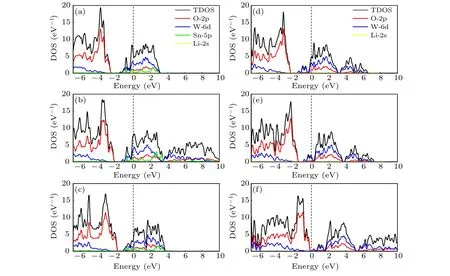
Fig. 5. The total density of states (TDOS) and project density of states (PDOS) of (a) Li0.66Sn0.33WO3, (b) Li0.33Sn0.33WO3, (c) Sn0.33WO3,(d)Li0.66WO3,(e)Li0.33WO3 and(f)WO3. The Fermi energy level is indicated by the vertical dashed lines at energy equal to zero.
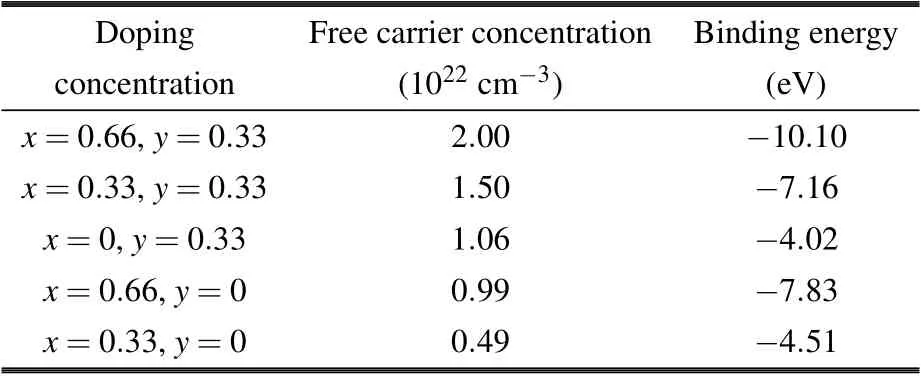
Table 2. Free carrier concentration and binding energy of five structures.
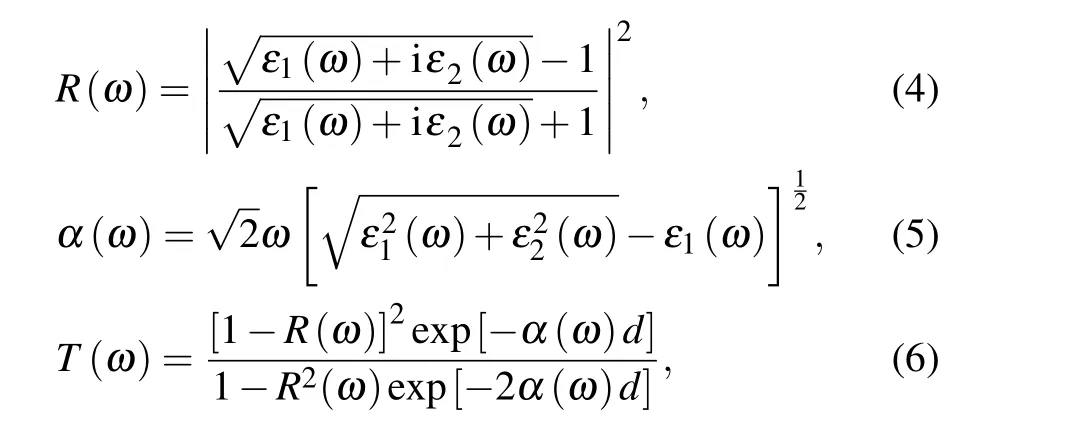
Besides,the codes behind the effects of doping Sn and Li atoms on the enhancement of free carrier concentration have been trying to decipher, which are crucial to understanding the changes in the NIR-shielding ability of various structures of LixSnyWO3. The number of free carriers (N) is given by Eq.(2),wheref(E)is the Fermi—Dirac distribution andg(E)is the density of states. At 0 K, the Fermi—Dirac distribution for electrons would simplify to either 0 (E >Ef) or 1(E <Ef). Although this assumption is only completely accurate at 0 K,the total concentration should relatively remain unchanged when temperature build-up. Therefore Eq.(2)can be simplified to Eq. (3), where theVis the unit cell volume and theEcis the bottom of the conduction band.[41]
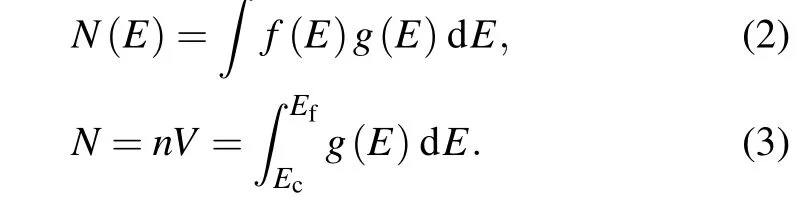
wheredis the thickness of the film, which is 50 nm in this calculation, andε1andε2are real part and imaginary part of the dielectric function,respectively. For simplicity,only conditions concerning optical properties along with thex-axis of LixSnyWO3are shown below.
To understand the change of optical properties,two models were introduced.The first model is the interband transition.In this module, the electrons can move or jump from a filled band to the higher empty band. The second is the intraband transition, which can describe the contribution of free carriers and always be concerned as the source of plasmonic resonances. And it always leads to a large of optical losses at low frequency.[44—46]In this model, the free carrier concentration plays a very important role. The dielectric function of intra-
3.3. Optical properties
In this investigation, the imaginary part of the dielectric function was concerned with the local field in the randomband transition can be described by the Drude model[44,47,48]
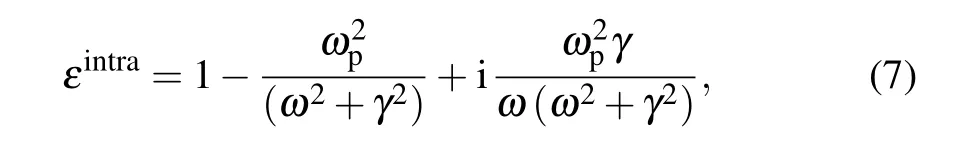
whereγis the Drude relaxation rate,which is inverse with the mean free path of electrons,[49]andωpis the plasma frequency which is scales with the free carrier concentration.[14]
As shown in Fig. 6(b), the imaginary part of the dielectric function of h-WO3goes to zero when the energy approaches 0 eV,which is in line with the previous result.[19,50]The plasma energy where the intersection points between the gray line where the real part equals zero, is corresponding to a fast abatement of the reflectivity as shown in Fig.7,respectively. In Fig. 6, when energy is less than 1, it can be found that with the increase of free carrier concentration,the imaginary part of the dielectric function increases first and then decreases, and the situation is quite reversed for the real part.This change is caused by the increase of free carrier concentrations and the decrease of the mean free path of electrons.The mean free path of electrons describes the average distance traveled by the electron. When the inserted atoms increase,the possibility of the electron being stopped by inserted ions increases. Therefore, the mean free path of electrons would decrease with the increase of doping concentration. And the Drude relaxation rate is inversed with the mean free path of electrons Therefore, when Li and Sn are codoped in the h-WO3, although the free carrier concentration would increase with the doping concentration increasing,the Drude relaxation rate would also increase. This explains the decline of the NIR shielding ability with excessive doping concentration.
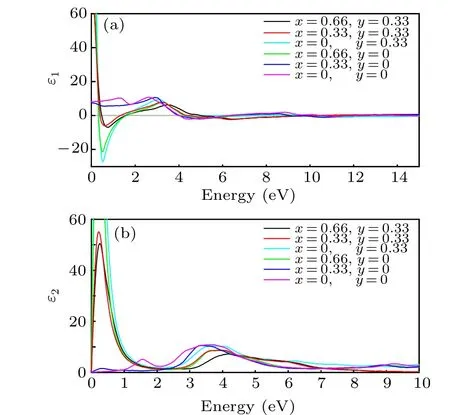
Fig. 6. The dielectric function of LixSnyWO3 (x=0,0.33,0.66 and y=0,0.33)at approximation including local field effects in the RPA.
In Fig. 7, the h-WO3shows nearly no NIR-shielding ability, which corresponds with the previous result.[30]The absorption coefficient and reflectivity of Sn0.33WO3sharply changed in the visible light range,which is also in good agreement with the experiment.[25]The peak of the absorption coefficient of h-WO3at 700 nm is originated due to the transition of electrons, which is in accordance with the transition between two bands in Fig.4(f). For other structures, the Fermi energy upshifted, and the electron occupied the bands that were empty before. That is why the absorption peaks of other structures disappeared. Compared with ITO,[16,17]which shows nearly zero absorption coefficient in the range from 780—1000 nm, the absorption coefficient of Sn0.33WO3is over 3×105at 1000 nm. In Fig. 8, except the Li0.33WO3is nearly the same as the h-WO3, other structures show pronounced improvement of reflectivity.
Figure 9 shows the transmittance of LixSnyWO3in the NIR and visible light range. The valley of transmittance of pure h-WO3can be found at 700 nm, which is according to the absorption coefficient and interband transition.The Sn0.33WO3shows the strongest NIR shielding ability and acceptable transmittance at the visible light range. For Sn0.33WO3, the maximum visible transmittance is over 60%at 562 nm which is larger than the VO2(36.2%)and the minimum NIR transmittance is nearly 10%over 1000 nm which is very close to the VO2.[13]The results show that the Sn0.33WO3can be applied as excellent NIR-shielding material with acceptable visible transmittance.
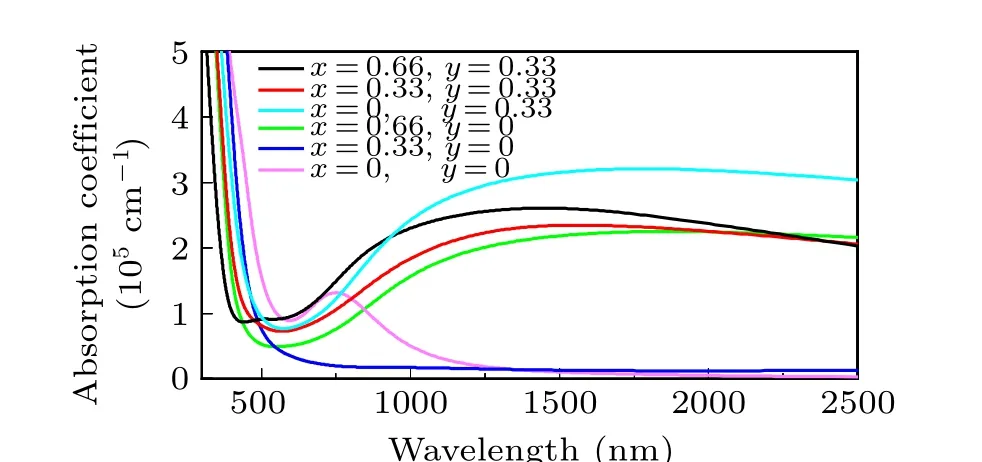
Fig. 7. The absorption coefficient of LixSnyWO3 (x=0,0.33,0.66 and y=0,0.33)at approximation including local field effects in the RPA.
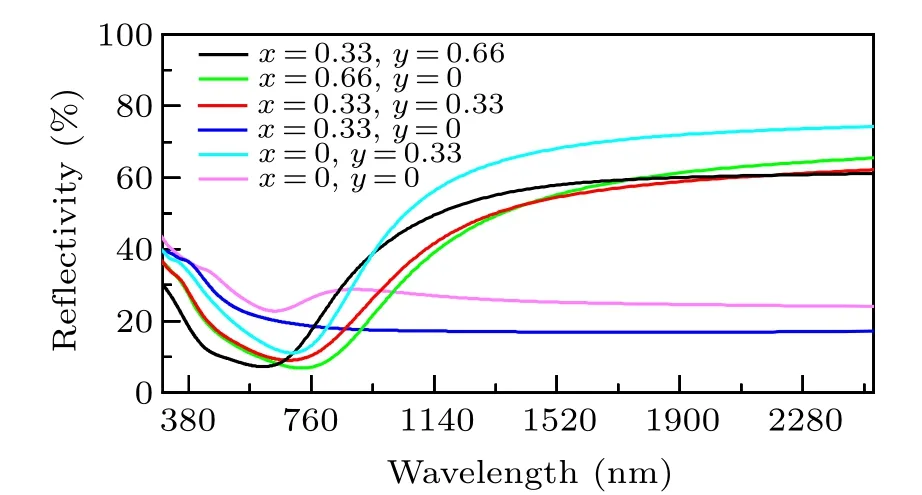
Fig.8. The reflectivity of LixSnyWO3 (x=0,0.33,0.66 and y=0,0.33)at approximation including local field effects in the RPA.
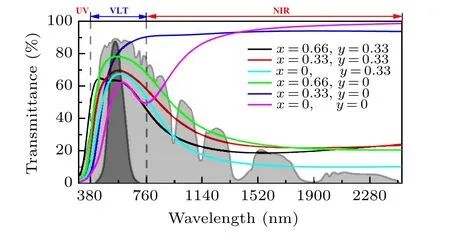
Fig.9. Comparing the transmittance of LixSnyWO3. The grey and light gray areas indicate the global tilt and extraterrestrial reference solar spectrum(ISO 9845-1,1992).
4. Conclusions
In summary, the binding energy, electric structure, and optical properties of LixSnyWO3were studied using the DFT calculation. After doping, the Fermi energy level entered into the conductor band and the electrons occupied the bands that were empty before doping,which indicates the metal-like characteristics of the doped material. The density of states shows that the Sn-5p electrons act as the free electrons in the conductor and Li-ions provide nearly no electron. Therefore,except the Li0.33WO3,other doped structures show metal-like characteristics. The optical properties show that except the Li0.33WO3exhibiting poor NIR shielding ability, other materials show excellent visible transmittance and NIR-shielding.Our results show that Sn0.33WO3is the material with satisfying chemical stability,excellent NIR-shielding ability,wide NIR-shielding range (780—2500 nm), and acceptable visible transmittance. It can be expected that our rational theoretical prediction should serve as an impetus for the pursuit of experimental realization of these(Li,Sn)codoped hexagonal tungsten bronze as NIR-shielding materials for the energy-saving smart window made in buildings.
Acknowledgment
Thanks to the Beijing Super Cloud Computing Center for assistance with calculation.
杂志排行
Chinese Physics B的其它文章
- A nonlocal Boussinesq equation: Multiple-soliton solutions and symmetry analysis
- Correlation and trust mechanism-based rumor propagation model in complex social networks
- Gauss quadrature based finite temperature Lanczos method
- Experimental realization of quantum controlled teleportation of arbitrary two-qubit state via a five-qubit entangled state
- Self-error-rejecting multipartite entanglement purification for electron systems assisted by quantum-dot spins in optical microcavities
- Pseudospin symmetric solutions of the Dirac equation with the modified Rosen–Morse potential using Nikiforov–Uvarov method and supersymmetric quantum mechanics approach
