麻醉和手术导致老年小鼠脑内能量代谢紊乱与mTOR信号通路的关系
2022-04-28路建陶小燕张宏利周红梅高苏楠
路建 陶小燕 张宏利 周红梅 高苏楠

[摘要] 目的 評价麻醉和手术导致老年小鼠脑内能量代谢紊乱与雷帕霉素靶蛋白(mTOR)信号通路的关系。 方法 选取健康雄性C57BL/6小鼠64只,18月龄,随机分为两组(n=32):对照组(C组)和麻醉手术组(A/S组)。于术后第3天进行Morris水迷宫测试;于术后第1和第3天时取海马组织,采用Western blot法检测GLUT1、mTOR和磷酸化mTOR(p-mTOR)的水平,计算p-mTOR/mTOR比值;采用ATP试剂盒检测海马ATP浓度;于术后第1天和第3天时取脑组织进行切片、免疫荧光染色,观察海马GLUT1和p-mTOR表达情况。 结果 两组小鼠潜伏期随时间变化呈降低趋势,其中A/S组小鼠在术后第6天潜伏期高于C组(P<0.01),两组小鼠潜伏期不存在时间和组间的交互作用(P>0.05)。术后第7天A/S组穿越平台次数和平台象限游泳时间均低于C组(P<0.01)。与C组比较,A/S组术后1、3 d时海马GLUT1表达减弱,p-mTOR表达增强(P<0.01)。与C组比较,A/S组术后1、3 d时海马GLUT1水平降低,p-mTOR/mTOR比值升高(P<0.01)。A/S组术后1 d和3 d时海马ATP水平低于C组(P<0.01)。 结论 麻醉手术导致老年小鼠脑内能量代谢紊乱的机制可能与mTOR信号通路活化有关。
[关键词] 葡萄糖转运蛋白1;麻醉手术;老年;认知障碍
[中图分类号] R741 [文献标识码] A [文章编号] 1673-9701(2022)09-0034-04
Relationship between energy metabolism disorders and mammalian target of rapamycin (mTOR) signaling pathway in elderly mice induced by anesthesia and surgery
LU Jian1 TAO Xiaoyan2 ZHANG Hongli1 ZHOU Hongmei1 GAO Su′nan1
1.Department of Anesthesiology, the Second Affiliated Hospital of Jiaxing University, Jiaxing 314000,China;2.Ward 29, the Second Affiliated Hospital of Jiaxing University, Jiaxing 314000,China
[Abstract] Objective To evaluate the relationship between energy metabolism disorders and mammalian target of rapamycin (mTOR) signaling pathway in elderly mice induced by anesthesia and surgery. Methods Sixty-four healthy male C57BL/6 mice aged 18 months were randomly divided into two groups (n=32),the control group (group the C) and the operation with anesthesia group (group the A/S). The Morris Water Maze test was performed on postoperative day 3.The levels of GLUT1, mTOR and phosphorylated mTOR (p-mTOR) were detected by Western blot, and the p-mTOR/mTOR ratio was calculated. The ATP concentration in hippocampus was detected by the ATP kit. The brain tissue was sectioned and stained with immunofluorescence on days 1 and 3 after operation. The expressions of GLUT1 and p-mTOR in hippocampus was observed. Results The incubation period of mice in the two groups decreased with the change of time, and the incubation period of mice in the A/S group was higher than that in the C group on the 6th day after operation (P<0.01),and no interaction existed between the two groups and each time points (P>0.05).The times of platform crossing and platform quadrant swimming time in the A/S group were lower than those in the C group on postoperative day 7 (P<0.01).Compared with the C group, the expressions of GLUT1 were decreased and the expressions of p-mTOR were increased in the A/S group at days 1 and 3 after operation (P<0.01).Compared with the C group, the levels of GLUT1 in hippocampus of the A/S group were decreased and the p-mTOR/mTOR ratios were increased at days 1 and 3 after operation (P<0.01). The levels of ATP in the hippocampus of the A/S group were lower than those of the C group at days 1 and 3 after operation (P<0.01). Conclusion The mechanism of energy metabolism disorder in the brain of elderly mice induced by operation with anesthesia may be related to the activation of mTOR signaling pathway.
[Key words] Glucose transporter 1 (GLUT1); Operation with anesthesia; Elderly; Cognitive disorder
术后认知功能障碍(postoperative cognitive dysfunction,POCD)是麻醉手术后中枢神经系统的常见并发症[1],可持续数天或数周,延长住院时间,增加住院花费。目前POCD具体的发病机制尚不清楚。围术期患者常出现血糖水平的波动,葡萄糖代谢紊乱与认知功能损害有关[2]。有学者认为大脑能量代谢紊乱是多种因素综合作用造成,并导致神经认知功能障碍[3],并且葡萄糖转运蛋白1(the glucose transporter 1,GLUT1)在维持脑内能量稳态中非常重要[4]。mTOR可以通过调控能量代谢等作用,参与调节突触可塑性、认知功能等[5]。麻醉手术导致脑内能量代谢紊乱的机制是否与mTOR信号通路有关尚待证明。本研究拟评价麻醉手术导致老年小鼠脑内能量代谢紊乱与mTOR信号通路活化的关系,现报道如下。
1 材料与方法
1.1 动物与分组
选取健康雄性C57BL/6小鼠64只,体重(35.6±2.5)g,18月龄,由湖南SJA实验动物公司提供[动物许可证号SYXK(鄂)2018-0101]。在实验前,小鼠适应新环境7 d。采用随机数字表法分为两组(n=32):对照组(C组)和麻醉手术组(A/S组)。对照组小鼠不做任何处理,麻醉手术组小鼠在异氟醚麻醉下行剖腹探查术。
1.2 动物造模
小鼠在异氟醚(麻醉诱导为4.0%和维持2.0%,鲁南贝特制药有限公司,国药准字H20020267,批号64191201)麻醉下行剖腹探查术[6]。首先行腹中线切开术(长度3 cm);其次,将无菌棉签浸入湿生理盐水中,依次探查肝、脾、肾、小肠等器官3 min,暴露3 min,整个过程需30 min,加温板保持温度在36~37 ℃之间。
1.3 Morris水迷宫实验
每组取12只小鼠,A/S组小鼠在术后休息2 d,第3天用水迷宫测试小鼠认知功能。定位航行试验测试4 d,然后进行空间探索试验1 d。
1.4 免疫荧光检测海马GLUT1和p-mTOR的表达
于术后第1天和第3天时,每组取4只小鼠,暴露心脏,灌注4%冷多聚甲醛。取脑、脱水、石蜡包埋。将切片与抗体结合,在4℃孵育过夜,洗涤。用共焦显微镜(Eclipse C1,尼康)获取图像。使用Image-Pro-Plus 6.0对荧光信号强度进行量化。
1.5 Western blot法检测海马GLUT1、p-mTOR和mTOR表达
于术后第1天和第3天时,每组取6只小鼠,取脑,分离海马。海马匀浆离心,取上清。转膜,加入抗体,曝光、扫描。采用Alpha Ease FC(Alpha Innotech)软件测定条带灰度值,以目的蛋白条带灰度值与β-actin条带灰度值的比值反映目的蛋白的表达水平。
1.6 ATP试剂盒检测海马ATP浓度
于术后第1天和第3天时,取海马组织,制备匀浆,离心取上清液。用ATP试剂盒(S0026B,Bwyotime)测定海马组织ATP浓度。
1.7 统计学方法
应用SPSS 20.0统计软件分析,正态分布的计量资料以均数±标准差(x±s)表示,不同时间点组间比较采用双因素方差分析,两组间比较采用t检验,P<0.05为差异有统计学意义。
2 结果
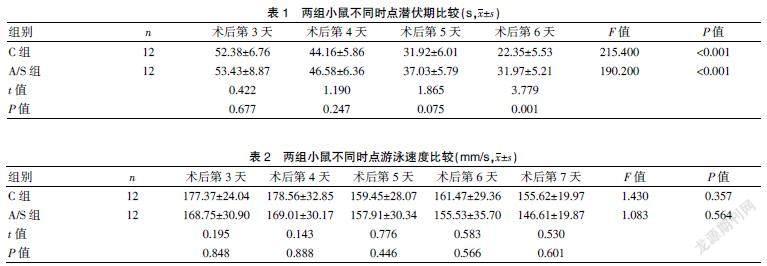
2.1 两组小鼠不同时点潜伏期和游泳速度比较
A/S组小鼠在术后第6天潜伏期高于对照组(P<0.01),两组小鼠潜伏期不存在时间和组间的交互作用(P>0.05);两组小鼠游泳速度比較,差异无统计学意义(P>0.05)。见表1~2。
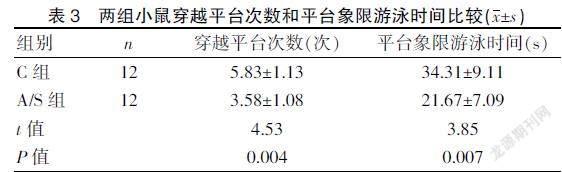
2.2 两组小鼠空间探索试验比较
A/S组小鼠穿越平台次数及平台象限游泳时间比C组降低(P<0.01)。见表3。
2.3 两组小鼠不同时点海马GLUT1和p-mTOR表达
GLUT1阳性细胞染色为红色,细胞核染色为蓝色;p-mTOR阳性细胞染色为绿色,细胞核染色为蓝色。C组海马GULT1高表达和p-mTOR低表达,A/S组术后第1天和第3天时海马GLUT1表达减弱,而p-mTOR表达增强。见封三图3~4。
2.4 两组小鼠不同时点海马GLUT1和p-mTOR免疫荧光定量比较
与C组比较,A/S组术后第1天、第3天时海马GLUT1表达减少,p-mTOR表达增加(P<0.01)。见表4。
2.5 两组小鼠不同时点海马GLUT1、ATP水平和p-mTOR/mTOR比较
与C组比较,A/S组术后第1天、第3天时海马GLUT1、ATP水平降低,p-mTOR/mTOR值升高(P<0.01)。见表5。
3 讨论
Morris水迷宫实验是测试小鼠认知功能的常用方法。逃避潜伏期反映小鼠空间学习能力,目标象限活动时间反映小鼠空间记忆能力。本研究结果表明,在第4天的定位航行试验中,A/S组小鼠潜伏期比C组小鼠明显延长,目标象限活动时间明显缩短,提示麻醉手术损害了老年小鼠的参考记忆能力。两组小鼠间游泳速度比较,差异无统计学意义(P>0.05),提示所有小鼠的运动能力没有差异。
在神经系统中能量的主要来源是葡萄糖[7]的有氧代谢,脑组织区域性葡萄糖代谢低下患轻度认知障碍的风险增加三倍[8]。大脑中的小血管疾病采用Fazekas评分量表进行量化,研究显示Fazekas评分与脑组织局部脑糖代谢呈负相关。脑糖代谢降低可能会导致AD患者的认知障碍[9]。提高大脑葡萄糖代谢水平,可以改善AD小鼠学习记忆能力[10]。而葡萄糖是极性分子,需要血脑屏障血管内皮细胞上的GLUT1的转运才能透过血脑屏障进入脑内。高血糖和低血糖显著影响人类的大脑健康,特别是认知功能。研究显示,脑微血管中GLUT1水平与脑葡萄糖摄取相关[11],且GLUT1表达减少加重AD的认知功能损害[12],提示GLUT1可能是治疗神经胶质细胞和神经元功能障碍的新靶点。本研究结果表明,麻醉手术创伤导致海马GLUT1蛋白表达下调,ATP水平下降。Sun等[13]研究显示,葡萄糖能减弱异氟醚诱导的ATP水平下降,提示葡萄糖可能减弱麻醉相关的神经毒性作用。上述研究结果提示,GLUT1可能是脑内葡萄糖代谢紊乱相关的神经认识功能障碍的重要治疗靶点。
mTOR信号通路在神经退行性疾病和术后认知功能障碍的发生中发挥重要作用[5,14]。最近mTOR被认为是AD早期的生物标志物,mTOR衰减可能会阻止AD的发生和发展[15]。因此,mTOR抑制剂可能具有治疗与认知功能减退性疾病的潜力。研究表明,mTOR过度活化引起磷酸化胰岛素受体[16],导致其内化引起胰岛素抵抗,影响葡萄糖的正常转运[17]。增加AD小鼠海马GLUT1表达,可改善脑糖代谢紊乱[18]。因此,mTOR水平的动态平衡是保持脑葡萄糖代谢正常的重要条件。本研究结果表明,麻醉手术后小鼠海马mTOR磷酸化水平升高,GLUT1和ATP水平下降,提示麻醉手术所致小鼠海马ATP水平下降的机制可能与mTOR信号通路过度活化有关。麻醉手术激活海马mTOR信号通路和抑制GLUT1表达的具体机制还需进一步研究。
综上所述,麻醉手术导致脑内能量代谢紊乱的机制可能与mTOR信号通路活化有关。
[参考文献]
[1] Olotu C.Postoperative neurocognitive disorders[J].Curr Opin Anaesthesiol,2020, 33(1):101-108.
[2] Díaz-Venegas C,Schneider DC,Myrskyl?覿 M,et al. Life expectancy with and without cognitive impairment by diabetes status among older Americans[J].PLoS One,2017, 12(12):e0190 488.
[3] Lin X,Chen Y,Zhang P,et al. The potential mechanism of postoperative cognitive dysfunction in older people[J].Exp Gerontol,2020,130:110 791.
[4] Koepsell H.Glucose transporters in brain in health and disease[J].Pflugers Arch,2020,472(9):1299-1343.
[5] Mueed Z,Mehta D,Rai PK,et al. Cross-interplay between osmolytes and mTOR in Alzheimer's disease pathogenesis[J]. Curr Pharm Des,2020,26(37):4699-4711.
[6] Qiu LL,Pan W,Luo D,et al. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca2+/calpain might contribute to postoperative cognitive dysfunction in aging mice[J]. J Neuroinflammation,2020,17(1):23.
[7] Butterfield DA,Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease[J].Nat Rev Neurosci,2019,20(3):148-160.
[8] Krell-Roesch J,Syrjanen JA,Vassilaki M,et al. Brain regional glucose metabolism,neuropsychiatric symptoms,and the risk of incident mild cognitive impairment:The mayo clinic study of aging[J].Am J Geriatr Psychiatry,2021, 29(2):179-191.
[9] No HJ,Yi HA,Won KS,et al. Association between white matter lesions and the cerebral glucose metabolism in patients with cognitive impairment[J]. Rev Esp Med Nucl Imagen Mol,2019,38(3):160-166.
[10] 曹瑾,唐銀杉,李昱颉,等.“通督启神”法电针治疗对APP/PS1双转基因小鼠脑葡萄糖代谢和学习记忆能力的影响[J].中华中医药,2016,31(5):1983-1987.
[11] Koepsell H. Glucose transporters in brain in health and dis- ease[J]. Pflugers Arch,2020,472(9):1299-1343.
[12] G?覬uchowska K,Pliszka M,Szablewski L. Expression of glu- cose transporters in human neurodegenerative diseases[J]. Biochem Biophys Res Commun,2021,540:8-15.
[13] Sun Y,Zhang Y,Cheng B,et al. Glucose may attenuate isoflurane-induced caspase-3 activation in H4 human neuroglioma cells[J].Anesth Analg,2014,119(6): 1373-1380.
[14] Gao S,Zhang S,Zhou H,et al. Role of mTOR-regulated autophagy in synaptic plasticity related proteins downregulation and the reference memory deficits induced by anesthesia/surgery in aged mice[J].Front Aging Neurosci,2021,13:628 541.
[15] Van Skike CE,Lin AL,Roberts Burbank R,et al. mTOR drives cerebrovascular, synaptic,and cognitive dysfunction in normative aging[J].Aging Cell,2020, 19(1):e13 057.
[16] Romanelli RJ,LeBeau AP,Fulmer CG,et al. Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt[J].J Biol Chem,2007,282(31):22 513-22 524.
[17] Wang J,Tong H,Wang X,et al. Tanshinone IIA alleviates the damage of neurocytes by targeting GLUT1 in ischaemia reperfusion model (in vivo and in vitro experiments)[J]. Folia Neuropathol,2020,58(2):176-193.
[18] Mana L,Feng H,Dong Y,et al. Effect of chinese herbal compound GAPT on the early brain glucose metabolism of APP/PS1 transgenic mice[J].Int J Immunopathol Pharmacol,2019,33:1-13.
[19] Geng J,Zhang Y,Li S,et al. Metabolomic profiling reveals that reprogramming of cerebral glucose metabolism is involved in ischemic preconditioning-induced neuroprotection in a rodent model of ischemic stroke[J].J Proteome Res,2019, 18(1):57-68.
[20] Vorhees CV,Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory[J]. Nat Protoc,2006,1(2):848-858.
(收稿日期:2021-03-09)
