Anxiety is a Risk Factor of AD at an Early Stage With Gender Differences in APP V717I Transgenic Mice*
2022-04-24TANYanWANGYaLeiPENGTianTianZHANGYaLiZHANGHuaWeiCHENWeiHangYANGKeZHANGJiaNiWANGXuWEIPengLIUZhaoHengYANGKaiYuLIUTongHuaHUAQian
TAN Yan,WANG Ya-Lei,PENG Tian-Tian,ZHANG Ya-Li,ZHANG Hua-Wei,CHEN Wei-Hang,YANG Ke,ZHANG Jia-Ni,WANG Xu,WEI Peng,LIU Zhao-Heng,YANG Kai-Yu,LIU Tong-Hua*,HUA Qian*
(1)School of Life Sciences,Beijing University of Chinese Medicine,Beijing 100029,China;2)School of Traditional Chinese Medicine,Beijing University of Chinese Medicine,Beijing 100029,China;3)School of Acupuncture-Moxibustion and Tuina,Beijing University of Chinese Medicine,Beijing 100029,China;4)Dalian The Fourth People’s Hospital,Dalian 210200,China)
Abstract Objective Alzheimer’s disease (AD) is the most common form of dementia. There are two main risk factors for developing AD—age and gender. By aging, preclinical and clinical symptoms are emerging in succession. Anxiety is one of the typical early symptoms during AD processing.Besides,the incidence of AD is higher in women than in men.However,although the differences were observed in the AD cohort, there is a lack of the assessment of AD experimental animal models within age and gender.Methods We choose the APP London mutation(Val717Ile)transgenic(Tg)mice for study.This is one of the first described mutations in APP, with the early onset of AD. To illustrate if the gender difference occurred in APP V717I mice during AD processing,we made use of animal behavior tests,such as open field test,step-down test and Morris water maze to evaluate the noncognitive symptom and cognitive symptom. Results In this study, we found that at 6-month age, female Tg mice showed significant anxiety, while male Tg mice only showed anxiety at 10-month age.At 6-month age, both male and female Tg mice did not show cognitive deficits; but, at 10-month age, both genders showed significant cognitive impairments. Conclusion These results indicated that APP V717I Tg mice showed anxiety-like activity before the occurrence of memory deficits; in the process of AD, APP V717I Tg mice have a clear difference in age and gender. This study provides favorable evidence for age and gender differences in the process of AD.The mouse model is also expected to become an essential tool for AD drug research,aiming to help precision medicine and distinguish gender differences in drug use.
Key words Alzheimer’s disease,anxiety,behavioral test,gender difference
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder leading to progressive decline in cognition. It accounts for 60%-80% of dementia cases (www. alz. org). Currently, over 46 million individuals are living with dementia worldwide,and this number is projected to increase to 131.5 million by 2050, accompanied by an enormous economic impact on society[1]. The main risk factors for AD are age and gender. The pathophysiological process of AD begins many years before the diagnosis of AD. In the cohort, epidemiological investigations pointed that anxiety and depression usually occurred before the occurrence of dementia[2]. Anxiety,characterized by enduring anticipation and permanent neurotoxic distress, is also a risk factor of dementia[3-5]. Besides, gender is crucial in the pathogenesis of AD. However, in the early stages of AD, whether there is a gender difference in anxiety has not been established in the animal model.Beginning at age 60-64 through the population over age 94, the incidence of AD is higher in women than in men; it is almost twofold increased risk in women versus men[6-8]. Besides family history, age, education,habits and other factors, the gender difference is a strong predictor of dementia.
However, although epidemiology has helped us map the age and gender to the disease course in AD,we have not discriminated the differences in AD treatments. In addition, some animal experiments did not distinguish genders[9-10]. In order to illustrate the age and gender differences of AD, we firstly focused on the London mutation of APP. This was one of the first described mutations in APP. It was initially identified in 1991 in an English kindred with earlyonset AD, identified as family F23. It appears to be one of the most common APP mutations worldwide,with approximately 30 families identified from various countries of origin, including the United States, England, Japan, Thailand, Germany, France,Italy, Australia, Belgium, Iran, and China. London mutation refers to the occurrence of valine (Val) -isoleucine (Ile) mutation at site 642 (calculated by APP 695) in the transmembrane region, which is only 4 amino acids away from the 42nd amino acid (γ secretase restriction site) of Aβ. The conformational change of the protein caused by the London mutation makes the APP protein more easily cleaved by γ secretase, leading to an increase in the production of Aβ through the amyloid metabolic pathway, and the abnormal aggregation of the increased Aβ leads to age spots and cognitive impairment[11-12].Therefore,in this study, we use the APP V717I London mutation mouse model to illustrate the age and gender difference during AD processing. This study will help researchers to extend the usage of choosing proper experimental animal models in AD research.
1 Materials and methods
1.1 Subjects and housing
We used APP V717I transgenic (Tg) mice of two different ages in this study, 6-month-old and 10-month-old. Each group contained 8-10 mice of both genders. The mice were group-housed for 2-4 per cage and allowed free access to food and drink.They were maintained on a 12 h light/dark cycle.Male and female mice were caged separately. The Animal Ethics Committee of Beijing University of Chinese Medicine approved all experimental procedures. The ethical approval number was No.BUCM-2016103101-1008.
1.2 Behavioral testing
There were three main behavioral tests used in this study. The test order was the open field test, the step-down test and the Morris water maze. There was a 3-5 days of interval for each experiment.
1.2.1 Open field test
The Plexiglas open field was 50 cm at the bottom, 50 cm at the top, and 50 cm in height, with a black wall and white bottom. Light dimly illuminated by a 60 W lamp placed above the box. Experiments were conducted in a sound-attenuated room. After each trial, the box was wiped with 75% ethanol to eliminate olfactory cues. A camera connected to the computer was used to record animal activities.
On the first day of training, the mouse freely moved and habituated the arena for 5 min.After 24 h of habitation, mice were submitted to a 5-min trial.The locomotor activity was recorded as the total distance within 5 min and average speed; the anxiety was concerned as time spent and moving distance in the center. The center area had a side of 25 cm in the middle of the arena.
1.2.2 Step-down test
The inhibitory avoidance apparatus was an acrylic box with length of 17 cm, width of 13.5 cm,and height of 32 cm, whose floor consisted of a grid of parallel copper network with a diameter of 0.3 cm and spacing of 1 cm. An insulated platform was placed in the center of the floor, 5 cm diameter and 5 cm high. The behavioral performance was recorded by infrared sensors and was transformed into a digital signal. After each mouse was removed, the box was cleaned with alcohol to avoid the smell of the later mouse.
a. Training test: the animal was put into the box for 3 min to habituate, then placed on the floor. An electric current of 36 V was delivered to the copper grid. The mice subjected to electric shocks would jump back to the platform to avoid noxious stimuli.The cut-off time was 300 s.The escape latency to step up to the platform with all four paws was measured.
b. Retention test: 24 h after the training test, the animal was first put on the central platform. No electricity was applied. The escape latency to step down to the floor with four paws was measured.
1.2.3 Morris water maze
The Morris water maze (MWM) is widely use to evaluate hippocampal-dependent spatial learning and memory.The pool was 100 cm diameter,with a 6 cmdiameter platform submerged 1 cm below the water surface.Water temperature is(22±1)℃.All trials were monitored by a video camera placed about 2 m above the center of the pool.The water was made opaque by the addition of non-toxic white paint. The pool was placed in a room surrounded by fixed spatial cues.
a. Habituation trial: each mouse received one habituation trial (1 trial/d) in the Morris water maze before the spatial training.
b. Spatial training: training on the hidden platform began within 24 h after the habituation trial.Hidden platform training was carried out over five consecutive days with three trials per day. The platform was set to the center of the target quadrant.During a given trial,the mouse was gently placed into the pool, at one of four quadrants randomly as start quadrant, and allowed 60 s to find the platform. If the animal did not find the platform within 60 s, it was placed on the platform for 10 s. After each trial, the mouse was patted gently with a towel to dry.Dependent measures acquired each trial are escape latency (in second), swimming distance (in centimeter).
c. Spatial memory testing: 24 h after the last trial of spatial training, a probe test was conducted in the same spatial training pool with the platform removed.All mice started the probe test from the opposite quadrant. The dependent measures were the percentage of escape latency and the percentage of dwelling time in the target quadrant.
1.3 Statistical analysis
Results are expressed asSEM. Student’st-test was used to compare two groups of Tg mice and their littermate WT control; one-way ANOVA was used for multiple groups in the same gender; two-way ANOVA was used for multiple groups of all groups.Statistical analysis was performed using GraphPad software. Statistical significance was defined asP<0.05.
2 Results
2.1 Locomotor activity reduced with aging, but no difference within genders at the same age
Open field test has been used to test locomotor activity to provide an overview of active situation of subjects. Average speed and distance have been evaluated in this test. For 6-month age, within both genders, there is no difference occurred in moving speed or total distance. For 10-month age, no difference happened among all groups (Figure 1). But with aging, both WT and Tg mice showed lower locomotor activity. The average speed is reduced by 57.92% and 47.02% in WT male and APP male comparing 10-month to 6-month age; WT female and APP female reduced by 32.08% and 35.21%,respectively. The moving distance is reduced by 68.65% and 62.61% in WT male and APP male comparing 10-month to 6-month age; WT female and APP female reduced by 45.77% and 51.57%,respectively.
2.2 Anxiety only appeared in female Tg mice at 6-month age,but was observed in Tg mice within both genders at 10-month age
In the open field test, anxiety has been evaluated in terms of moving distance and time spent in the center areas during AD processing.At 6-month of age,only female Tg mice showed evident anxious activity(Figure 2a, b). Interestingly, there is no difference between male and female WT mice, but female Tg mice showed more anxiety compared with male Tg mice (Figure 2a). At 10-month of age, both genders showed significant anxiety (Figure 2c, d).Furthermore, during the AD process, for the changes of center activity in mice from 6-month age to 10-month age, we found Tg male exhibited relatively fast processing (Table 1). In terms of moving distance and time spent in the center areas, Tg male mice reduced by 49.50% and 76.05%, respectively; while Tg female mice reduced by 52.47% and 62.34%,respectively.

Table 1 Changes of moving distance and time spent from 6-month to 10-month of age
2.3 Short-term memory was normal in both genders at 6-month of age, but impairment observed at 10-month age
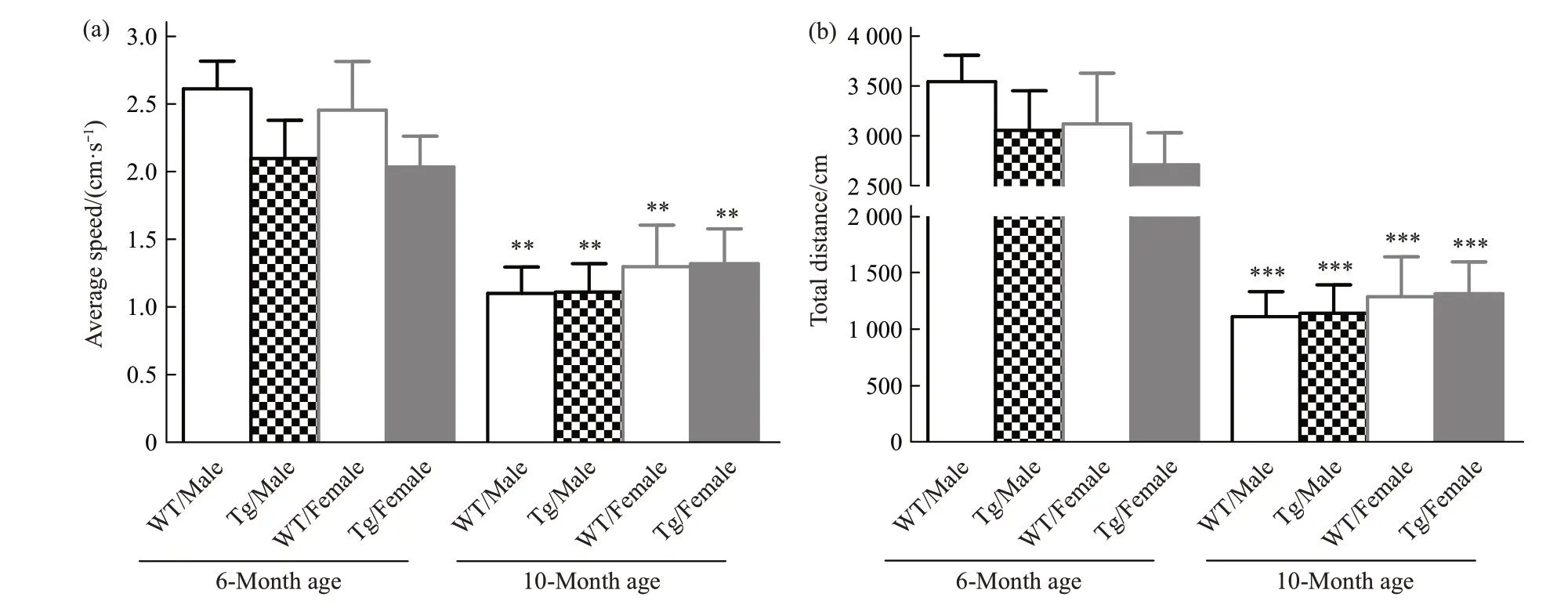
Fig.1 Locomotor activity in all groups
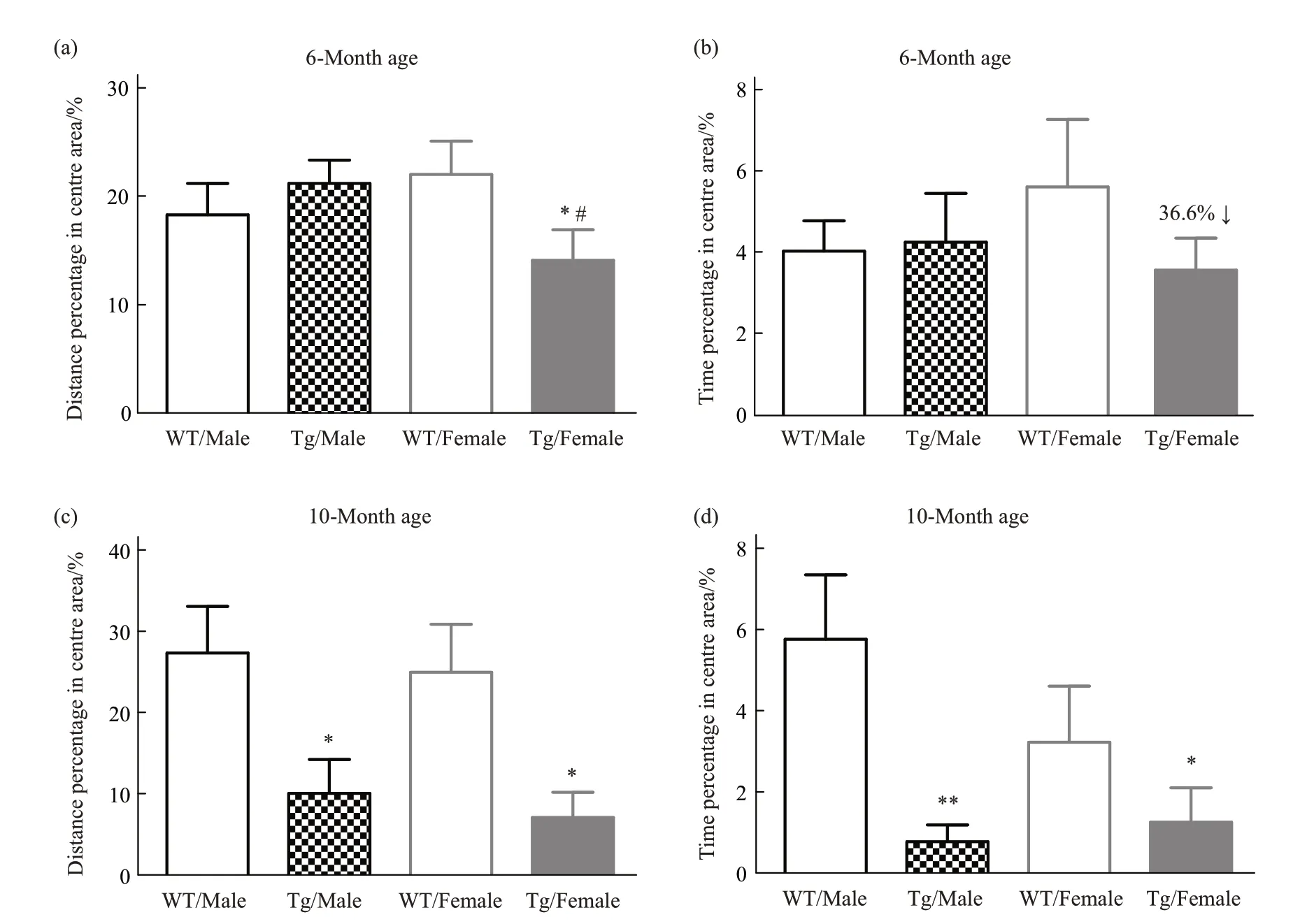
Fig.2 Anxiety has been evaluated from 6-month to 10-month of age
In the step-down test, short-term memory has been evaluated. At 6-month of age, there is no memory deficits in terms of latency to the step-down platform and time spent in the safety zones among all groups (Figure 3a, b). But, at 10-month of age, both female and male Tg mice showed significant memory loss compared with their WT controls (Figure 3c);although there is no difference observed in time spent in the safety zone, a reduced duration tendency was occurred in both Tg mice compared with their littermate WT controls(Figure 3d).

Fig.3 Short-term memory has been evaluated from 6-month to 10-month of age
2.4 Spatial learning and memory were normal in both genders at 6-month of age,but impairment observed at 10-month age
In the Morris water maze test, spatial learning and memory have been evaluated.At 6-month of age,there is no cognition impairment occurred in all groups (Figure 4a, b). Further, at 10-month of age,both female and male Tg mice showed significant spatial learning deficits compared with their WT controls; interestingly, male Tg mice exhibited more severe cognitive impairment compared with female Tg mice at the fifth training day (Figure 4c). Again,both Tg genders showed spatial memory deficits in the trial testing(Figure 4d).

Fig.4 Spatial learning and memory have been evaluated from 6-month to 10-month of age
3 Discussion
Psychological symptoms often happened in AD patients during the clinical course,or even at the early stage before cognition impairments occurred, such as anxiety and depression. Overlapped with depression,agitation, anxiety is seen in 25%-75% of the patients with AD[13]; Clinical studies have confirmed the relationship between anxiety and dementia. A metaanalysis study identified a positive association between anxiety and AD (n=26 193 out of 7 studies;hazard ratio 1.53, 95% CI 1.18-2.01,P<0.01)[3-4]. In addition, several other research reported a higher risk of AD in people with a history of anxiety[14-15]; the prevalence of anxiety highly seems to increase the early phase of cognitive decline[16]. The consistent observations also have been found in several experimental AD animals, such as the TgF344-AD rat model and APP/PS1 transgenic mice, they exhibit anxiety-like behavior, even before the occurrence of spatial memory defects[17-19]. In addition, accumulated evidence suggests that anxiety exists with gender difference at the early phase of AD[2]. Anxiety symptoms were evident in 30.1% of the female ApoE ε3/ε4 heterozygotes compared with 7.3% of the male patients[11]. In animal studies, researchers also found the gender difference occurred in AD experimental models, such as APP/PS1, 5×FAD mice[20]. Some studies suggested that APP V717I mice develop later in life abundant amyloid plaques in the brain parenchyma[21], as well as vascular deposits[22].Nevertheless, in another study using male mice of these models, they have observed early differences in exploratory behavior, anxiety-like behavior, as well as inflexibility in hippocampus-dependent learning in two mouse models that reproduce certain pathological features of human AD[23]. However, most studies have used only males. In our previous study, we found that female and male APP V717I mutation Tg mice responded medically differently[24], suggesting distinguishing treatments in genders should be under consideration. In order to extend our previous observation, we further assessed the age and gender difference in APP V717I mice.In this study,we found gender differences in APP V717I Tg mice:a. locomotor activity significantly reduced with aging,but there was no gender difference at their same age;b. compared with male Tg mice, female mice showed significant anxiety at 6-month age;while both genders showed anxiety at 10-month age; c. in terms of the short-term memory, the impairment was only observed at 10-month age in both genders, but normal at 6-month age; d. again, consistent with the shortterm memory, the special learning and memory were only impaired at 10-month age in both genders.These results showed that APP V717I Tg mice exhibited a clear behavioral difference with age and gender,suggesting APP V717I Tg mice can be a good candidate to study the gender differences of AD.
As we know, estrogen is a neuroactive steroid,and its decline can cause damage to brain function and mechanism[25]. Estrogen is considered to be an effective neurogenic and plasticity stimulus, and changes in its level and activity are associated with damage to neuroplasticity and insufficient neuronal renewal in the dentate gyrus of the hippocampus,leading to memory and learning disabilities.Therefore, in female patients, hippocampal atrophy is associated with memory and cognitive decline[26].Experiments have shown that the ventral hippocampal corticotropin-releasing factor 1 (CRF1) system underlies the early anxiety-like phenotype in TGF344-AD rats and APP/ haβ/PS1 mice[27]. CRF signaling also shows particular differences according to the sex of the organism. From the perspective of HPA axis activation,women seem to have a higher sensitivity to CRF[28-29]and the concentrations of CRF in the portal vein system and hypothalamus of elderly female rats were higher than those of male rats.Proteomic studies have found a direct link between female-dominated CRF1 signaling and pre-AD pathway activation,which is not found in men[30]. These results suggest that the mechanism of gender differences in anxiety in early AD may be related to the release of estrogen and CRF1.
4 Conclusion
Anxiety is an early behavioral change in the AD process, especially for female ones. In this study, we extend the observationin vivo, we found that APP V717I Tg mice showed clear age and gender differences in the process of AD.The study can better guide the gender differences in pre-clinical studies.Earlier than cognitive deficits, anxiety in AD patients is underdiagnosed and undertreated in clinical practice[31], so the lack of early prevention is a risk in AD processing. Approaches to mitigate psychotropic medication use need to consider different prescribing habits observed in women and men with AD[32]. Still,estrogen and CRF signaling play a critical role,further research is needed on the mechanism.