Phytochemical,anti nutrient,invitro-protein digestibility and functional properties of(Balanites aegyptiaca(L.)Delile)Aduwa protein meals,Protein Concentrate and Isolate
2022-05-10AkamaFridayOgoriAbrahamTertengerGirgihMichealOjotuEkeJosephOnehAbuAkinsolaAlbertFamuwagun
Akama Friday Ogori ,Abraham Tertenger Girgih ,Micheal Ojotu Eke ,Joseph Oneh Abu ,Akinsola Albert Famuwagun
1Department of Home Sciences,Faculty of Agriculture,Federal University,Gashua,Gashua P.M.B.1005,Yobe State,Nigeria.2Department of Food Science and Technology,Federal University of Agriculture,Makurdi P.M.B 2373,Benue State,Nigeria.3Department of Food Science and Technology,Joseph Ayo Babalola University,Ikeji-Arakeji P.O.BOX 315,Osun State,Nigeria.
Abstract Background:Protein meals,Concentrate and Isolates are widely use as bioactive ingredients in functional food.In this study,the effect of toasting of Aduwa (Balanites aegyptiaca) seed on the phytochemicals,Anti nutrient,in vitro protein digestibility and functional properties of Aduwa Protein Meals,Protein Concentrate and Isolate were investigated.Methods: Aduwa seeds toasted at 70°C to make Aduwa protein meal (APM)yield extensive resolved biomaterials.The meal were resolved into DAPM,APC and API.Results:Phytochemicals analysis revealed reduction as APM sample is being resolved to protein concentrate and isolate.Similar trend was also observed in Anti-nutrients content significant reduction as material meal are resolved into protein concentrate and isolate.The in-vitro protein digestibility showed that APM (59.81%) and API (76.41%) had high percentage protein digestibility.Functional properties declined as meals materials were been resolved into protein concentrate and isolate.Swelling capacity revealed that resolved and unresolved samples leached biomolecules.Conclusion:The results obtained showed nutritional potential,and safety of biomaterials from Aduwa meals,an alternative food ingredient for protein supplementation.
Keywords: Balanites aegyptiaca;meal;resolved meal;phytochemicals;protein digestibility;functional properties
Introduction
Desert dateBalanites aegyptiacatree is also popularly calledAduwatree in Hausa language in Northern part of Nigeria.The tree produces leaves and seeds which are used as food and folders in animal feeds.The fruit mesocarp and the seed have been reported to be potentially rich in minerals and other bioactive nutrients [1].The use ofAduwais very wide,some use it as food folder for domestic animal like donkeys,goat and camels.Reports have also indicated that it used in local medicine to treat jaundice,intestinal worms,and wound healing.According to [2]Aduwafruit is used to treat liver disease,also as a purgative.The fleshy mesocarp is eaten by children as a confectionary mint.The leaf extract and saponin isolated from the kernel cakes have been reported to be anti-bactericidal [3,4],high larvicidal activity[5].Many flavanoids,saponins and other important phytochemicals have been reportedly found inAduwatree portion [1,6].When alcohol was used to extract its raw pulp and kernel,the raw pulp and kernel were found to contain sterols,terpenes,saponins,tannins,alkaloids and resins [7].The oil obtained fromAduwaseed has been used especially in Northern Nigeria to substitute for groundnut oil which is relatively expensive [1,8].According to Obidah et al [8],Balanites aegyptiacaoil is used to fry food and also add flavor to food and tea in addition to their medicinal use in the treatment of skin diseases as well as rheumatism.Roasted pre-treated seed has the best physicochemical,biochemical,haematological,carcase,and growth performance with better anti-nutritional properties [1,9],however previous work hinged on only the roasted seed meals and not defatted meals,protein concentrate and protein isolate.With the wide knowledge ofBalanites aegyptiaca,there are limited literature on the effect of toasting on the phytochemical,Anti nutrients,in vitro-protein digestibility and functional properties ofBalanites aegyptiaca.(Aduwa)seed protein meals,protein concentrate,and isolates which could have extended application at home,food and pharmaceutical industries.This work aims to explore un-resolved meal produced and resolved meals such as defatted meal,protein concentrate and isolate from toasted seed ofBalanites aegyptiacato ascertainBalanites aegyptiaca.wider scope for its functional and nutraceutical applications.
Materials and Methods
Material
Two kilograms (2 kg) of mature (B.aegyptiacaL) fruits used for this study were bought from Gashua market in Yobe State of Nigeria.They were transported immediately to the biochemistry laboratory of the Federal University Gashua .Two kilogramms (2 kg) of cracked seed kernels ofBalanites aegyptiacaseeds were weighted using a weigh balance and moisture value ascertained at 15% using moisture prop(E20 USA Model 121).The weighted sample were given to toasting treatment under dry heat at 70°C for 30 minutes and was allowed to cool.
Seed processing and meal making
The seed kernels were subjected to toasting pre-treatment before milling;oil extraction and meal caking.The cakes from the seed milled flours were mechanically expelled using a centrifugal screw which is semi-automated for meal making and oil expelled from the entrapped increase surface area of the kernel at 60 revolutions per minute Ogori et al[9].
The cakes made were dried to 10% MC using the solar cabinet dryer and well packaged and transported to the Federal University of Agriculture,Makurdi,Food Chemistry and Cereal laboratories for analysis.
Preparation of defatted Aduwa protein meal(DAPM)
Preparations of DefattedBalanites aegyptiacaprotein meal were prepared from toasted seed made from meal samples.The method of Sathe [10] as modified by Gbadamosi et al [11] was used to defatBalanites aegyptiacameal.The flour sample was defatted with cold(4°C) acetone using a flour to solvent ratio of 1:5 w/v.The mixture was stirred over a magnetic stirred for 4 h.The slurry was then filtered through a Whatman No.1 filter paper.The residue was reextracted twice in a similar fashion.The defatted flour was desolventized by drying in a fume hood at room temperature,and the dried flour was finally ground in a blender to obtain homogeneous defattedBalanites aegyptiacaflour and stored in an air-tight plastic bottle for use.
Preparation of Aduwa Protein Isolate (API)
AduwaProtein Isolate (API) was produced from DAMF according to the method of Tang et al [12],with slight modifications (Girgih et al.2011a).DAMF was dispersed in deionized water (1:20,w/v) and the dispersion was adjusted to pH 10.0 using 2 M NaOH while stirring for 2 h at 37°C.The mixture was centrifuged at 10,000xg,for 30 min at 10°C,the supernatant adjusted to pH 5.0 by adding 2M HCl and then centrifuged at 10,000xg for 20 min at 10°C.The resulting precipitate was dispersed in water,adjusted to pH 7.0 using 2M NaOH and freeze-dried to obtain the protein isolate.
Methods
Determination of Phytochemical properties of Aduwa protein meals,concentrates,and isolates
Determination of alkaloids

Alkaloids was quantitatively estimated using the method of Harborne[13].Five grams of the sample was extracted in 200 mL of 10% acetic acid in ethanol in a 250 mL beaker.Samples were incubated for 4 h at room temperature,then filtered,and the filtrate concentrated on a water bath to one-quarter of the original volume.The extracts were then precipitated by the addition of drops of concentrated ammonium hydroxide and allowed to settle.The precipitates were washed with dilute ammonium hydroxide and then filtered.The residue comprised of the alkaloid,was dried and weighed.The alkaloid content was determined using the formula:
Total Phenolic Content (TPC)
Total phenolic content of the sample was determined according to the Folin-Ciocalteau method of Roghini and Vijayalakshmi[14].The sample was extracted by making a suspension of 500 mg of the sample and garlic acid in 10 mL of distilled water.The sample was vortex every 10 minutes for 1 H.The mixture was centrifuged to obtain the extract.Briefly,0.1 mL of the extract was diluted with 1.9 mL of distilled water and 1 mL of Folin-Ciocalteau’s reagent (which has been previously diluted 10 folds) added.The mixture was incubated for 5 min and 1 mL of 7% sodium carbonate was added.The reaction mixture was incubated for 2 h in the dark and the absorbance of the mixture was measured at 765 nm.Similarly the blank was prepared in the following manner but without the samples.The standard garlic extract was prepared in different concentrations (100,200,400,600,800 and 1000 µg/mL) to obtain the calibration curve and the total phenolic content of the sample was extrapolated from the standard curve and expressed as mg of garlic equivalents per gram of extract.
Determination of saponin
The saponin content was determined according to the method of Obadoni and Ochuko [15].One gram of the sample was mixed with 10 mL of 20% ethanol.The sample was heated over hot water for 1 h,with continuous stirring at 55°C.The mixture was filtered and the residue reextracted with another 20 mL of 20% ethyl alcohol.The combined extracts were reduced to 10 mL over a water bath at about 90°C.The concentrate is then transferred into a 250 mL separating funnel and 10 mL of diethyl ether is added to the extract and vigorously shaken.The aqueous layer is recovered while the diethyl ether layer is discarded.12 mL of n-butanol were added and the combined n-butanol extract was washed twice with 10 mL of 5%sodium chloride.The remaining solutions were then heated in a water bath and after evaporation;the samples were dried in the oven to a constant weight and values were expressed as mg/g of extract.
Determination of Total Flavonoid Content
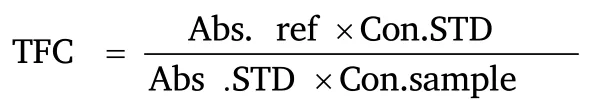
The total flavonoid content of the sample was determined using a modified method reported by Meda et al [16].0.5 mL of diluted samples were mixed with 500 µL methanol,50 µL of 10% AlCl3,50 µL of 1 M potassium acetate,and 1.4 mL distilled H2O,this was allowed to incubate at room temperature for 30 min.Thereafter,the absorbances of the reaction mixtures were subsequently measured at 415 nm.Quercetin was used a standard flavonoid,and the total flavonoid content was calculated as quercetin equivalent.
Determination of Tannins Content (TC)
Tannins were determined by the method of Peri and Pompei [17] and described by Roghini and Vijayalakshmi [14].1 mL of the sample extract of concentration 1mg/mL in distilled water was taken in a test tube and 1 mL of water served as the blank.To this,0.5 mL of Folin’s phenol reagent (1:2) followed by 5 mL of 35% sodium carbonate were added and kept at room temperature for 5 min.Blue colour was formed and the colour intensity was read at 640 nm.A standard graph(catechin 0.1 to 1 mg/mL) was plotted and from which the tannin content of the extract was determined.The total tannin content was expressed in mg/g of extract.See the graph in Figure 5.
Calculation:
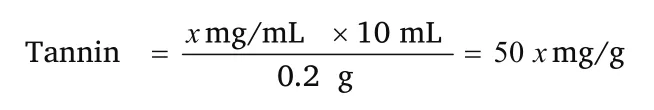
Wherex-value obtained from standard catechin graph Preparation of trypsin solution 0.5 mg/mL in 0.001 N HCl
Phytate Determination
Five (5 mL) of the sample was mixed and cured for 5 h and filtered.Aliquot of 2500 mL of the filtrate in a conical flask was added to 5 mL of 0.30% ammonium thiocynate.The mixture was titrated with standard iron (III) chloride solution to a persistent brownish yellow coloration that persisted for 4 min.The amounts of phytates were calculated with the equation below [18]
Phytic acid=Titre value×0.00195×1.9×100
Determination of oxalate
Oxalate will be determined by the method of Oke [19].Four grams (4 g) of the sample will be weighed in triplicate into 250 mL conical flasks and will be extracted with 190 mL distilled water and 10 mL 6M HCl.The suspensions were placed in boiling water for 2 H and filtered and made up to 250 mL with water in a volumetric flask.To a 50 mL aliquots,10 mL 6 M HCl was added and filtered and the precipitates were washed with 10 mL of hot water.The filtrate and wash water will be combined and titrated with concentrated NH4OH until the salmon pink colour of the methyl red indicator changed to faint yellow.The solution was heated to 90°C and 10 mL 5 % (w/v) CaCl2solution will be added to precipitate the oxalate overnight.The precipitate was washed free of calcium with distilled water and then washed into a 100 mL conical flask with 10 mL hot 25% (v/v) H2SO4and then with 15 mL distilled water.The final solution was heated to 90 °C and titrated against a standard 0.05 M KMnO4until a faint purple solution persisted for 30 s.The oxalate was calculated as the sodium oxalate equivalent as shown in equation 1 mL of 0.05 M KMnO4=2 mg sodium oxalate equivalent/g of sample)
In-vitro protein digestibility (IVPD)
In-vitroprotein digestibility of the samples was measured according to the method described by Chavan et al [20].Two hundred and fifty milligrams of the sample were suspended in 15 mL of 0.1 M HCl containing 1.5 mg pepsin,followed by gentle shaking for 1 h at room temperature.The resultant suspension was neutralized with 0.5 M NaOH and treated with 4.0 mg pancreatin in 7.5 mL of phosphate buffer (0.2 M,pH 8.0).The mixture was shaken for 2 h at room temperature.The mixture was then filtered using Whatman No 1 filter paper and the residue washed with distilled water,air-dried,and used for protein determination using Kjeldahl method of protein determination [21].
Protein Determination by Kjeldahl Method
The protein content of the residue of the residue was determined using the AOAC [21] method.The digested residue was weighed into a Kjeldahl flask.Ten millilitres of concentrated sulphuric acid were added followed by one Kjeltec tablet.The mixture was digested with a heating racket to obtain a clear solution.The digestate was cooled,and made up to 75 mL with distilled water and transferred into kjeldahl distillation unit followed by the addition of 50 mL of 40%sodium hydroxide solution.The mixtures were then distilled and the ammonia formed in the mixture is subsequently distilled into 25 mL 2% boric acid solution containing 0.5 mL of the mixture of 100 mL of bromocresol green solution (prepared by dissolving 100 mg of bromocresol green in 100 mL of methanol) and 70 mL of methyl red solution (prepared by dissolving 100 mg of methyl red in 100 mL methanol) as indicators.The distillate collected was titrated with 0.05 M HCl.Blank determination was carried out by excluding the sample from the above procedure
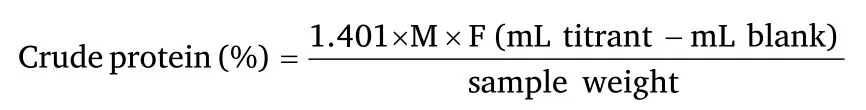
Where:
M=Molarity ofacid=0.05
F=kieldahl factor=6.25
Then,the protein digestibility was calculated using the equation below

Functional properties of Aduwa protein meals,concentrates and isolates
Bulk densityFive(5)grams of flour sample was poured into a 100 mL measuring cylinder.The cylinder was tapped continuously until a constant volume was obtained.The bulk density (g/cm3) was calculated as the weight of flour (g) divided by the volume of flour(cm3) [22].
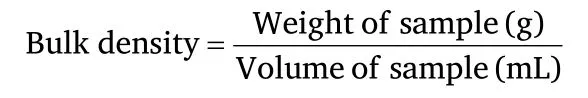
Least gelation concentration (LGC)LGC was determined according to the method of [23] with slight modification.I gram sample was suspended in water at different concentrations (2% to 20%,w/v,protein weight basis).The mixture was vortexes and placed in a water bath at 95°C for 1 h,cooled rapidly under tap water,and left in the refrigerator (4°C) for 2-14 h.The sample concentration at which the gel did not slip when the tube was inverted was taken as the LGC.
Water Absorption Capacity (WAC)The WAC was determined using the method of [23] with slight modifications.Protein sample of (1 g)was dispersed in 10 mL distilled water in a 15 mL preweighed centrifuge tube.The dispersions were vortexed for 1 min,allowed to stand for 30 min,and then centrifuged at 4000xg for 25 min at room temperature.The supernatant was decanted,excess water in the upper phase was drained for 15 min,and tube containing the protein residue were weighed again to determine the amount of water retained per gram of sample.
Oil Absorption Capacity (OAC)The OAC was determined using the method of Adebiyi and Aluko [23] with slight modifications.Protein sample (1 g) was dispersed in 10 mL pure canola oil in a 15 mL preweighed centrifuge tube.The dispersions were vortexed for 1 min,allowed to stand for 30 min,and then centrifuged at 7000xg for 25 min at room temperature.The supernatant was decanted,the excess oil in the upper phase drained for 15 min,and tube containing the protein residue was weighed again to determine the amount of water or oil retained per gram of sample
Swelling capacitySwelling index was determined according to the method of Takashi and Sieb [13],One (1) grams of the sample (W1)was poured into preweighted centrifuged tubes and 10 mL of distilled water added and mixed gently using a glass stirring rod.The slurries formed were heated separately at a constant temperature of (60,70,80,90 and 100°C) in a water bath for 15 minutes.During the heating process continues,gentle stirring is done to prevent lumping.At the end of the use of 15 minutes,the tubes were centrifuged at 4000xg for 10 minutes.The supernatant was discarded immediately and the tubes dried at 50°C for 30 minutes,cooled and then weighed W3.Centrifuge containing sample before adding distilled water is weighted as(W2).
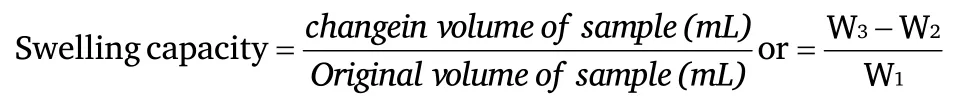
W3=weight of tube+sample after centrifuge and drying
W2=weight of tube +Sample before centrifuging
W1=weight of sample
Determination of Percentage DispersabilityPercentage dispersability was determined by using the method described by [24].10 g of the samples were weighted into a 100 mL measuring cylinders and water added to make up to 100 mL.The set-up was stirred vigorously and allowed to stand for three hours.The volumes of settled particles were taken and subtracted from 100.Dispersability (%)=100- Volume of settled particle
Results and Discussion
Phytochemical Properties of (Balanites aegyptiaca) Aduwa protein meals,protein concentrate and isolate
phytochemical properties of (Balanites aegyptiaca)Aduwaprotein meal,defatted meal,protein concentrate,and isolates are shown in Figure 1.The amounts of Alkaloids inBalanites aegyptiacaprotein meal APM (2.70 mg/g) and defatted meal DAPM (3.2 mg/g) were significantly higher at (P<0.05) than the amount present in protein concentrate APC (1.5%) and isolate API (0.7 mg/g).These values obtained from APM,DAPM and APC were higher than the values reported by Ogori et al[9] on balanite aeqyptiaca (Aduwa) meal flour when given the following pretreatments;boiled (1.06 mg/g),roasted(1.01 mg/g) and soaked (0.65 mg/g) respectively.These variations in the understudy might be due to the treatments employed.Alkaloid is an antimicrobial bio actives [9] however,the alkaloid is reduced inAduwaprotein meal (APM) and resolvedAduwaprotein isolate(API)having the lowest amount of alkaloid(0.7 mg/g) respectively.
Phenols are conjugated bioactive materials,but vary depending on exposed hydroxyl sites in food systems [9].Report by [25] revealed that in human nutrition,phenolic compounds have been reported to release wide range of biological effects like anti-bacterial,anti-inflammatory and antioxidant properties.The phenolic content in this study decreased significantly as protein meal material were resolved from APM (2.2 mg GAE/g) to DAPM (0.7 mg GAE/g),APC(0.31 mg GAE/g)and API(0.20 mg GAE/g)respectively.The obtained results under this study were lower than the values reported by Ogori et al[9]for soakedBalanites aegyptiacameal flour(2.8 mg/100 g)and 4 mg/100 g in roasted samples.These indicates that pre-treatment did affect phenolic profile.Phenolic content have been reported to have antioxidant potentials and α-amylase and α–glycosidase enzymes inhibitory and binding abilities [25].
The work of Ogori et al [9] on the assessment of phytochemical and functional properties of pretreated (Balanites aegyptiaca) seed meal flour revealed saponin contents of raw,roasted,soaked and boiled seed meal flours as 8.63 mg/100 g,5.81 mg/100 g,5.96 mg/100 g,and 7.07 mg/100 g respectively,thus higher than the toasted(Balanites aegyptiaca)Aduwaprotein meals,APM (0.14 mg/g),DAPM(0.09 mg/g),APC 0.04 mg/g) and API (0.02 mg/g) saponin contents respectively under this study.According to Okwu [27] saponin in biosystems confers coagulating,precipitating abilities and as well as bitterness in fluidly systems,This revealed that saponin contents in defatted meal (0.09 mg/g)Balanites aegyptiacaprotein concentrate(0.04 mg/g) andAduwaprotein isolates (0.02 mg/g) were reduced relative toBalanites aegyptiacaprotein meals (0.14 mg/g).
Tannin confers bitterness and inhibitory potential in food systems,hence reduces palatability as well as certain micro-nutrient absorptions.Tannin are known to alter digestive tract and their metabolites are known to be toxic [28].Tannin content under this study reduced significantly at (P<0.05) as material samples were resolved fromBalanites aegyptiacaprotein meal to BBalanites aegyptiacaprotein isolate (2.73 mgCAT/g-0.11 mgCAT/g) .The amount of tannin that can cause depression in man is quit unknown however the values obtained from tannin report on fruits by [29]were between 0.03 mg/100g-1.72 mg/100g which were within APC and API tannin values under this study.The reduction in anti-nutrient factors of tannin in resolvedAduwamaterial samples were comparable to the values reported forAduwa(Balanites aegyptiaca) seed flour (2.0 mg/100 g) and guava(0.00 mg/100g) [9,30,31].
Flavonoid in food system has been attributed to antioxidant and microbial inhibitory potentials [30].Flavonoid content in the samples reduced significantly as APM sample is been resolved to protein concentrate and protein isolate.High flavonoid content were observed in APM (15.95 mg/g),but low in API sample (9.95 mg/g).However there existed no significant differences at(P<0.05) with DAPM (9.45 mg/g).The amounts of flavonoids reported by [30] in soaked and boiledAduwa(Balanites aegyptiaca) cake samples were (13.40 mg/100 g) and (12.40 mg/100g) respectively,but differ from the values under study for APM (15.95 mg/g) DAPM (9.45 mg/g),APC (5.79 mg/g)and API(9.95 mg/g).Anti-oxidant capacity is an index of the scavenging compounds like flavonoids,therefore it could be concluded that APM and API samples have the higher total antioxidant competency.

Figure 1 Phytochemical composition of (Balanites aegyptiaca) Aduwa protein meals,protein concentrate and protein isolate
Anti Nutrient composition of (Balanites aegyptiaca) Aduwa protein meals,protein concentrate and isolate
Anti Nutrient composition of (Balanites aegyptiaca)Aduwaprotein meals,protein concentrate and isolate are presented in Figure 2.Oxalate,anti-nutrient moiety that causes intestinal hyperoxaturia and in turn increases development of kidney stones and thus interfere with a lot of metabolic processes.The level of occurrence of oxalate as an anti-nutritional factor in the sample under study is very low.However,samples APM (5.00 mg/g) and (DAM 3.50 mg/g) showed no significant difference (P<0.05) but varied when compared to APC(1.80 mg/g) and (API 1.60 mg/g) samples .The values between 3-5 g has been peg by [32] to be a lethal level [29,33].There was a significant decrease at (P<0.05) in oxalate content as material samples were been resolved to protein concentrate and protein isolate.These values were within safety bench mark by [33].Implying that the use of acid and base precipitations had reduced effect on anti-nutritional factors.
Phytate in food system chelates divalent cationic like calcium ion,magnesium and zinc ions and also inhibit enzyme activity [29].The phytate value under this study reduced significantly at (P<0.05) as the samples were been resolved to protein concentrate and protein isolate .Phytate content of APM (5.24 mg/g) and DAPM (1.94 mg/g)were low compared to APC (0.55 mg/g) and API (0.20 mg/g).These values are lower than the values reported for chicken peas (601.80 to 709.74 mg/100 g) in soaked varieties of chicken pea [34,35].The toasting pre-treatment process,oil expulsion mechanism fromAduwaseed meal and solar drying to reduced moisture content to (10%) has been attributed to the reduction in phytate content [29].It has been reported that phytic acid intake of 4-9 mg/100 g is said to reduce iron absorption by 4 to 5 folds in human [33].According to Wang et al[29],leaching out or migration of phyate ions depends on the concentration gradient,which governs the kinetics of phytate reduction and this has been observed in cowpea by [36],faba bean[37],mung beans [38],dry beans [39].
In-vitro protein digestibility of (Balanites aegyptiaca) Aduwa protein meals,Protein concentrate and isolate
In-vitro protein digestibility of (Balanites aegyptiaca)Aduwaprotein meals,Protein concentrate,and isolate are shown in Figure 3.The in vitro protein digestibly increased significantly at (P<0.05) asAduwaprotein meals were resolved and API sample having the highest percentage in vitro protein digestibility,API (76.41%).APM (59.81%),DAPM (46.43 %) and APC (40.54%),respectively.The high value of protein digestibility observed in APM and API may be due to their native and resolved proteins,within their biomolecules.This value agreed with the (73.69-84.35%) ranged pressured cooked chicken peas reported by [34].The increase in in vitro protein digestibility experienced in API samples may be due to the reduction in the levels of anti-nutritional factors like polyphenols and phytic acid [40,41].These anti-nutrients are known to inhibit α-amylase activities [26].The observed decrease in values in DAM and APC could be due to the processing approach employed during defatting of the sample and acid–base precipitation.The observed values for the sample under study were similar with values of (63%-77%) range by [34] for in vitro protein digestibility of chicken peas under the process treatment of soaking,dehulling and pressure cooking.According to [42]ant-nutritional factors and processing approaches when employed could influence protein digestibility.Figure 3.

Figure 2 Anti Nutrient composition of (Balanites aegyptiaca) Aduwa protein meals,protein concentrate and isolate APM,Aduwa protein meal;DAPM,defattaed aduwa protein meal;APC,Aduwa;protein concentrate;API,Aduwa protein isolate
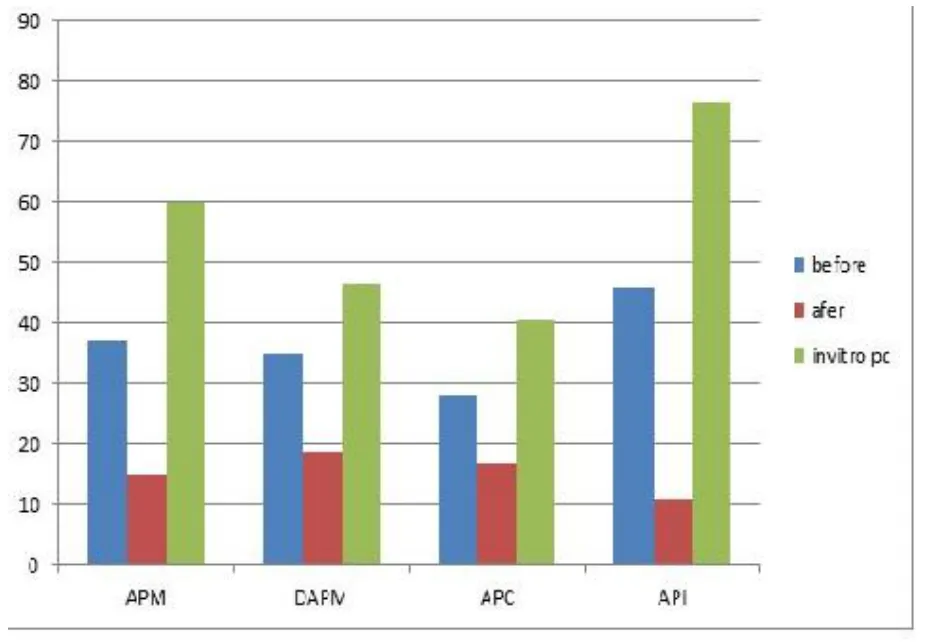
Figure 3 Invitro protein digestibility of (Balanites aegyptiaca) Aduwa protein meals,protein concentrate and protein isolate APM,Aduwa protein meal,DAPM,defattaed aduwa protein meal,APC,Aduwa protein concentrate;API,Aduwa protein isolate
Functional properties of Aduwa(Balanaites aegyptiaca)seed meal,deffated meal,Protein concentrate and isolate
The Bulk density,WAC,OAC,LGC,and Dispersibility of (Balanaites aeqyptiaca.l)Aduwaseed meal,deffated meal,protein concentrate and isolates water absorption capacity and oil absorption capacity are shown in Figure 4.Bulk density is applied to packaging.The APM sample had high bulk density significantly compared to DAPM and API samples.APM and API samples had good packaging properties which is followed by DAM (0.78%) hence good weight and space relationship.This observation could be due to the many air spaces created.
The ability of protein material micelles to hold water molecules depends on the conformational position of the protein material,size and shape [20].According to [43] this behaviour is attributed to the hydrophilic and hydrophobic balance of the residual amino acid in the material,polar amino acid,ionic strength and lipids.WAC in APM(9.66 g/g) is significantly (P>0.05) higher compared to other samples under study.This increase was followed significantly by API and DAM samples and APC had the least WAC content.The values obtained on WAC for APM and API(9.32-12) g/g were lower than that reported by [43] on hemp meal and isolate.However,the present values were higher than the value reported by [44] which was 2.7 g/100g and 3.4g/100 g by [13].This variation observed inAduwaprotein meal samples may be attributed to the extraction method and solar drying approach employed and lipid interaction [45].The OAC increased significantly(P>0.05) from sample AM(6.94) g/g through DAPM to APC samples.Protein isolate in sample API (5.56) g/g had the least value at (P<0.05).The value of APC sample agreed with value obtained by [47] on protein concentrate fromAduwaseed.However,the value did not agree with the value obtained from peas,chicken peas and lentils concentrates at these range (1.10-2.3 g/g).According to [47],walnut protein concentrate (2.50)g/g).According to [48],this may suggest thatAduwaprotein samples has good nonpolar amino acids,greater surface area of macro molecules,charges,hydrophobicity and better extraction method employed.The least gelation concentration of DAM is significantly higher than APC.The least gelation concentration value in APM (12%) is not significantly different atP>0.05% with API sample.The least gelation concentration values from APM,DAPM,APC and API obtained from this study were similar with the values obtained by[43,49] on hemp seed meal and protein isolate .The APM and APC values were far favoured than 14% for soybean protein isolate reported by[23].These protein sample materials can form an easy 3-dimensional structural material for food application.The low least gelation concentration in APM and API could be due to protein aggregation,although APM was not precipitated but underwent heat annealing during oil expulsion.The aggregation of protein at the isoelectric point could have reduced the sample protein flexibility to form a mesh networks [43].The ability of the material samples to disperse easily in solution decreased significantly.APM (72%) had the highest value followed by DAPM,APC and API with the least dispersibility index.The constitution and reconstitution ability of API in aqueous medium were low compared to APM and DAPM,however APM and DAPM were not resolved via acid or base precipitation.
Swelling capacity of (Balanites aegyptiaca) Aduwa protein meals,protein concentrate and isolate
Swelling capacity of (Balanites aegyptiaca)Aduwaprotein meals,protein concentrate,and isolate are shown in Figure 5.The APC and APM samples were observed to swell gradually as the swelling temperature increases (60-90°C).This was also observed in DAPM and API but sharply fell at 80°C temperature rise.These changes could be attributed to material leaching during heating.TheBalanites aegyptiacaprotein concentrate APC and meal APM possibly had more leachable biomaterials compared toBalanites aegyptiacadefatted meal DAPM and protein isolate API.The swelling capacity of food material confers the ability to form bulkiness when incorporated into the food system.The APM and APC samples were significantly high in swelling capacity indicating that they could be better preferred for fillers and extenders in food systems,hence better samples for analogues and API preferred for enrichment purposes.
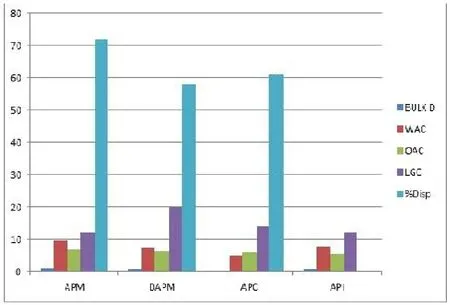
Figure 4 Functional properties of(Balanites aegyptiaca) Aduwa protein meals,protein concentrate and isolateAPM,Aduwa protein meal;DAM,defattaed aduwa meal;APC,Aduwa protein concentrate;API,Aduwa protein isolate
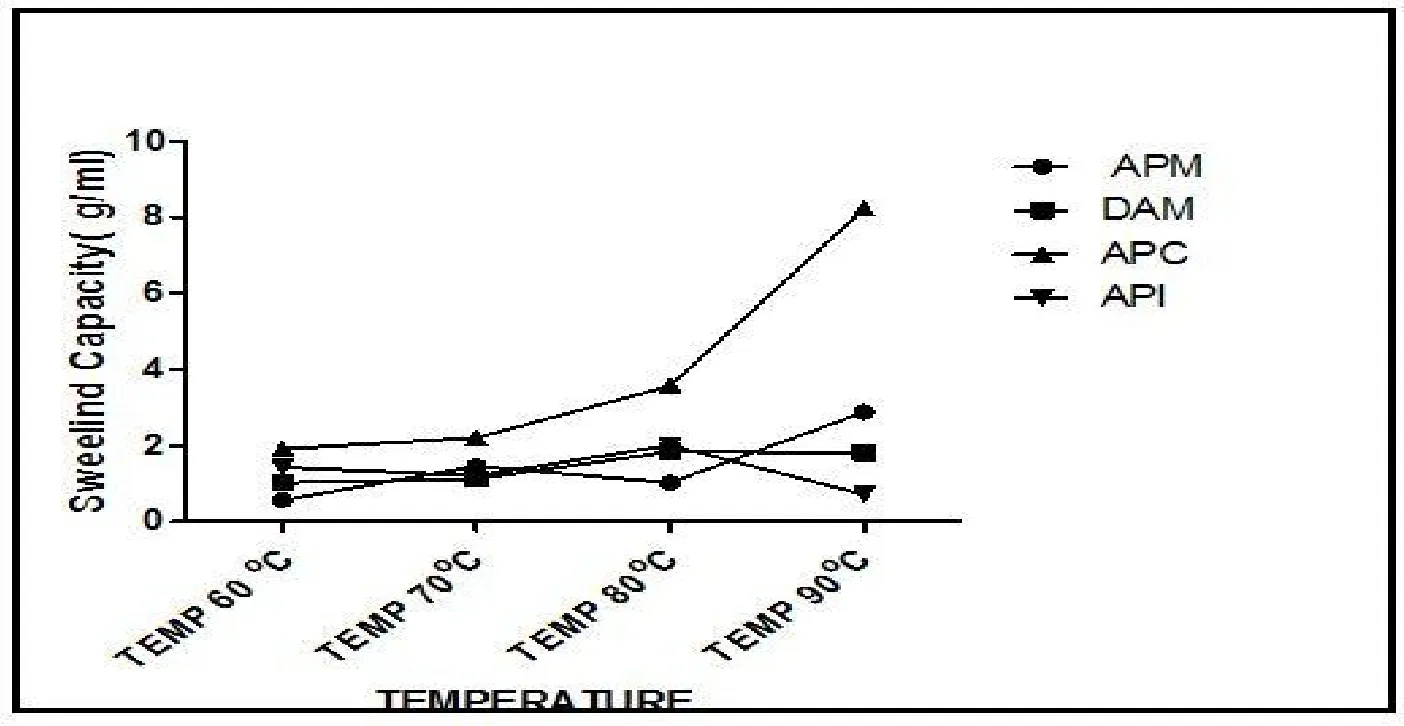
Figure 5 Swelling capacity of(Balanites aegyptiaca) Aduwa protein meals,protein concentrate and isolate.APM,Aduwa protein meal;DAM-DAPM,defattaed aduwa protein meal,APC,Aduwa protein concentrate;API,Aduwa protein isolate
Conclusion
The study revealed that (Balanites aegyptiaca)AduwaProtein Meals,Protein Concentrate,and Isolate could be an alternative food ingredients for protein supplementation when compared to some other oil seeds and nut meals.The low anti-nutrient contents observed in APC and API samples showed their reliability as resolved meal material (bio-safe) as a good source of functional meal to enrich children and adults foods.The phytochemical,protein digestibility,and functional properties of the samples indicated that it is edible and functionally good to enrich starch and other non-protein based foods.
